SUMMARY
Drug-induced immune thrombocytopenia (DITP) can be triggered by a wide range of medications. Although many cases of DITP are mild, some are characterized by life-threatening bleeding symptoms. The pathogenesis of DITP is complex in that at least six different mechanisms have been proposed by which drug-induced antibodies can promote platelet destruction. It is possible in many cases to identify antibodies that react with platelets in the presence of the sensitizing drug, but the required testing is technically demanding and not widely available. Therefore, a decision on whether to discontinue an implicated medication in a patient suspected of having DITP must be made on clinical grounds. An algorithms is available that can be helpful in assessing the likelihood that a particular drug caused thrombocytopenia, but the most important single determinant in managing a patient is a high index of suspicion and a careful history of drug exposure in an individual who presents with acute, often severe thrombocytopenia of unknown etiology. How drugs induce platelet-reactive antibodies and how, once formed, the antibodies cause platelet destruction following exposure to the drug is poorly understood. Further studies to address these issues and characterize more completely the range of drugs and drug metabolites that can cause DITP are needed.
INTRODUCTION
Thrombocytopenia is a recognized side effect of treatment with a wide range of medications. Certain agents, particularly those used for chemotherapy and regulation of immunity, tend to suppress hematopoiesis and produce pancytopenia. A few preferentially inhibit megakaryocytopoiesis to produce isolated thrombocytopenia[1, 2]. However, many medications lower platelet levels by accelerating platelet clearance through immune and (less often) non-immune [1, 2] mechanisms. In patients who experience an acute drop in platelet levels, usually within a week or two of starting a new medication, antibody-mediated platelet destruction should be suspected.
INCIDENCE
Epidemiologic studies performed in the US and Europe suggest that about 10 persons per million are affected by DITP annually[3], but the incidence could be higher in elderly and hospitalized persons more likely to be exposed to medications. A study done in the Eastern United States estimated that DITP occurred in persons treated with sulfamethoxazole/trimethoprim or quinidine/quinine at the rates of 36 and 28 persons per million per week of exposure[4]. Since these drugs are among the most common causes of DITP, the incidence of this condition in persons treated with most other medications is undoubtedly lower. Comparable data are not available for drug-induced immune hemolytic anemia. However, samples referred to our center for drug-dependent platelet antibody testing regularly exceed referrals for neutrophil and erythrocyte antibody testing by a ratio of more than 10:1, consistent with the likelihood that platelets are targeted by drug-induced antibodies considerably more often than other blood cell types.
PATHOGENESIS
The etiology of drug-induced immune thrombocytopenia is complex, at least six distinct pathologic mechanisms having been identified (Table 1). Heparin-induced thrombocytopenia (HIT) is technically the most common cause of drug-associated thrombocytopenia, but the drop in platelet levels in patients with HIT is rarely sufficient to provoke bleeding and thrombosis is the major clinical complication. HIT has a unique pathogenesis, has been extensively reviewed elsewhere[5, 6] and will be mentioned only briefly. Excluding heparin-induced thrombocytopenia, “quinine-type” immune thrombocytopenia and thrombocytopenia induced by platelet GPIIb/IIIa inhibitors are most likely to be responsible for a drop in the platelet count in patients seen clinically.
Table 1.
