Abstract
Inhalant abuse is a world-wide public health concern among adolescents. Most preclinical studies have assessed inhalant effects in adult animals leaving unclear how behavioral effects differ in younger animals. We exposed adolescent (postnatal day [PN] 28) and adult (PN90) male rats to toluene using 1 of 3 exposure patterns. These patterns modeled those reported in toluene abuse in teens and varied concentration, number and length of exposures, as well as the inter-exposure interval. Animals were exposed repeatedly over 12 days to toluene concentrations of 0, 8,000 or 16,000 parts per million (ppm). Locomotor activity was quantified during toluene exposures and for 30 min following completion of the final daily toluene exposure. For each exposure pattern, there were significant toluene concentration-related increases and decreases in locomotor activity compared to the 0-ppm “air” controls at both ages. These changes depended upon when activity was measured – during or following exposure. Compared to adults, adolescents displayed greater locomotor activity on the first day and generally greater increases in activity over days than adults during toluene exposure. Adults displayed greater locomotor activity than adolescents in the “recovery” period following exposure on the first and subsequent days. Age group differences were clearest following the pattern of paced, brief (5-min) repeated binge exposures. The results suggest that locomotor behavior in rats during and following inhalation of high concentrations of toluene depends on age and the pattern of exposure. The results are consistent with dose-dependent shifts in sensitivity and sensitization or tolerance to repeated toluene in the adolescent animals compared to the adult animals. Alternate interpretations are possible and our interpretation is limited by the range of very high concentrations of toluene used. The results imply that both pharmacological and psychosocial factors contribute to the teen prevalence of inhalant abuse.
Keywords: toluene, adolescent, locomotor activity, sensitization, tolerance, rodent (rat)
1. Introduction
Overall, illicit substance abuse decreased slightly in the United States between 2002 and 2008 (SAMHSA, 2009). However, the prevalence of inhaling chemical vapors for the purpose of intoxication had not changed appreciably among American youth between 12 and 17 year olds during that time and was exceeded only by alcohol, cigarettes and marijuana use (Ding et al., 2009, Johnston et al., 2009, SAMHSA, 2009). Over 70% of people who used inhalants for the first time in 2008 were under age 18. While the number of new “inhalant initiates” each year has decreased since 2003, the average age of first-time inhalant users is getting younger (SAMHSA, 2009). Inhalant abuse also remains a well-recognized problem among adolescents throughout the world (Chakroun et al., 2008, Kurtzman et al., 2001, NIDA, 2005, Yarnold, 1996, Zebrowski and Gregory, 1996).
Animal studies have been beneficial for understanding the neurobehavioral consequences of inhalant exposure, but nearly all of these studies have used adult animals. Inhaled toluene in adult rodents dose-dependently increases locomotor activity at concentrations ranging from 500 ppm to 5,000 ppm with the greatest increases occurring during exposures to between 2,000 ppm and 5,000 ppm (Bowen and Balster, 1998a, Hinman, 1987, Riegel and French, 1999). Higher concentrations of toluene (6,000 ppm to 15,000 ppm) result in initial increases in activity which quickly progress to motor impairment, sedation (Bowen and Balster, 1998a, Himnan, 1984, Yavich et al., 1994), and ultimately death by respiratory depression if exposures to these concentrations are prolonged (Moser and Balster, 1985). Studies of the effects of inhaled toluene early in development have focused on gestational exposure followed by later assessment of toluene's effects in pre-weanling and older animals [see reviews (Bowen et al., 2006, Bowen and Hannigan, 2006, Hannigan and Bowen, 2010)]. For example, results from our laboratory and others have shown that repeated, brief (i.e., 15-min), high-concentration binge maternal toluene exposures adversely impacted prenatal development and postnatal maturation of pups (PN22 – PN63), as well as spontaneous exploration and amphetamine-induced locomotor activity (Bowen et al., 2007c). These results suggest that exposure to high concentrations of toluene during early development may be more deleterious than exposure during adulthood.
In rodents and humans, adolescence is a time of extensive neural reorganization/pruning, so the potential for long-term deleterious effects due to drug exposures is greater in adolescence than at later times in life (Spear, 2000a, Spear, 2000b). Previous research into other forms of drug abuse common to adolescents, such as alcohol, have found benefits in using adolescent animals to model drug abuse in adolescent humans (Spear, 2000a, Spear, 2000b). Both adolescent humans and adolescent rodents exhibit differential neurobehavioral responses to commonly abused drugs, such as alcohol and cocaine, than their adult counterparts (Laviola et al., 1995, Spear, 2000a, Spear, 2000b). For example, peri-adolescent rats are reported to exhibit patterns of sensitization following repeated cocaine administration that differ from adult animals given equivalent drug injections (Laviola, Wood, 1995). While repeated exposure to toluene has been shown to result in cross-sensitization to the locomotor-activating effects of cocaine in adult rodents (Beyer et al., 2001), it is unclear whether the same pattern of effects would be seen in adolescent animals given equivalent cocaine injections. Since inhalants are abused primarily by teenagers and young adults, delineation of age-dependent differences during this developmental stage is important for understanding use and abuse of these drugs.
Inhalant abuse in adolescents typically involves variable patterns of binge exposures (Watson, 1982). Such exposure patterns are brief and repeated – ranging perhaps from a few to a dozen deep breaths over several to 30 minutes, to breathing vapors over hours or in repeated bouts throughout a day – and all at very high concentrations of solvent (Brouette and Anton, 2001, Bukowski, 2001, Oliver and Watson, 1977, Watson, 1977, 1980). For toluene, estimates of the ambient concentration encountered during abuse range from 5,000 parts per million (ppm) to 10,000 ppm or greater (Bukowski, 2001, Cavender, 1993). Toluene and many of the other commonly abused volatile organic solvents can have neurobehavioral effects at concentrations well below these levels (e.g., (Cruz and Bowen, 2008, Iregren et al., 2002) and can result in motor incoordination, as well as euphoria and cognitive and behavioral effects (Bukowski, 2001, Cavender, 1993, Williams and Storck, 2007). Also, while repeated inhalations – both across and within days – are common in inhalant abuse, little is known about how behavioral responses change following different patterns of repeated exposures. Modeling the exposure patterns and levels reported in binge toluene abuse, and clarifying differences in these responses between adolescents and adults, is important for understanding the dynamics of inhalant abuse.
The purpose of the current study was to evaluate age- and dose-dependent differences in responsivity to the locomotor activating effects of different patterns of repeated binge toluene exposure. Inhaled toluene's concentration-dependent effects on activity in adult rodents have been characterized previously as progressing from initial and low-dose motor excitation to sedation, motor impairment and anesthesia as concentration and duration of exposure increase (Bowen et al., 2006, Evans and Balster, 1991). Also, assessing changes in locomotor activity with repeated exposures over days allows evaluation of possible tolerance (i.e., decreasing effects of toluene – a dose-response shift downward and/or to the right”), or sensitization (i.e., increasing effects of toluene over days – a dose-response shift upwards and/or “to the left”). Tolerance or sensitization can be indicative of the abuse potential of toluene. One current model suggests that locomotor sensitization may indicate a neural adaptation consistent with a concomitant increase in the sensitivity of dopaminergic reward pathways (Robinson and Berridge, 1993, 2003). According to this sensitization theory, emergence of locomotor hypersensitivity over days could lead to increased “craving” for the drug (Robinson and Berridge, 1993, 2003), resulting in increased use. The vast neural and hormonal differences in adolescents versus adults, including ontogenetic changes in dopamine system function at this age range (cf., (Spear, 2000b), may account for the different sensitivity, sensitization or tolerance, and withdrawal responses seen in adolescence.