Mechanisms of drug-induced immune thrombocytopenia and commonly implicated medications *
| Designation | Mechanism | Examples |
|---|---|---|
| Hapten-dependent antibody | Drug (hapten) links covalently to membrane protein and induces a drug- specific immune response. | Penicillin, pipericillin? cephalosporin antibiotics? |
| Drug-dependent antibody | Drug induces antibody that binds to membrane protein only in the presence of soluble drug. | Quinine, many antibiotics, non-steroidal anti- inflammatory drugs, anti- convulsants |
| Fiban-induced thrombocytopenia | Drug (ligand) reacts with membrane glycoprotein IIb/IIIa and induces a conformational change recognized by naturally-occurring antibody? | Epitifibatide, tirofiban, |
| Drug-specific antibody | Naturally occurring or induced antibody is specific for the murine component of a abciximab, a chimeric Fab fragment specific for GPIIIa. | Abciximab |
| Autoantibody induction | Drug induces antibody that reacts with platelets in the absence of drug. | Gold salts, L-Dopa, procainamide |
| Immune complex | Drug binds to platelet factor 4 (PF4) to produce a complex for which antibody is specific. The resulting immune complex activates platelets via Fc receptors. | Heparin |
Adapted from RH Aster (2)
Hapten-induced antibodies
Early immunologic studies suggested that small molecules like drugs trigger an immune response only when linked covalently to a macromolecule such as a protein, in which form they act as a “hapten,” to induce a humoral immune response. The resulting antibodies recognize the carrier molecule only where the “hapten” is attached covalently. Accordingly, when drug-induced immune thrombocytopenia was first recognized as a clinical entity, it was suspected that the drug became immunogenic by being linked covalently to a cell membrane protein, thereby becoming capable of inducing a classical “hapten-dependent” antibody. Upon re-exposure of a sensitized individual to drug, it was presumed that the drug-protein complex was re-formed, providing a target for antibody and enabling it to cause platelet destruction. This mechanism probably accounts for immune hemolytic anemia formerly seen in a subset of patients treated with massive doses of penicillin[7], a drug that reacts covalently and spontaneously with free amino groups on protein by virtue of containing a reactive beta lactam structural element. A recent report indicates that the widely used penicillin derivative pipericillin can induce hapten-specific antibodies reactive with pipericillin-coated RBCs, but whether these antibodies actually cause the hemolytic anemia seen in some patients treated with pipericillin is uncertain[8]. A similar process may account for thrombocytopenia seen rarely in patients treated with penicillin[9], pipericillin[10] and cephalosporin[11, 12] antibiotics, but this has not been confirmed experimentally. As will be discussed, the usual in vitro behavior of drug-dependent antibodies (DDAbs) found in patients with DITP is distinctly different from that expected of classical “hapten-specific” antibodies.
“Quinine-type” immune thrombocytopenia
More than a century ago, it was recognized that patients treated with quinine for malaria sometimes experienced acute, severe bleeding that resolved when quinine was discontinued. It was later found that such patients had virtually no circulating platelets, despite having adequate numbers of megakaryocytes in the bone marrow. Other medications were subsequently shown to be capable of producing a similar clinical picture. In the early 1950s, JF Ackroyd and colleagues studied a series of patients who developed severe thrombocytopenia while taking the sedative allylisopropyl-acetylurea (Sedormid). They found that serum from such individuals contained a factor that caused platelet agglutination and lysis in the presence of the drug and showed that re-exposure to drug after recovery led to a recurrence of thrombocytopenia[13]. In the 1950s, NR Shulman and associates showed that the drug-dependent serum factor acting on platelets was an antibody that did not behave as if it were “hapten-specific”, since it reacted with cells only in the presence of soluble drug, its binding was not inhibited by drug at the highest concentrations achievable, and it did not react with cells pre-treated with drug and then washed[14, 15]. In a series of studies remarkable for their time, Shulman suggested that DDAbs react directly with the sensitizing drug itself to form an “immune complex” and proposed that this complex reacts with the target cell to cause its destruction as an “innocent bystander”[15]. In later studies, however, the hypothesized drug-antibody complexes could not be demonstrated experimentally and it was found that DDAbs react with targets through their Fab, not their Fc domains, as would be expected of an immune complex[16, 17]. Accordingly, the view became favored that drug reacts non-covalently with target proteins to somehow prime them for recognition by DDAbs[18–20].
Recent studies suggest that DDAbs produced by patients sensitive to quinine and other drugs may be derived from a naturally occurring pool of immunoglobulins that are weakly autoreactive with epitopes on platelet membrane glycoproteins and that drugs capable of causing DITP possess structural elements that improve the fit between these autoantibodies and their targets, raising the association constant for antibody binding to a value that allows drug, at pharmacologic concentrations, to promote antibody binding and cause cell destruction[20, 21]. This model has not been directly validated, but is consistent with studies showing that quinine is “trapped” on the platelet surface when antibody binds[22] and with the finding that quinine-dependent, platelet-reactive monoclonal antibodies bind drug at the 1:2 ratio expected for a specific antibody:drug interaction[23]. If confirmed by future studies, the model shown in Figure 1 would reconcile seemingly conflicting hypotheses developed to explain drug-dependent antibody binding, since whether the drug reacts first with antibody or first with the target glycoprotein could depend simply on its relative affinity for one or the other of the two macromolecules. An alternative concept proposed to explain drug-dependent antibody binding is that certain drugs induce a structural change in a target glycoprotein, leading to the creation of neoepitopes elsewhere in the protein for which the antibodies are specific. This mechanism is unlikely to explain antibody binding induced by drugs like quinine, which interact only weakly with proteins[14, 24] and would not be expected to stabilize an alternative structure. However, it could explain thrombocytopenia in patients treated with platelet inhibitors of the fiban class, which bind tightly to the RGD recognition site of GPIIb/IIIa and “activate” the integrin (see below).
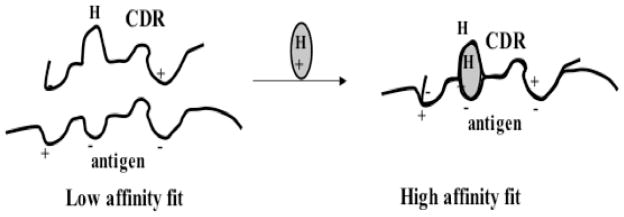
Figure 1. A model for drug-dependent antibody binding.
Left: Antibodies capable of causing drug-dependent thrombocytopenia recognize an epitope on a platelet glycoprotein but the reaction is too weak for significant numbers of antibody molecules to bind in the absence of drug. Right: The sensitizing drug contains structural elements that are complementary to charged (+/-) or hydrophobic domains (H) on the glycoprotein epitope and the complementarity determining region (CDR) of the antibody and improves the “fit” between the two proteins, increasing the KA to a value that permits binding to occur at levels of antibody, antigen and drug achieved in the circulation after ingestion of the drug. From DW Bougie et al, 2006 [21] with permission of the publisher.
The great majority of platelet-specific DDAbs recognizes the IIb/IIIa and/or Ib/IX membrane glycoprotein complexes. Restricted domains of GPIb/IX[25–27] and GPIIIa[28] appear to be favored targets for antibodies induced by the drugs quinine, quinidine, rifampin and ranitidine. Why platelets are so often targeted by DDAbs and how this remarkable class of antibodies is induced upon exposure of some individuals to certain drugs is presently unresolved.
Thrombocytopenia induced by RGD-mimetic platelet inhibitors
RGD mimetic drugs (fibans) are synthetic agents that bind tightly to the arginine-glycine-aspartic acid (RGD) recognition site on GPIIb/IIIa and prevent the formation of platelet thrombi by blocking the reaction of the activated integrin with fibrinogen and other ligands. Two drugs of this class, tirofiban and eptifibatide, are widely used to reduce complications following percutaneous transluminal coronary angioplasty (PTCA). In initial trials and in subsequent clinical experience, it was found that between 0.1 and 2% of patients treated with these agents experienced acute, often severe, thrombocytopenia within a few hours of the first exposure to one of these drugs[29, 30]. Serologic studies indicate that this complication is caused by antibodies that can be naturally occurring and recognize GPIIb/IIIa in a complex with the particular ligand-mimetic that caused thrombocytopenia[29]. It is known that both RGD peptide and peptide-mimetic drugs induce structural changes in GPIIb/IIIa recognized by monoclonal antibodies specific for “ligand-induced binding sites” (LIBS) on the integrin[31, 32]. It has been suggested that antibodies from patients with fiban-induced thrombocytopenia are specific for similar ligand-induced determinants on GPIIb/IIIa but this has not been formally proved. In general, antibodies from patients with tirofiban-induced thrombocytopenia do not recognize GPIIb/IIIa in a complex with eptifibatide (and vice versa) and neither type of antibody reacts with GPIIb/IIIa associated with RGD peptide[29]. These observations suggest that each ligand may induce slightly different structural changes in the integrin that can be distinguished by the patient antibodies. Why some individuals should have naturally occurring antibodies specific for conformational changes induced in GPIIb/IIIa by RGD-mimetic drugs is an interesting and unresolved question.
Abciximab-induced thrombocytopenia
Abciximab, the first chimeric (mouse/human) monoclonal antibody approved for human use, is a Fab fragment specific for a peptide loop in the beta A domain of GPIIIa[33]. Because this epitope is close to the RGD recognition site, abciximab blocks the reaction of fibrinogen with activated GPIIb/IIIa, thereby inhibiting platelet thrombus formation. About 2% of patients given abciximab for the first time[34] and 10–12% given this agent a second time[35] develop acute, often severe thrombocytopenia within a few hours of starting treatment. In such patients, antibodies can usually be detected that react strongly with normal platelets coated with abciximab and appear to be specific for mouse sequences in the drug that confer specificity for GPIIIa[36]. Laboratory identification of such antibodies is complicated by the fact that many normal persons have naturally occurring antibodies, apparently benign, that are specific for the papain cleavage site introduced at the C-terminus of abciximab in the manufacturing process and therefore bind to abciximab-coated platelets. The latter antibodies can be distinguished from pathogenic antibodies specific for mouse sequences by showing that their binding can be inhibited by Fab fragments prepared from normal IgG[36, 37]. A subgroup of patients given abciximab maintains normal platelet levels for 6–8 days before experiencing acute thrombocytopenia. Platelet destruction in these individuals is caused by antibodies produced in response to the intitial one-day infusion of the drug[37]. The newly formed antibodies are able to cause platelet destruction because platelet-bound abciximab persists in the circulation for up to two weeks after treatment[38].