Consistent with the pervasive clinical picture of greater toluene use/abuse in human teens than in adults, we used a clinically relevant, validated animal model of binge toluene use. We hypothesized that adolescent rats would show greater sensitivity to the acute locomotor stimulatory effects of binge-like toluene exposures than adult rats. We also hypothesized that repeated exposure would result in sensitization to the locomotor stimulatory effects in both age groups, and that – consistent with previous work comparing adolescent and adult animals (Spear, 2000a, Spear, 2000b) – the sensitization would be greater in the adolescent rats. Finally, modeling the practices reported in human teen binge toluene abuse, we explored different exposure patterns (5 min versus 15 min) using shorter intervals between exposures (30 min rather than 2 hrs) to examine effects of toluene's locomotor stimulatory effects in both the adolescent and adult rats.
2. Materials and Methods
2.1. Animals
The Wayne State University Institutional Animal Care and Use Committee approved all animal procedures with each measure conducted in accordance with the NIH “Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy Press 1996; NIH Publication No. 85-23, revised 1996). Male Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI, USA) on postnatal day 24 (PN24; weighing ~50g on arrival; “Adolescent”) or PN86 (weighing ~350g on arrival; “Adult”). Following arrival, animals were weighed and group-housed (4 adolescent animals/cage and 2 adult animals/cage) in polypropylene cages (52 × 28 × 22-cm) with hardwood chip bedding and had ad lib access to Rodent Lab Diet 5001 (PMI, Nutrition International, Inc., Brentwood, MO) and water when in their home cages. Access to food and water was only limited when animals were in the lab for their toluene exposures. The AAALAC-certified vivarium was maintained on a 12-h light/dark cycle (lights on at 0700) with temperature controlled at 22 ± 2°C. Animals from each age group acclimated to the new environment for a period of 4 days prior to any experimental manipulations. The spacing of arrival, exposures, and behavioral testing was identical for animals at both age groups.
2.2. Toluene inhalation exposure
Rats were transported to the laboratory at least one hour prior to the start of daily toluene exposure. Body weights were measured daily during this pre-session period of acclimation to the experimental environment. Toluene vapor exposures were conducted in sealed 36-liter transparent cylindrical glass jars with acrylic lids, described in detail by (Bowen et al., 2005). Briefly, the lids were equipped with an injection port, fan, and a stainless steel mesh box holding filter paper. During exposures, animals were placed onto a steel grid floor 20 cm from the bottom and 30 cm from the filter paper in the lid of the chamber. For air-only, 0-ppm exposures, the lid was sealed, and the fan turned on. For daily toluene exposures, after the lid was sealed a calculated amount of toluene was injected onto filter paper suspended below the sealed lid. The fan was then turned on which volatilized and distributed the solvent throughout the chamber. Toluene (T-324, Fisher Scientific, Fairlawn, NJ, USA) was purchased commercially (purity ≥ 99.5%). Toluene vapor concentrations were confirmed periodically by single wavelength-monitoring infrared spectrometry (Miran 1A, Foxboro Analytical). Mean ambient nonzero concentrations of toluene were within 3% of nominal ~2.5 min after toluene was added and remained within 2% of the nominal concentrations throughout the sessions. Levels of CO2 and water vapor were monitored during pilot studies and changes during sessions were negligible.
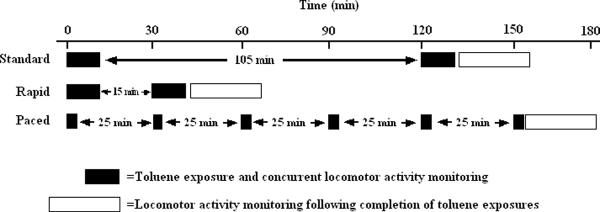
Animals were assigned randomly to 1 of 3 exposure patterns. The different patterns represented some of the variability in patterns of use by human teens (Brouette and Anton, 2001, Bukowski, 2001, Oliver and Watson, 1977, Watson, 1977, 1980). In the first “Standard” pattern, animals were exposed to toluene using two 15-min exposures (for a total of 30 min/day) separated by a 120-min interval. The total time from initiation of the first exposure to completion of the final exposure was 135 mins. This pattern is labeled “Standard” only because it has been used in our prior studies (e.g., (Bowen et al., 2005, Bowen et al., 2009a, Bowen et al., 2007b, Bowen et al., 2009b, Bowen et al., 2007c, Jarosz et al., 2008, O'Leary-Moore et al., 2009)). In the second “Rapid” pattern, animals were similarly exposed to toluene using two 15-min exposures (also for a total of 30 min/day), but separated by a shorter 30-min interval. The total time for the “Rapid” pattern, from initiation of the first exposure to completion of the final exposure, was 45 mins. In the third “Paced” pattern, animals were exposed to toluene using six 5-min exposures (for a total of 30 min/day) with 30-min intervals separating the beginning of consecutive exposures. The total time from initiation of the first exposure to completion of the final exposure for the “Paced” pattern was 155 mins. This “Paced” pattern may mimic the practice of “cuffing” where teens inhale more briefly but repeatedly during the day from solvent-soaked cuffs or cloth bracelets (Cruz and Bowen, 2008, Hannigan and Bowen, 2010). We emphasize that the total cumulative toluene exposure time for all patterns was 30 min per day; the patterns of exposure episodes and total duration varied (see Figure 1).
Figure 1.
Schematic of toluene exposures and locomotor activity testing for each of the 12 days of toluene exposures. Solid boxes indicate toluene exposure and concurrent locomotor activity monitoring. Open boxes indicate a “recovery period” monitoring spontaneous locomotor activity in a toluene-free environment for 30 min immediately following the final toluene exposure each day. Total cumulative exposure time each day – at 30 min – is equivalent among the three exposure patterns, “Standard,” “Rapid” or “Paced.”
Within each of the 3 exposure patterns, animals were assigned randomly to 1 of 3 toluene concentration groups: 0, 8,000 or 16,000 ppm, with a final cell size of 8 animals/cell (total N = 144). These concentrations (functionally equivalent to “doses”) were chosen because, as detailed above, they represent the very high concentrations seen typically in inhalant use/abuse among teens. Binge toluene exposures for adolescent and adult rats began on PN28 or PN90, respectively. Exposures to toluene occurred 6 days/week for 2 consecutive weeks. A given animal was exposed to the same concentration of toluene in the same pattern for all 12 days of exposures. Each individual rat was tested at approximately the same time each day; however, test times for rats for all groups were distributed throughout the day.
2.3. Locomotor Activity Testing
Locomotor activity was measured within the static exposure chamber via 3 sets of 16-beam infrared (I/R) emitter-detector arrays (Med Associates, St. Albans, VT) mounted on Plexiglas bases around the sides of the exposure chambers. Interruptions of I/R beams were recorded automatically (Open Field Activity Software [SOF-811], Med Associates, St. Albans, VT) and quantified total beam breaks in the horizontal plane, encoding a measure of distance traveled (cm). Activity was recorded in 5-min time Blocks over the duration of each session for all patterns. The processing of photobeam break counts was scaled automatically to reflect differences in the sizes of the animals such that activity field for the adolescent animals was 60% of the size for the adult animals (i.e., settings of 3 for adolescents and 5 for adults; Med Associates software). Each day, following completion of all toluene exposures, animals were removed from the exposure chambers and placed into separate, toluene-free clear Plexiglas boxes (70 × 70 × 30 cm high; Model ENV-515; Med Associates, St. Albans, VT, USA) where activity was assessed for an additional 30 min via interruptions of photo beams as described above. After this last assessment, animals were returned each day to the vivarium in their home cages.