Drug-induced autoantibodies
Clinical and laboratory observations suggest that drugs occasionally trigger the production of platelet-specific autoantibodies, leading to a clinical picture indistinguishable from spontaneous, autoimmune thrombocytopenia (AITP)[39]. A condition similar to AITP occurred in 1–2% of patients treated with gold salts for rheumatoid arthritis[40]. Other medications implicated as possible triggers for AITP include L-dopa, procaine amide, penicillamine, and sulfamethoxazole (Figure 2)[1, 2, 39]. How selected medications induce an autoimmune response against platelets is unknown. The suggestion has been made that some drugs perturb the processing of platelet glycoproteins by macrophages, leading to the generation of cryptic peptides not ordinarily seen by the immune system[39] but there is little direct evidence for this. In recent years, various reports have described an AITP-like clinical picture in patients treated for malignant or immune conditions with the chimeric monoclonal antibodies infliximab (anti-TNF alpha), rituximab (anti-CD20), etanercept (anti-TNF alpha receptor) and efalizumab (anti-CD11a)[41–44]. Most cases resolved after weeks or months of treatment with corticosteroids. Whether this complication is related to the immunomodulatory effects of these agents is unknown.
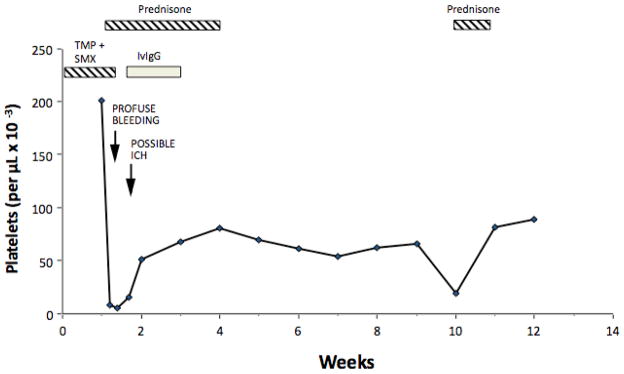
Figure 2. Development of chronic autoimmne thrombocytopenia in a patient who presented initially with thrombocytopenia caused by sulfamethoxazole (SMX)-dependent, platelet-reactive antibodies.
SMX-dependent antibodies were identified in acute phase serum together with GPIIb/IIIa-specific non-drug-dependent autoantibodies. Persistent non-drug-dependent antibodies reactive with autologous platelets were identified during Weeks 1, 5, and 9. SMX = sulfamethoxazole; ICH = intracranial hemorrhage; IVIgG = intravenous gamma globulin. From RH Aster, 2000 [38] with permission of the publisher.
Heparin-induced thrombocytopenia (HIT)
About 5% of patients given unfractionated heparin and a smaller percentage of those treated with low molecular weight heparin for at least 5–7 days develop low-grade thrombocytopenia, which in itself is rarely symptomatic. A subset of patients, however, experience venous or arterial thrombosis, which can be life-threatening[45]. HIT is caused by antibodies that recognize complexes of heparin and platelet factor 4 (PF4), a 32 kD CXC chemokine found in platelet alpha granules. Recent reports suggest that HIT antibodies can recognize platelet factor 4 released from platelets and bound to chondroitin sulfate, the major glycosaminoglycan (GAG) present on the platelet surface[46]. The thrombotic tendency associated with HIT may be the result of procoagulant microparticles released from platelets upon activation of Fc receptors[47]. Other mechanisms may also be operative[48]. Further discussion of HIT is beyond the scope of this review. For detailed information, readers are referred to recent publications[5, 6].