It is important to note our rationale for a key feature of this design, assessing behavior both during and after the toluene exposure. With other routes of administration (e.g., i.p., p.o. or s.c.), the time course of behavioral observation following an injection or intubation in rats will likely include the time required for absorption and distribution (although this can be ignored) plus a sometimes extended period during which circulating levels of drug are declining due to metabolism and elimination. With inhalation, in contrast, absorption is extremely rapid and administration continues throughout the entire duration of the exposure. Behavior after exposure, which is during the “falling limb” of the time-concentration curve, is often ignored. A pharmacologically complete and clinically relevant examination of toluene's effects on locomotion must include assessment of these post-exposure “recovery” sessions. Consideration of behavior during both time periods is necessary to judge age differences in sensitivity and sensitization or tolerance to toluene.
2.4 Statistical Analyses
For the first day of exposure, to assess initial differences in sensitivity to toluene between ages and among exposure patterns, locomotor activity – measured as distance traveled – was analyzed during the toluene-exposure sessions and separately for the post-exposure “recovery” session using 2 × 3 × 3, three-way analysis of variance (ANOVA) with Age (Adolescent or Adult), Toluene treatment (0 ppm, 8,000 ppm & 16,000 ppm), and exposure Pattern (Standard, Rapid, Paced) as the between-subjects factors.
Across repeated exposures over Days, for each of the 3 exposure patterns, locomotor activity was analyzed during the toluene-exposure sessions and separately for the post-exposure “recovery” session. Data were analyzed using 2 × 3 × 12, three-way, repeated-measures ANOVAs with Age (Adolescent or Adult) and Toluene treatment (0 ppm, 8,000 ppm & 16,000 ppm) as the between-subjects factors, and Day (12) as the within-subjects factor. Results from initial analyses that had also included 5-min Block repeated factor nested within Day were consistent with findings regarding differences in concentration-related toluene sensitivity and differences in how behavior changed over days between adolescents and adults. Therefore, for greater clarity, analyses involving the 5-min Block factor are not reported.
Baseline activity levels were determined by averaging locomotor activity on the control air-only test sessions and responses of all animals were expressed as percent of these control baseline levels. An alpha level of p<0.05 determined statistical significance. Tukey's B post hoc contrasts and simple main effects analyses were used to determine the locus of significant main effects and interactions.
2.5. Chemicals
Toluene was purchased from Fisher Scientific (T-324, Fisher Scientific Co., Fairlawn, NJ). A measured amount of toluene was drawn directly from the bottle into a glass syringe and injected into the static vapor exposure system.
3. Results
The mean (± SEM) distance traveled (cm) during the first day of air-only (i.e., 0 ppm) sessions testing for the adolescent and adult animals, respectively, for each of the exposure patterns were: “Standard” − 2222.0 ± 230.8 & 1416.7 ± 297.8; “Rapid” − 1637.8 ± 201.4 & 957.4 ± 198.4; and “Paced” − 1467.8 ± 238.7 & 1194.5 ± 241.3. There was a significant main effect for Age F(1,42) = 9.18, p<0.001, with adolescents being significantly more active at baseline than the adults. Differences among the exposure patterns did not reach statistical significance, p=0.06.
None of the animals exposed to toluene showed signs of prolonged toxicity due to these brief toluene exposures following any pattern of exposure. Adolescent animals exposed to toluene never lost their righting reflex, whereas adult animals exposed to either 8,000 ppm or 16,000 ppm of toluene lost the righting reflex during the final exposures of each day (data not shown). Adult animals regained the righting reflex within a few minutes following removal from the exposure chambers (<5 min), during the recovery period.
3.1 “Standard” Exposure Pattern
3.1.1 Acute Activity during Initial Toluene Exposure (Day 1 only)
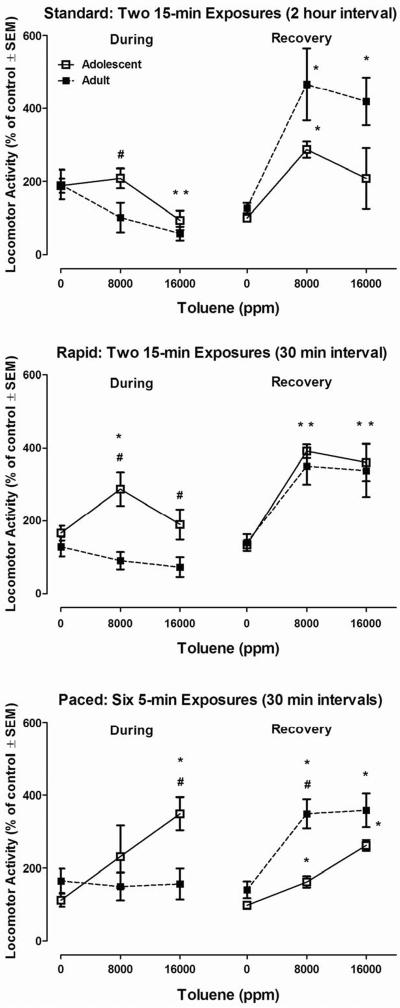
The comparisons on Day 1 assessed initial concentration-dependent differences in sensitivity to acute toluene across the two ages (see Figure 2, top left panel). The main effect for Age during the first day's Standard exposure approached but did not reach significance (F(1,42) = 3.61, p<0.06). There was, however, a significant effect of Toluene concentration (F(2,42) = 7.55, p<0.002) with post hoc tests showing that the 16,000 ppm-group had lower locomotor activity than the 0-ppm control group (p<0.05). The Toluene × Age interaction was not significant (p=0.19).
Figure 2.
Effects of inhaled 0, 8,000 ppm, or 16,000 ppm toluene during exposures (left side) on rat locomotor activity, and in the “recovery” period following exposure (right side) expressed as percent of 0-ppm air control. In the top panel, mean (± s.e.m.) total distance traveled during a “Standard” exposure pattern summed across two 15-min sessions (separated by a 120-min interval); in the middle panel, mean (±s.e.m.) total distance traveled during a “Rapid” exposure pattern summed across two 15-min sessions (30-min interval); in the bottom panel, mean (± s.e.m.) total distance traveled during a “Paced” exposure summed across six 5-min sessions (with 30-min intervals). Mean (± s.e.m.) total distance traveled in a 30-min post-exposure “recovery” period is similarly expressed as percent of 0-ppm air control in all three panels. * Significantly different from 0 ppm (p≤ 0.05). # Significantly different across age (p≤ 0.05). N=8 rats per concentration.
3.1.2 Activity during Repeated “Standard” Toluene Exposure
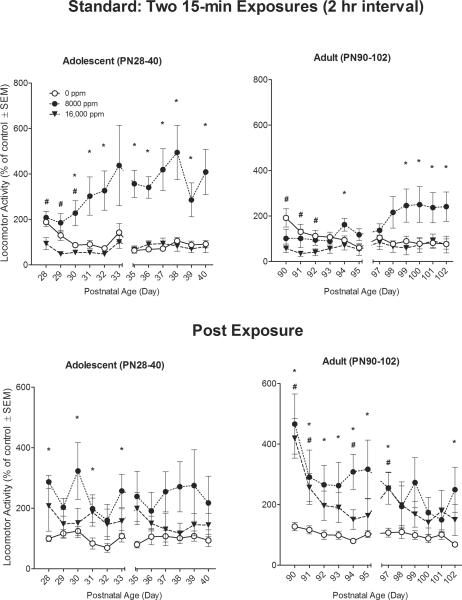
The primary comparisons of interest were among the toluene concentration-effect curves (i.e., “dose-response curves”) across the two ages and days. There were significant main effects for Age (F(1,42) = 5.56, p<0.05), and Toluene (F(2,42) = 19.79, p<0.001). As seen in the top panels of Figure 3, toluene-induced increases in activity for the adult animals were less overall than for the adolescents across all toluene concentrations tested (p<0.05). Toluene at 8,000 ppm increased locomotor activity at both ages, whereas exposure to the 16,000-ppm concentration resulted in non-significant decreases compared to the air-controls. The Toluene × Age interaction was significant (F(2,42) = 4.62, p<0.05), with adolescent animals showing greater increases in activity than adult animals during exposure to the 8,000-ppm toluene concentration (p<0.01). A significant main effect was also observed for Day (F(11,462) = 2.65, p<0.01), with distance traveled changing significantly across sessions (top panels of Figure 3). Finally, there was a significant Toluene × Day interaction (F(22,462) = 3.99, p< 0.001) with the 8,000-ppm animals in both ages showing significant increases in locomotion beginning 2 to 4 days after repeated exposure and continuing across days.
Figure 3. Standard Exposure Pattern.
Effects of inhaled 0, 8,000 ppm, or 16,000 ppm toluene exposures (top panel) on rat locomotor activity, and post-exposure (bottom panel). Mean (±s.e.m.) total distance traveled during exposure (top panel) summed across two 15-min sessions (with a 120-min interval) is expressed as percent of 0-ppm air control. Mean (±s.e.m.) total distance traveled in a 30-min post-exposure session (bottom panel) is similarly expressed as percent of 0-ppm air control. * 8,000 ppm significantly different from 0 ppm (p≤ 0.05). # 16,000 ppm significantly different from 0 ppm (p≤ 0.05). N=8 rats per concentration.
3.1.3 Acute Activity following Initial Toluene Exposure (Day 1 only)
Day 1 concentration-dependent differences in sensitivity following acute toluene across the two ages are seen in Figure 2, top right panel. There were significant main effects for Age (F(1,42) = 7.99, p<0.007) and Toluene concentration (F(2,42) = 10.35, p<0.0001) following the first day's Standard exposure. Adult rats had greater locomotor activity than the adolescents with both 8,000 ppm and 16,000 ppm toluene significantly increasing locomotion. The Toluene × Age interaction, however, was not significant (p=0.28).
3.1.4 Activity following Repeated “Standard” Toluene Exposure
Analysis of locomotion in the 30-min recovery period following the last daily Standard toluene exposure revealed a significant main effect for Toluene (F(2,42) = 7.68, p<0.001). After exposure, toluene resulted in increases in locomotor activity across both ages as compared to the air-only controls. There was no main effect for Age (p=0.54) nor a Toluene × Age interaction (p=0.91). A significant main effect was observed for Day (F(11,462) = 4.01, p<0.001): locomotor activity decreased significantly across days (bottom panels of Figure 2), but no Toluene × Day interaction (p= 0.21). Finally, there was a significant Age × Day (F(11,462) = 2.33, p< 0.001) interaction with adult animals recovering from toluene by showing significantly greater activity compared to their adolescent counterparts.
3.2 “Rapid” Exposure Pattern
3.2.1 Acute Activity during Initial Toluene Exposure (Day 1 only)
The comparisons on Day 1 assessed initial concentration-dependent differences in sensitivity to acute toluene across the two ages (Figure 2, middle left panel). There was a significant main effect for Age during the first day's Rapid exposure (F(1,42) = 19.58, p<0.0001), with the adolescent animals being more active than adults overall. There was no main effect of Toluene concentration (p=0.19) with the Toluene × Age interaction approaching significance (F(2,42) = 3.03, p=0.059).
3.2.2 Activity during Repeated “Rapid” Toluene Exposure
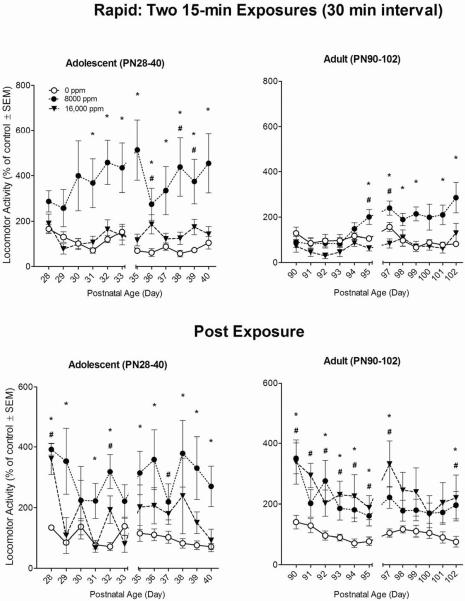
There were significant main effects for Age (F(1,42) = 8.58, p<0.05), and Toluene (F(2,42) = 13.34, p<0.001) during the Rapid exposure pattern. As seen in the top panels of Figure 4, the adult animals were less active overall than the adolescents across all toluene concentrations tested (p<0.05). Toluene increased locomotor activity at both ages, with the 8,000-ppm animals exhibiting significant increases compared to both the air controls and 16,000-ppm groups (p<0.05). The Toluene × Age interaction was also significant (F(2,42) = 4.01, p<0.05), with adolescent animals being more active than adult animals at the 8,000-ppm toluene concentration. A significant main effect was also observed for Day (F(11,462) = 3.61, p<0.001), with locomotor distance traveled changing significantly across days (top panels of Figure 3). Finally, there was a significant Toluene × Day interaction (F(22,462) = 2.95, p< 0.001) such that for 8,000 ppm animals, the adult showed significant intermittent increases in activity beginning on Day 6 of the repeated exposure to toluene, whereas for the adolescent animals, that significant increase was greater, consistent, and appeared earlier, on Day 3.
Figure 4. Rapid Exposure Pattern.
Effects of inhaled 0, 8,000 ppm, or 16,000 ppm toluene exposures (top panel) on rat locomotor activity, and post-exposure (bottom panel). Mean (±s.e.m.) total distance traveled during exposure (top panel) summed across two 15-min sessions (with a 30-min interval) is expressed as percent of 0-ppm air control. Mean (±s.e.m.) total distance traveled in a 30-min post-exposure session (bottom panel) is similarly expressed as percent of 0-ppm air control. * 8,000 ppm significantly different from 0 ppm (p≤ 0.05). # 16,000 ppm significantly different from 0 ppm (p≤ 0.05). N=8 rats per concentration.
3.2.3 Acute Activity following Initial Toluene Exposure (Day 1 only)
The only significant main effect was for Toluene concentration following the first day's Rapid exposure, (F(2,42) = 17.47, p<0.0001), with both the 8,000 ppm and 16,000 ppm-groups having higher locomotor activity than the 0-ppm control group. See Figure 2, middle right panel.
3.2.4 Activity following Repeated “Rapid” Toluene Exposure
In the recovery period following Rapid exposure, there was no significant main effect for Age (p=0.75), although a significant main effect was observed for Toluene (F(2,42) = 9.32, p<0.001). As seen in the bottom panels of Figure 4, recovery from either toluene dose resulted in increases in locomotor activity at both ages as compared to the air-only controls. A significant main effect was also observed for Day (F(11,462) = 5.55, p<0.001), with locomotor distance traveled decreasing significantly as the post-exposure sessions progressed (bottom panels of Figure 4). There were no significant Toluene × Day (p=0.47), Age × Day (p=0.26) or Toluene × Age × Day interactions (p=0.12).
3.3 “Paced” Exposure Pattern
3.3.1 Acute Activity during Initial Toluene Exposure (Day 1 only)
The comparisons on Day 1 assessed initial concentration-dependent differences in sensitivity to acute toluene across the two ages (Figure 2, bottom left panel). The main effects for Age (F(1,42) = 3.48, p=0.069) and Toluene concentration (F(2,42) = 2.78, p=0.07) during the first day's Paced exposure approached but did not reach significance. There was a significant Toluene × Age interaction (F(2,42) = 3.22, p=0.05) such that during the 16,000-ppm exposure the adolescents were more active than the adults.