IMPLICATED DRUGS
Selected drugs implicated as causes of immune thrombocytopenia by the several mechanisms described above are listed in Table 1. In some conditions, only a few agents, e.g, fiban drugs, abciximab, and heparin and heparin derivatives are known to be responsible. However, at least 100 different medications have been implicated as possible causes of drug-dependent, “quinine-type” immune thrombocytopenia[1, 2]. Many of the cases have been described only as anecdotal case reports from which it is hard to judge whether drug sensitivity was involved, but some drugs have been implicated sufficiently often to make a cause-and-effect relationship almost certain. George and co-workers analyzed cases of drug-induced thrombocytopenia reported through 2008 and assembled a database listing implicated drugs that is periodically updated and can be accessed on line at http://www.ouhsc.edu/platelets. They also devised a set of four clinical criteria to assess the likelihood that individual drugs are capable of causing DITP (Table 2). About 51 drugs were considered “definite” (Level 1) and 17 others “probable” (Level 2) causes of DITP on the basis of having met four or three of these criteria, respectively[49–52]. Classes of drugs implicated include the cinchona alakloids quinine and quinidine, non-steroidal anti-inflammatory agents, various antibiotics, especially sulfamethoxazole and vancomycin, anticonvulsants and sedatives, and the platelet inhibitors tirofiban, eptifibatide and abciximab. A recent finding of interest is that drugs used in the treatment of cancer such as platinum-containing compounds can cause acute, severe, immune thrombocytopenia in addition to their well known tendency to lower platelet counts by suppressing hematopoiesis[53]. Anecdotal, but well documented reports, including some in which thrombocytopenia recurred after challenge, indicate that folk medicines, herbal preparations and even foods occasionally trigger acute thrombocytopenia[54–56], but whether immune mechanisms are responsible is uncertain.
Table 2.
Criteria used to evaluate causative relationships in drug-induced thrombocytopenic purpura*
| Criterion | Description |
|---|---|
| 1 | Therapy with the candidate drug preceded thrombocytopenia. |
| 2 | Recovery from thrombocytopenia was complete and sustained after therapy with the drug was discontinued. |
| 2 | The candidate drug was the only drug used before the onset of thrombocytopenia or other drugs were continued or re-introduced after discontinuation of therapy with the candidate drug with a sustained normal platelet count. |
| 3 | Other causes for thrombocytopenia were excluded. |
| 4 | Re-exposure to the candidate drug resulted in recurrent thrombocytopenia. |
| Level of Evidence | |
| I | Definite: Criteria 1, 2, 3 and 4 are met. |
| II | Probable: Criteria 1, 2, and 3 are met. |
| III | Possible: Criterion 1 met. |
| IV | Unlikely: Criterion 1 not met. |
Adapted from JN George et al (49)
Although the George criteria are useful in defining agents that are capable of causing thrombocytopenia, they do not provide a means of identifying the causative agent in any particular patient, short of carrying out a diagnostic challenge with the suspect drug after recovery. In many, but not all cases of DITP, it is possible to identify an immunoglobulin in the patient’s serum that binds to normal platelets in the presence, but not in the absence of the implicated drug[20], but there is not universal agreement as to whether detection of such an antibody provides conclusive evidence that the drug for which it is specific actually caused thrombocytopenia. In a recent study of vancomycin-induced thrombocytopenia, von Drygalski et al identified vancomycin-dependent antibodies in 29 patients who satisfied at least three of the four George criteria (Table 2)[57]. Comparable antibodies were not found in 500 normal subjects or in 25 patients given vancomycin for at least one week who did not experience thrombocytopenia[57]. This study, together with other reports documenting the presence of drug-dependent, platelet-reactive antibodies in patients meeting most of the George criteria[2, 20] suggests that detection of a DDAb by an experienced laboratory significantly increases the likelihood that sensitivity to that drug caused or contributed to thrombocytopenia. Table 3 lists drug-dependent antibodies identified using a standard flow cytometric assay[21, 53] in patients referred to our laboratory for testing because of a suspicion of DITP over a ten-year span. All positive test results were confirmed by repeat testing. As already noted, detection of a drug-dependent antibody increases the likelihood, but does not prove unequivocally that a particular drug is the cause of thrombocytopenia.
Table 3.
Drug-dependent, platelet-reactive antibodies detected 1998–2008
| Drug category | Number | Individual drugs |
|---|---|---|
| ACE inhibitor | 1–3 | Lisinopril |
| Analgesic | 1–3 | Acetaminophen*, propoxyphene |
| Antibiotic | >15 | Sulfamethoxazole, vancomycin |
| 4–15 | Ceftriaxone, levofloxacin, nafcillin, pipericillin, rifampin, trimethoprim | |
| 1–3 | Ampicillin, amoxicillin, cefazolin, cefadroxil, cefepime, cefpodoxime, ceftazidime, ceftizoxime, cefpodoxime, ciprofloxacin, ethambutol, lisinopril, loracarbef. metronidazole, nitrofurantoin, sulfisoxazole | |
| Anticonvulsant | >15 | Carbamazepine |
| 4–15 | Phenytoin | |
| 1–3 | Lamotrigine, lorazepam, valproic acid | |
| Antidepressant, anti- psychotic | 1–3 | Amitriptyline, bupropion, haldol, olanzapine, paroxetine, sertraline |
| Antithyroid | 1–3 | Propylthoiuracil |
| Beta blocker | 1–3 | Atenolol, propranolol |
| Cardiac | 4–15 | Amiodarone |
| 1–3 | Dobutamine | |
| Chemotheraputic agent | 4–15 | Oxaliplatin |
| 1–3 | Geldanamycin, irinotecan, suramin | |
| Cinchona alkaloid | >15 | Quinine, quinindine |
| Diuretic | 4–15 | Furosemide |
| GPIIb/IIIa inhibitor | >15 | Abciximab, eptifibatide, tirofiban |
| 4–15 | Orbofiban, xemolifiban | |
| Histamine receptor antagonist | 1–3 | Fexofenadine, ranitidine |
| Narcotic | 1–3 | Fentanyl |
| Non-steroidal anti- inflammatory | 4–15 | Naproxen* |
| 1–3 | Celecoxib, ibuprofen, ibuprofen*, oxaprozin | |
| Proton pump inhibitor | 1–3 | Esomeprozole, lansoprazole, pantoprazole |
| Thrombin inhibitor | 1–3 | Argatroban |
| Vasodilator | 1–3 | Papaverine |
Italics indicate DDAbs specific for drug metabolites only.