3.3.2 Activity during Repeated “Paced” Toluene Exposure
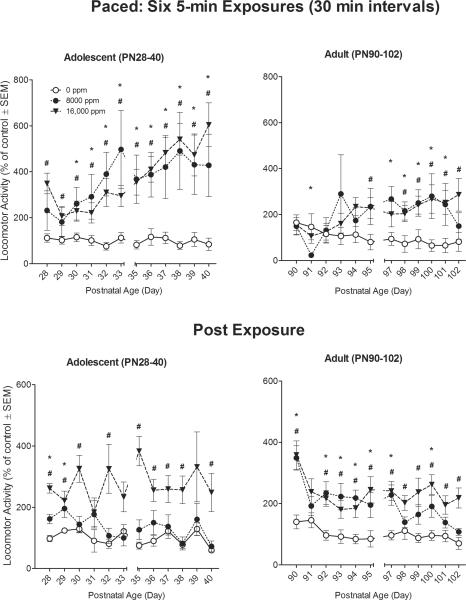
As was observed with the other exposure patterns, there were significant main effects for Age (F(1,42) = 6.07, p<0.05), and Toluene treatment (F(2,42) = 7.66, p<0.001). As seen in the top panels of Figure 5, the adult animals were less active overall than the adolescents across all toluene concentrations tested (p<0.05). Unlike the other, 15-min exposure patterns, the 5-min toluene inhalations resulted in concentration-dependent increases in locomotor activity across both ages. A significant main effect was also observed for Day (F(11,462) = 7.67, p<0.001), with locomotor distance traveled increasing significantly as the sessions progressed (top panels of Figure 5). Finally, there were significant Day × Age (F(11,462) = 2.02, p< 0.05) and Toluene × Day (F(22,462) = 4.83, p< 0.001) interactions with adolescents showing greater activity across days and animals in both ages showing significant increases in activity ~4 days after repeated toluene exposure. The Toluene × Day × Age interaction was not significant (p=0.49).
Figure 5. Paced Exposure Pattern.
Effects of inhaled 0, 8,000 ppm, or 16,000 ppm toluene exposures (top panel) on rat locomotor activity, and post-exposure (bottom panel). Mean (±s.e.m.) total distance traveled during exposure (top panel) summed across six 5-min sessions (with 30-min inter-exposure intervals) is expressed as percent of 0-ppm air control. Mean (±s.e.m.) total distance traveled in a 30-min post-exposure session (bottom panel) is similarly expressed as percent of 0-ppm air control. * 8,000 ppm significantly different from 0 ppm (p≤ 0.05). # 16,000 ppm significantly different from 0 ppm (p≤ 0.05). N=8 rats per concentration.
3.3.3 Acute Activity following Initial Paced Toluene Exposure (Day 1 only)
The comparisons on Day 1 assessed initial concentration-dependent differences in sensitivity after acute toluene across the two ages (see Figure 2, bottom right panel). Significant main effects were observed for both Age (F(1,42) = 22.05, p=0.0001) and Toluene concentration (F(2,42) = 24.16, p=0.0001) during the first day's Paced exposure. There was also a significant Toluene × Age interaction (F(2,42) = 3.32, p<0.05) with adults being more active than adolescents following the 8,000-ppm exposure.
3.3.4 Activity after Repeated “Paced” Toluene Exposure
Analysis of the post-exposure period revealed that there was a significant main effect for Toluene (F(2,42) = 25.41, p<0.001) with recovery from toluene treatment producing increases in locomotor activity across both ages. While there was no main effect for Age (p=0.69), the Toluene × Age interaction was significant (F(2,42) = 3.13, p=0.05), with adult animals being more active than adolescent animals at the 8,000 ppm toluene concentration (p<0.05). A significant main effect was observed for Day (F(11,462) = 4.44, p<0.001), with locomotor distance traveled decreasing significantly as the sessions progressed (bottom panels of Figure 5). Finally, there were significant Age × Day, (F(22,462) = 3.23, p< 0.001), Toluene × Day (F(22,462) = 1.87, p< 0.05) and Age × Day × Toluene, (F(22,462) = 1.59, p< 0.05) interactions, with adult animals initially showing significant increases in activity as compared to their adolescent counterparts and the adult 8,000-ppm toluene group displaying more activity than their adolescent counterparts.
4. Discussion
The present data demonstrate that adolescent and adult rats differed in locomotor activity during and following acute and repeated binge exposures to toluene that modeled the levels and patterns of solvent vapor exposure reported clinically in inhalant use/abuse by human teens. In general, adolescent rats showed higher levels of locomotor activity than adults during the three patterns of exposure. In contrast, the adult rats showed levels of locomotor activity that were higher than adolescents in the recovery period following exposures.
The results for behavior during each of the three patterns of exposure are consistent with our a priori hypothesis that there would be greater sensitivity to the locomotor stimulatory effects of acute binge-like toluene exposure in adolescent rats than in adult rats. This increased sensitivity is evident at Day 1, is clearer during the Rapid and Paced exposure patterns than during the Standard exposure, and depends on the concentration of exposure (left panels, Figure 2). Similarly, the changes in behavior over days during each of the three patterns of exposure are also consistent with our a priori hypothesis that there would be greater sensitization to the locomotor stimulatory effects of repeated binge-like toluene exposure in adolescent rats than in adult rats (top panels, Figures 3, 4 & 5). There were significant increases over days in locomotor activity during exposure to 8,000 ppm toluene, for both adolescents and adults, in all three exposure patterns.
The magnitude of the increases over days during exposure was significantly greater for adolescents than for adults. This sensitization to toluene-induced locomotion activity appeared 3 to 6 days sooner for the adolescents than for the adults. This age-difference in sensitization to either 8,000 ppm or 16,000 ppm of toluene was clearer during the repeated Paced pattern of exposure than during the Standard or Rapid patterns. Adolescent and adult male rats were exposed to binge patterns modeling the practices reported in human teen binge toluene abuse (Brouette and Anton, 2001, Bukowski, 2001, Oliver and Watson, 1977, Watson, 1977, 1980). The three exposure patterns varied in frequency, duration and the interval between individual exposures over two weeks while keeping the total cumulative exposure time, at 30 min/day, identical for all three exposure patterns. In addition to the toluene-induced increases in locomotor activity at both ages, and the greater increases in locomotor activity in the adolescent animals than the adults during the 8,000-ppm exposure, there were differences among exposure patterns at the 16,000-ppm dose. There were no significant increases in locomotion during the two 15-min exposures to 16,000-ppm toluene (i.e., the “Standard” or “Rapid” patterns – the 2-hr or 30-min ITI, respectively) for either the adolescent or adult rats indicating a biphasic response. During the shorter 5-min-exposure “Paced” pattern with 30-min intervals, however, rats at both ages showed significant increases in locomotion to the 16,000-ppm toluene dose (Figure 5, top panels). Here again, the adolescents showed greater relative increases in locomotor activation over days than the adults, consistent with the hypothesis that the adolescent animals were showing greater sensitivity as well as apparent sensitization to the locomotor activating effects of toluene during exposures at these concentrations. While the 16,000-ppm concentration of toluene is higher than what has been examined previously in rodents, these results are in general agreement with previous studies using adult rats (Himnan, 1984, Hinman, 1987, Yavick et al., 1994) and adult mice (Bowen and Balster, 1998a, Bushnell et al., 1985, Wood and Colotla, 1990) in which the acute locomotor effects of inhaled toluene were shown to be biphasic, with excitation at concentrations below 4,000 ppm and sedation with motor impairment at concentrations above 6,000 ppm. However, to our knowledge, this is the first study to study shorter 5-min repeated binge toluene exposures, perhaps more faithfully modeling some abuse patterns in teens (including “cuffing”), in comparison to the longer exposure patterns used in previous investigations (Balster et al., 1997, Bowen, 2009, Bowen and Balster, 1996, 1997, 1998a, b, Bowen and McDonald, 2009, Bowen et al., 1996a, Bowen et al., 1996b, Himnan, 1984, Hinman, 1987).