CLINICAL PRESENTATION
As a rule, patients will have been exposed to the sensitizing medication for at least one week before thrombocytopenia becomes clinically evident. As noted, eptifibatide, tirofiban, and abciximab, are exceptions to this rule since naturally occurring antibodies specific for these drugs can cause thrombocytopenia within a few hours of the first exposure. Once an antibody has been produced, exposure to the sensitizing drug sometimes produces systemic symptoms such as faintness, chills, fever and nausea[58–60]. Hypotension and even syncope sometimes occur in patients with high titer antibodies[58, 59, 61]. Occasional patients present with immune hemolytic anemia or neutropenia in addition to thrombocytopenia[62, 63]. In such cases, DDAbs specific for red cells or neutrophils can sometimes be detected in addition to platelet-specific antibodies.
The severity of bleeding tends to be inversely related to the platelet count, although patients with profound thrombocytopenia occasionally have no bleeding symptoms. It is not rare for severely affected individuals (platelets less than 10,000/uL) to present with extensive purpuric lesions on the skin and mucosal surfaces, hematuria and gastrointestinal hemorrhage (“wet purpura”). Those sensitive to quinine sometimes have microangiopathic hemolytic anemia and renal failure typical of the hemolytic uremic syndrome (HUS) [60, 64] or thrombotic thrombocytopenic purpura (TTP)[64]. Rarely, other drugs appear to trigger HUS in association with drug-induced thrombocytopenia[65]. The pathogenesis of renal failure in these cases is not understood[66].
After the sensitizing medication is discontinued, bleeding symptoms usually subside within one or two days. Platelet counts often return to normal in 4–8 days but, rarely, thrombocytopenia persists for several weeks. Catastrophic bleeding is unusual, but fatal intracranial[67] and intrapulmonary[68] hemorrhage has been described. Patients who present with HUS often require hemodialysis but usually regain normal renal function within a few weeks.
DIAGNOSIS
Drug-induced immune thrombocytopenia should be considered in any patient who presents with acute thrombocytopenia of uncertain etiology. A careful history of drug exposure should be taken and patients should be asked specifically about quinine (still used for prevention and treatment of nocturnal leg cramps) and sulfonamide antibiotics. It should be kept in mind that quinine present in beverages can trigger DITP[69]. It is not rare for DITP to be diagnosed as acute autoimmune thrombocytopenia (AITP) at the initial presentation. Since the sensitizing medication is usually discontinued when the patient is hospitalized, a rise in platelet count may be attributed to treatment given for AITP, creating the possibility of a recurrence when the patient is re-exposed to the sensitizing drug at a later time. Examples of patients with DITP who were hospitalized for “recurrent AITP” as many as five times and were subjected to diverse treatments, including splenectomy, before the correct diagnosis was established have been described[58, 70]. Because the onset of thrombocytopenia is sometimes associated with chills and fever[58–60], it may initially be assumed that the low platelet count is an indicator of bacterial sepsis[59]. DITP triggered by medications taken surreptitiously has been reported[71].