These data during exposure are consistent with an interpretation of greater sensitivity and sensitization to toluene in adolescents than in adults. However, this conclusion must be qualified because an alternate interpretation of the underlying dose-response curves is supported by behavior in the recovery periods following toluene exposure. Locomotor activity following acute binge-like toluene exposure was greater in adult than in adolescent rats following the Standard and Paced (but not Rapid) exposure patterns, and following either 8,000 ppm or 16,000 ppm of toluene (right panels, Figure 2). Further, the changes in behavior over days following each pattern of repeated binge toluene exposure were different for the adults and adolescents (bottom panels, Figures 3, 4 & 5). Adolescent behavior appeared more variable within and over days than adults who were more active at 8,000 ppm than adolescents following the Standard and Paced patterns. For adults but not adolescents, there were significant decreases in toluene-induced activity across days following the Standard and Paced patterns. These data following exposure may support an interpretation of greater sensitivity and sensitization to toluene in adults rather than in adolescents.
Because of the unique dynamics of inhalation routes of exposure, which are directly related to pattern of exposure (e.g., administrations lasting 5–15 minutes, contrasted to relatively instantaneous i.p., s.c. or p.o. administrations), behavior in the 30-min “recovery” period can be equated to behavior assessed through the “falling limb” phase with any drug administration. Behavior following toluene exposure, as blood concentrations and effects of toluene diminish, has been reported previously with lower concentrations (Bowen and Balster, 1998a, Hinman, 1987, Wood and Colotla, 1990). The age-dependent differences in locomotion measured during and following binge exposure to the high concentrations used here must, therefore, reflect some broader dose-or concentration-response characteristics than those seen in either period alone. Behavior in “recovery” may be inconsistent with our interpretation of adolescent animals being more sensitive to toluene than adults. In contrast to effects during exposure, when adolescents appeared more sensitive to toluene and showed greater sensitization with repeated exposures than adults, levels of locomotor activity for adults following exposure were equivalent to or greater than adolescents. The results taken together highlight the age dependence of bi-phasic dose-response characteristics and could support the alternate hypothesis that adult rats are more sensitive to toluene than adolescents, that is, a “right-ward” shift in the dose-response curve for adolescents relative to adults. The results following exposure are consistent with the notion that as blood toluene levels are reduced during the post-exposure recovery period, adults are moving from sedation to locomotor activation on this biphasic dose-response curve, while adolescents are moving from levels producing activation to no effects. This interpretation is also supported by behavior following the 5-min “Paced” exposure pattern. Specifically, the adolescents showed their highest levels of locomotion following the 5-min 16,000-ppm concentration exposures, when blood levels would be dropping into the range producing locomotor activation, whereas the shorter exposure to the 8,000-ppm concentration did not support persistent activity during recovery in adolescents. In contrast to the adolescents, following the 5-min exposures to toluene in the “Paced” pattern, adults show higher relative increases in locomotion over days after both 8,000 ppm and 16,000 ppm toluene, consistent with a greater sensitivity to the locomotor increasing effects of toluene in adults than in adolescents.
The only other study to date that has examined locomotor activity immediately following high-dose toluene exposures at concentrations likely to be encountered in abuse settings is one by Hinman (Hinman, 1987). In the present study, separate apparatuses were used to measure locomotor activity during the recovery phase than those that were used to measure locomotor behavior during toluene exposures. The study by Hinman (Hinman, 1987) used the same chambers for measuring locomotor activity both during and after 60-min exposures to estimated concentrations of 2,500 ppm to 15,000 ppm toluene. Hinman also reported increased activity during single acute exposures to 5,000 ppm or 10,000 ppm toluene in adult male Long-Evans rats and a biphasic effect with reduced locomotion towards the end of the period during 10,000 ppm toluene and beginning about 15 min into the 15,000 ppm exposure. Also similar to the present study, Hinman reported recovery over 30 min to 90 min after toluene when rats exposed to concentrations ≤5,000 ppm toluene returned to a resting state of inactivity and rats exposed to ≥10,000 ppm returned to baseline only after a 15-min to 30-min increase in locomotor activity. Despite the considerable differences in exposure parameters and rat strain, our results are generally consistent with those of Hinman in reporting biphasic dose-response relationships following high-dose exposure to toluene in adult rats (Hinman, 1987).
The increases in locomotor activity with repeated exposure to 8,000-ppm toluene in the present study are similar to what has been previously reported for toluene and other abused inhalants (see reviews (Bowen et al., 2006, Evans and Balster, 1991). Taken in isolation it could be reasonable to interpret such increases over days (cf., top panels in Figures 2, 3 & 4) as sensitization to toluene. A handful of previous studies have reported development of sensitization after repeated exposure of adult male rodents to inhaled toluene (Bowen et al., 2007a, Himnan, 1984). Other studies demonstrating sensitization following systemically administered intraperitoneal (Riegel et al., 2003, Riegel et al., 2004, Riegel and French, 2002) or oral toluene (Wiaderna and Tomas, 2000) are difficult to reconcile with responses following inhalation. While there is consistency between the results for adult male rats in this study and those of previous reports, there is a discrepancy between the results for the adolescent rats in this study and a previous report which utilized Long-Evans rats (Bowen et al., 2007a). There are a number of factors known to contribute to differences in behavioral effects of solvents or other drugs on locomotor activity, including age, sex, species, and strain, as well as task and exposure parameters. Several studies have shown strain differences in the CNS effects of inhaled anesthetics and pesticides in adult male rodents (Moser et al., 1991, Sonner et al., 1999), indicating that strain may have played a role in the discrepancies noted above, and other differences were noted above in the discussion of Hinman, 1987 (Hinman, 1987).
Toluene has also been shown to enhance the locomotor activating affects of cocaine or diazepam (Beyer et al., 2001, Wiley et al., 2002), suggesting common neurochemical pathways with other drugs of abuse, probably involving essential dopaminergic systems in the acute and long-term effects of toluene on locomotor activity (Riegel et al., 2003, Riegel, Ali, 2004, Riegel and French, 2002). Further, cross-sensitization has also been demonstrated with cocaine and toluene (Beyer et al., 2001). The role of dopaminergic systems in the effects of toluene remains to be determined, and may prove useful in clarifying the pharmacodynamic responses – e.g., sensitization versus tolerance – to repeated binge toluene exposure. Sensitization theory (Robinson and Berridge, 1993, 2003) suggests that the development of increased locomotor activation to toluene could lead to increased “craving” for toluene and greater risk for dependence to toluene, particularly in adolescents.
While the age differences in the patterns of sensitization over days to the locomotor activating effects of toluene suggest that adolescents are more sensitive to toluene, several factors – including the age differences in activity among the three exposure patterns, age differences in dose-response characteristics in the “recovery” period following exposure, and the apparently clear biphasic effects of toluene at different ages in the present and previous studies – do not allow us to exclude the possibility that adolescents are less sensitive to toluene or that animals at either age may be developing tolerance to some effects. For example, during the recovery period for all exposure patterns for both adolescents and adults, the size of toluene-induced elevations in locomotor activity tended to decrease over days (Figures 3, 4 & 5, bottom panels).
Age-dependent differences in sensitivity to the effects of toluene intoxication extend our previous studies of the effects of acute, high-dose toluene exposure on regional brain neurochemistry in juvenile (i.e., postnatal day 21 [PN21]), adolescent (PN35) and young adult rats (PN56;(O'Leary-Moore et al., 2007, O'Leary-Moore et al., 2009). Those findings suggested significant CNS region- and dose-dependent differences in neurochemical profiles after acute toluene measured by the highly sensitive HR-MAS 1H MRS technique. The neurochemical profiles differed among juvenile, adolescent and adult animals (O'Leary-Moore et al., 2007, O'Leary-Moore et al., 2009). For example, juveniles showed increases in alanine levels in frontal cortex (like adults), and in striatum and anterior cingulate (like adolescents), whereas lactate levels in frontal cortex and striatum were increased for juveniles and adults, while only high-dose toluene elevated lactate in frontal cortex in adolescents. The present behavioral findings of dose-, age- and exposure pattern-dependent effects of binge toluene have implications for the neural effects of toluene and perhaps for the potential for age-dependent abuse, dependence and/or brain damage.
In summary, this study demonstrates that repeated exposure to abuse patterns of high concentrations of toluene via inhalation can significantly alter toluene-induced locomotor behavior in rats over days. There are clear findings that adolescent rats develop a greater sensitization than adults over days to these locomotor activating effects of repeated toluene exposure, and that the expression of these age-dependent changes also vary with the concentration of toluene and the pattern of exposure. However, it is not possible to conclude with confidence whether adolescents are more or less sensitive to toluene than adults – that is whether there was a dose-response shift to the left or right – because of the biphasic dose-response curves operating during and following exposure. These results imply that adolescents may be more likely than adults to abuse toluene, and perhaps other organic solvents, because of a pharmacodynamically mediated differential sensitivity rather than psychosocial factors alone. These results indicate the need for additional studies to evaluate neurobehavioral, metabolic and neurochemical activity in young animals exposed to wider concentration ranges of toluene to determine the degree to which these factors may play a role in the observed age-dependent differences in abuse.
Acknowledgements
This work was supported in part by NIDA R01 DA015951 to S.E. Bowen and was part of the doctoral dissertation of J.C. Batis at Wayne State University. We appreciate the excellent criticisms from two anonymous reviewers which helped us revise the manuscript and focus the “frame of reference” for biphasic dose-response curves. Dr. Batis is currently at Molecular NeuroImaging, L.L.C., New Haven, CT. Preliminary reports of a portion of this study were presented at the annual scientific meetings of the College on Problems of Drug Dependence, Scottsdale, AZ, 2005, and the Behavioral Toxicology Society, Little Rock, AR, 2006.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Balster RL, Bowen SE, Evans EB, Tokarz ME. Evaluation of the acute behavioral effects and abuse potential of a C8–C9 isoparaffin solvent. Drug and alcohol dependence. 1997;46:125–35. doi: 10.1016/s0376-8716(97)00055-0. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Stafford D, LeSage MG, Glowa JR, Steketee JD. Repeated exposure to inhaled toluene induces behavioral and neurochemical cross-sensitization to cocaine in rats. Psychopharmacology. 2001;154:198–204. doi: 10.1007/s002130000614. [DOI] [PubMed] [Google Scholar]
- Bowen SE. Time course of the ethanol-like discriminative stimulus effects of abused inhalants in mice. Pharmacology, biochemistry, and behavior. 2009;91:345–50. doi: 10.1016/j.pbb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. Effects of inhaled 1,1,1-trichloroethane on locomotor activity in mice. Neurotoxicol Teratol. 1996;18:77–81. doi: 10.1016/0892-0362(95)02024-1. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. A comparison of the acute behavioral effects of inhaled amyl, ethyl, and butyl acetate in mice. Fundam Appl Toxicol. 1997;35:189–96. doi: 10.1006/faat.1996.2278. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Experimental and clinical psychopharmacology. 1998a;6:235–47. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. The effects of inhaled isoparaffins on locomotor activity and operant performance in mice. Pharmacology, biochemistry, and behavior. 1998b;61:271–80. doi: 10.1016/s0091-3057(98)00108-7. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Mohammadi MH, Hannigan JH. Abuse pattern of gestational toluene exposure and early postnatal development in rats. Neurotoxicol Teratol. 2005;27:105–16. doi: 10.1016/j.ntt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 2006;28:636–47. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Charlesworth JD, Tokarz ME, Wright MJ, Jr., Wiley JL. Decreased sensitivity in adolescent vs. adult rats to the locomotor activating effects of toluene. Neurotoxicol Teratol. 2007a;29:599–606. doi: 10.1016/j.ntt.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Hannigan JH. Developmental toxicity of prenatal exposure to toluene. The AAPS journal. 2006;8:E419–24. doi: 10.1007/BF02854915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Hannigan JH, Cooper PB. Abuse pattern of gestational toluene exposure alters behavior in rats in a “waiting-for-reward” task. Neurotoxicol Teratol. 2009a;31:89–97. doi: 10.1016/j.ntt.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Hannigan JH, Irtenkauf S. Maternal and fetal blood and organ toluene levels in rats following acute and repeated binge inhalation exposure. Reproductive toxicology (Elmsford, NY. 2007b;24:343–52. doi: 10.1016/j.reprotox.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Irtenkauf S, Hannigan JH, Stefanski AL. Alterations in Fetal Morphology Following Abuse Patterns of Toluene Exposure. Reproductive toxicology (Elmsford, NY. 2009b;27:161–9. doi: 10.1016/j.reprotox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, McDonald P. Abuse pattern of toluene exposure alters mouse behavior in a waiting-for-reward operant task. Neurotoxicol Teratol. 2009;31:18–25. doi: 10.1016/j.ntt.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Mohammadi MH, Batis JC, Hannigan JH. Gestational toluene exposure effects on spontaneous and amphetamine-induced locomotor behavior in rats. Neurotoxicol Teratol. 2007c;29:236–46. doi: 10.1016/j.ntt.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Balster RL. The effects of abused inhalants on mouse behavior in an elevated plus-maze. European journal of pharmacology. 1996a;312:131–6. doi: 10.1016/0014-2999(96)00459-1. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Evans EB, Tokarz ME, Balster RL. Functional observational battery comparing effects of ethanol, 1,1,1-trichloroethane, ether, and flurothyl. Neurotoxicol Teratol. 1996b;18:577–85. doi: 10.1016/0892-0362(96)00064-5. [DOI] [PubMed] [Google Scholar]
- Brouette T, Anton R. Clinical review of inhalants. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2001;10:79–94. doi: 10.1080/105504901750160529. [DOI] [PubMed] [Google Scholar]
- Bukowski JA. Review of the epidemiological evidence relating toluene to reproductive outcomes. Regul Toxicol Pharmacol. 2001;33:147–56. doi: 10.1006/rtph.2000.1448. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Evans HL, Palmes ED. Effects of toluene inhalation on carbon dioxide production and locomotor activity in mice. Fundam Appl Toxicol. 1985;5:971–7. doi: 10.1016/0272-0590(85)90178-2. [DOI] [PubMed] [Google Scholar]