Detection of an antibody that reacts with normal platelets in the presence of a drug being taken by the patient can be helpful in diagnosis although, as noted, the requisite testing is not widely available. A convenient assay for DDAbs involves incubation of normal platelets with patient serum in the presence and absence of the implicated drug followed by washing in the presence of drug and detection of platelet-bound immunoglobulin by flow cytometry[21, 72]. Other methods used for DDAb detection include complement fixation[14], platelet lysis[73], induction of platelet procoagulant activity[74] and passive hemaglutination[75]. The relative sensitivity of these techniques for DDAb detection is uncertain. Unfortunately, drug-dependent antibodies cannot always be detected, even in patients in whom DITP is considered to be “likely” or “definite” according to the George criteria (Table 2). One reason for this is that many drugs are highly insoluble in aqueous solution and are therefore difficult to work with in vitro. Another is that drug metabolites produced in vivo can be the sensitizing agents[76, 77]. Metabolites shown to be capable of causing sensitization leading to DITP include glucuronide conjugates of non-steroidal anti-inflammatory drugs[76] and acetaminophen[78] as well as acetaminophen sulfate[76, 77]. Drug-induced antibodies remain present for at least a month after the acute episode and sometimes persist indefinitely. A diagnostic challenge with the implicated drug following restoration of normal platelet levels can be considered in exceptional cases where DDAb testing is negative and an implicated drug is essential for a patient’s medical care. If performed, a challenge should start with very small doses of the medication, i.e., one or two mg, while platelet counts are carefully monitored since administration of a full dose can produce life-threatening bleeding[79].
MANAGEMENT
A decision on whether to discontinue medication in a patient with possible DITP must be based on clinical considerations, since testing for DDAbs requires time and is not widely available. When necessary, pharmacologically equivalent drugs with a different chemical structure can usually be substituted safely for medications essential to a patient’s medical care. It is reasonable to give platelet transfusions to severely thrombocytopenic patients who have “wet purpura” because of their increased risk of intracranial hemorrhage[80]. However, the effectiveness of transfusions has not been formally studied. It is a common practice to administer corticosteroids, but whether they are beneficial in patients with DITP is not established. Since most drugs are cleared within a few days, platelet levels often start to increase within a day or two of hospital admission. Rarely, thrombocytopenia and bleeding symptoms persist for several weeks. Such patients have been treated with intravenous IgG[81, 82] and even plasma exchange[83] with possible, but not proven benefit. Although identification of a platelet-reactive DDAb is not useful in acute management for practical reasons, antibody studies can help to implicate a specific drug as the cause of a thrombocytopenic episode and provide justification for measures taken to prevent future exposure.
Acknowledgments
Supported by Grants HL-13629 and HL-44612 from the National Heart Lung and Blood Institute
References
- 1.Aster RH, George JN. Drug-induced thrombocytopenia. In: McCrae K, editor. Thrombocytopenia. New York: Marcel-Dakker Inc; 2006. pp. 145–77. [Google Scholar]
- 2.Aster R. Drug-induced thrombocytopenia. In: Michelson A, editor. Platelets. New York: Academic Press; 2006. pp. 887–902. [Google Scholar]
- 3.van den Bemt PM, Meyboom RH, Egberts AC. Drug-induced immune thrombocytopenia. Drug Saf. 2004;27:1243–52. doi: 10.2165/00002018-200427150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman DW, Kelly JP, Johannes CB, Sandler A, Harmon D, Stolley PD, Shapiro S. Acute thrombocytopenic purpura in relation to the use of drugs. Blood. 1993;82 :2714–8. [PubMed] [Google Scholar]
- 5.Davoren A, Aster RH. Heparin-induced thrombocytopenia and thrombosis. Am J Hematol. 2006;81:36–44. doi: 10.1002/ajh.20490. [DOI] [PubMed] [Google Scholar]
- 6.Warkentin TE. Heparin-induced thrombocytopenia: diagnosis and management. Circulation. 2004;110:e454–8. doi: 10.1161/01.CIR.0000147537.72829.1B. [DOI] [PubMed] [Google Scholar]
- 7.Garratty G, Petz LD. Drug-induced immune hemolytic anemia. Am J Med. 1975;58 :398–407. doi: 10.1016/0002-9343(75)90606-3. [DOI] [PubMed] [Google Scholar]