- Cavender F. Aromatic Hydrocarbons. In: Clayton FC G, editor. Patty's Industrial Hygiene and Toxicology. Wiley; New York: 1993. p. 1329. [Google Scholar]
- Chakroun R, Faidi F, Hedhili A, Charbaji K, Nouaigui H, Laiba MB. Inhalant abuse detection and evaluation in young Tunisians. J Forensic Sci. 2008;53:232–7. doi: 10.1111/j.1556-4029.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- Cruz SL, Bowen SE. Inhalant Abuse. In: Ubach MM, Mondragon-Ceballos R, editors. Neural Mechanisms of Action of Drugs of Abuse and Natural Reinforcers. Research Signpost; Kerala, India: 2008. pp. 61–87. [Google Scholar]
- Ding K, Chang GA, Southerland R. Age of inhalant first time use and its association to the use of other drugs. J Drug Educ. 2009;39:261–72. doi: 10.2190/DE.39.3.c. [DOI] [PubMed] [Google Scholar]
- Evans EB, Balster RL. CNS depressant effects of volatile organic solvents. Neuroscience and biobehavioral reviews. 1991;15:233–41. doi: 10.1016/s0149-7634(05)80003-x. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Bowen SE. Reproductive toxicology and teratology of abused toluene. Syst Biol Reprod Med. 2010;56:184–200. doi: 10.3109/19396360903377195. [DOI] [PubMed] [Google Scholar]
- Himnan DJ. Tolerance and reverse tolerance to toluene inhalation: effects on open-field behavior. Pharmacology, biochemistry, and behavior. 1984;21:625–31. doi: 10.1016/s0091-3057(84)80048-9. [DOI] [PubMed] [Google Scholar]
- Hinman DJ. Biphasic dose-response relationship for effects of toluene inhalation on locomotor activity. Pharmacology, biochemistry, and behavior. 1987;26:65–9. doi: 10.1016/0091-3057(87)90535-1. [DOI] [PubMed] [Google Scholar]
- Iregren A, Andersson M, Nylen P. Color vision and occupational chemical exposures: I. An overview of tests and effects. Neurotoxicology. 2002;23:719–33. doi: 10.1016/S0161-813X(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Jarosz PA, Fata E, Bowen SE, Jen KL, Coscina DV. Effects of abuse pattern of gestational toluene exposure on metabolism, feeding and body composition. Physiology & behavior. 2008;93:984–93. doi: 10.1016/j.physbeh.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Volume I: Secondary school students (NIH Publication No 09-7402) National Institute on Drug Abuse; Bethesda, MD: 2009. Monitoring the Future national survey results on drug use, 1975–2008. [Google Scholar]
- Kurtzman TL, Otsuka KN, Wahl RA. Inhalant abuse by adolescents. J Adolesc Health. 2001;28:170–80. doi: 10.1016/s1054-139x(00)00159-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. The Journal of pharmacology and experimental therapeutics. 1995;275:345–57. [PubMed] [Google Scholar]
- Moser VC, Balster RL. Acute motor and lethal effects of inhaled toluene, 1,1,1-trichloroethane, halothane, and ethanol in mice: effects of exposure duration. Toxicology and applied pharmacology. 1985;77:285–91. doi: 10.1016/0041-008x(85)90328-x. [DOI] [PubMed] [Google Scholar]
- Moser VC, McDaniel KL, Phillips PM. Rat strain and stock comparisons using a functional observational battery: baseline values and effects of amitraz. Toxicology and applied pharmacology. 1991;108:267–83. doi: 10.1016/0041-008x(91)90117-w. [DOI] [PubMed] [Google Scholar]
- NIDA . Inhalant abuse among children and adolescents: Consultation on building an international research agenda. National Institutes of Health; Washington, D.C.: 2005. Available on http://international.drugabuse.gov/meetings/inhalant_presentations.html2005. [Google Scholar]
- O'Leary-Moore SK, Galloway MP, McMechan AP, Hannigan JH, Bowen SE. Region-dependent alterations in glutamate and GABA measured by high-resolution magnetic resonance spectroscopy following acute binge inhalation of toluene in juvenile rats. Neurotoxicol Teratol. 2007;29:466–75. doi: 10.1016/j.ntt.2007.03.062. [DOI] [PubMed] [Google Scholar]
- O'Leary-Moore SK, Galloway MP, McMechan AP, Irtenkauf S, Hannigan JH, Bowen SE. Neurochemical changes after acute binge toluene inhalation in adolescent and adult rats: a high-resolution magnetic resonance spectroscopy study. Neurotoxicol Teratol. 2009;31:382–9. doi: 10.1016/j.ntt.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JS, Watson JM. Abuse of solvents “for kicks”. A review of 50 cases. Lancet. 1977;1:84–6. doi: 10.1016/s0140-6736(77)91092-3. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Ali SF, French ED. Toluene-induced locomotor activity is blocked by 6-hydroxydopamine lesions of the nucleus accumbens and the mGluR2/3 agonist LY379268. Neuropsychopharmacology. 2003;28:1440–7. doi: 10.1038/sj.npp.1300193. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Ali SF, Torinese S, French ED. Repeated exposure to the abused inhalant toluene alters levels of neurotransmitters and generates peroxynitrite in nigrostriatal and mesolimbic nuclei in rat. Annals of the New York Academy of Sciences. 2004;1025:543–51. doi: 10.1196/annals.1316.079. [DOI] [PubMed] [Google Scholar]
- Riegel AC, French ED. Acute toluene induces biphasic changes in rat spontaneous locomotor activity which are blocked by remoxipride. Pharmacology, biochemistry, and behavior. 1999;62:399–402. doi: 10.1016/s0091-3057(98)00062-8. [DOI] [PubMed] [Google Scholar]
- Riegel AC, French ED. Abused inhalants and central reward pathways: electrophysiological and behavioral studies in the rat. Annals of the New York Academy of Sciences. 2002;965:281–91. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- SAMHSA SAaMHSA . Office of Applied Studies NSH-, HHS Publication No. SMA 09-4434. Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Sonner JM, Gong D, Li J, Eger EI, 2nd, Laster MJ. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesthesia and analgesia. 1999;89:1030–4. doi: 10.1097/00000539-199910000-00039. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000a;24:115–23. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000b;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Watson JM. `Glue-sniffing' in profile. Practitioner. 1977;218:255–9. [PubMed] [Google Scholar]
- Watson JM. Solvent abuse by children and young adults: a review. Br J Addict. 1980;75:27–36. doi: 10.1111/j.1360-0443.1980.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Watson JM. Solvent abuse: presentation and clinical diagnosis. Human toxicology. 1982;1:249–56. doi: 10.1177/096032718200100307. [DOI] [PubMed] [Google Scholar]
- Wiaderna D, Tomas T. Effects of repeated exposure to toluene or amphetamine on locomotor activity in rats. International journal of occupational medicine and environmental health. 2000;13:317–24. [PubMed] [Google Scholar]
- Wiley JL, Fagalde RE, Buhler KG, LaVecchia KL, Balster RL. Evaluation of 1,1,1-trichloroethane and flurothyl locomotor effects following diazepam treatment in mice. Pharmacology, biochemistry, and behavior. 2002;71:163–9. doi: 10.1016/s0091-3057(01)00645-1. [DOI] [PubMed] [Google Scholar]
- Williams JF, Storck M. Inhalant abuse. Pediatrics. 2007;119:1009–17. doi: 10.1542/peds.2007-0470. [DOI] [PubMed] [Google Scholar]
- Wood RW, Colotla VA. Biphasic changes in mouse motor activity during exposure to toluene. Fundam Appl Toxicol. 1990;14:6–14. doi: 10.1016/0272-0590(90)90226-a. [DOI] [PubMed] [Google Scholar]
- Yarnold BM. Use of inhalants among Miami's public school students, 1992. Psychol Rep. 1996;79:1155–61. doi: 10.2466/pr0.1996.79.3f.1155. [DOI] [PubMed] [Google Scholar]
- Yavich L, Patkina N, Zvartau E. Experimental estimation of addictive potential of a mixture of organic solvents. Eur Neuropsychopharmacol. 1994;4:111–8. doi: 10.1016/0924-977x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Zebrowski PL, Gregory RJ. Inhalant use patterns among Eskimo school children in western Alaska. J Addict Dis. 1996;15:67–77. doi: 10.1300/J069v15n03_05. [DOI] [PubMed] [Google Scholar]