- 8.Leger RM, Arndt PA, Garratty G. Serological studies of piperacillin antibodies. Transfusion. 2008;48:2429–34. doi: 10.1111/j.1537-2995.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 9.Murphy MF, Riordan T, Minchinton RM, Chapman JF, Amess JA, Shaw EJ, Waters AH. Demonstration of an immune-mediated mechanism of penicillin-induced neutropenia and thrombocytopenia. Br J Haematol. 1983;55:155–60. doi: 10.1111/j.1365-2141.1983.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Vazquez A, Pastor JM, Riancho JA. Immune thrombocytopenia caused by piperacillin/tazobactam. Clin Infect Dis. 1998;27:650–1. doi: 10.1086/517143. [DOI] [PubMed] [Google Scholar]
- 11.Aljitawi OS, Krishnan K, Curtis BR, Bougie DW, Aster RH. Serologically documented loracarbef (Lorabid)-induced immune thrombocytopenia. Am J Hematol. 2003;73:41–3. doi: 10.1002/ajh.10307. [DOI] [PubMed] [Google Scholar]
- 12.Grossjohann B, Eichler P, Greinacher A, Santoso S, Kroll H. Ceftriaxone causes drug-induced immune thrombocytopenia and hemolytic anemia: characterization of targets on platelets and red blood cells. Transfusion. 2004;44:1033–40. doi: 10.1111/j.1537-2995.2004.03378.x. [DOI] [PubMed] [Google Scholar]
- 13.Ackroyd JF. Allergic purpura, including purpura due to foods, drugs and infections. Am J Med. 1953;14:605–32. doi: 10.1016/0002-9343(53)90377-5. [DOI] [PubMed] [Google Scholar]
- 14.Shulman NR. Immunoreactions involving platelets. I. A steric and kinetic model for formation of a complex from a human antibody, quinidine as a haptene, and platelets; and for fixation of complement by the complex. J Exp Med. 1958;107:665–90. doi: 10.1084/jem.107.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman NR. A Mechanism of Cell Destruction in Individuals Sensitized to Foreign Antigens and Its Implications in Auto-Immunity. Combined Clinical Staff Conference at the National Institutes of Health. Ann Intern Med. 1964;60:506–21. doi: 10.7326/0003-4819-60-3-506. [DOI] [PubMed] [Google Scholar]
- 16.Christie DJ, Mullen PC, Aster RH. Fab-mediated binding of drug-dependent antibodies to platelets in quinidine- and quinine-induced thrombocytopenia. J Clin Invest. 1985;75:310–4. doi: 10.1172/JCI111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith ME, Reid DM, Jones CE, Jordan JV, Kautz CA, Shulman NR. Binding of quinine- and quinidine-dependent drug antibodies to platelets is mediated by the Fab domain of the immunoglobulin G and is not Fc dependent. J Clin Invest. 1987;79:912–7. doi: 10.1172/JCI112901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller-Eckhardt C, Salama A. Drug-induced immune cytopenias: a unifying pathogenetic concept with special emphasis on the role of drug metabolites. Transfus Med Rev. 1990;4:69–77. doi: 10.1016/s0887-7963(90)70249-0. [DOI] [PubMed] [Google Scholar]
- 19.Shulman NR, Reid DM. Mechanisms of drug-induced immunologically mediated cytopenias. Transfus Med Rev. 1993;7:215–29. doi: 10.1016/s0887-7963(93)70142-x. [DOI] [PubMed] [Google Scholar]
- 20.Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580–7. doi: 10.1056/NEJMra066469. [DOI] [PubMed] [Google Scholar]
- 21.Bougie DW, Wilker PR, Aster RH. Patients with quinine-induced immune thrombocytopenia have both "drug-dependent" and "drug-specific" antibodies. Blood. 2006;108:922–7. doi: 10.1182/blood-2006-01-009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christie DJ, Aster RH. Drug-antibody-platelet interaction in quinine- and quinidine-induced thrombocytopenia. J Clin Invest. 1982;70:989–98. doi: 10.1172/JCI110710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bougie DW, Birenbaum J, Rasmussen M, Poncz M, Aster RH. Quinine-dependent, platelet-reactive monoclonals mimic antibodies found in patients with quinine-induced immune thrombocytopenia. Blood. 2009;113:1105–11. doi: 10.1182/blood-2008-09-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frostell-Karlsson A, Remaeus A, Roos H, Andersson K, Borg P, Hamalainen M, Karlsson R. Biosensor analysis of the interaction between immobilized human serum albumin and drug compounds for prediction of human serum albumin binding levels. J Med Chem. 2000;43:1986–92. doi: 10.1021/jm991174y. [DOI] [PubMed] [Google Scholar]
- 25.Burgess JK, Lopez JA, Berndt MC, Dawes I, Chesterman CN, Chong BH. Quinine-dependent antibodies bind a restricted set of epitopes on the glycoprotein Ib-IX complex: characterization of the epitopes. Blood. 1998;92:2366–73. [PubMed] [Google Scholar]
- 26.Gentilini G, Curtis BR, Aster RH. An antibody from a patient with ranitidine-induced thrombocytopenia recognizes a site on glycoprotein IX that is a favored target for drug-induced antibodies. Blood. 1998;92:2359–65. [PubMed] [Google Scholar]
- 27.Asvadi P, Ahmadi Z, Chong BH. Drug-induced thrombocytopenia: localization of the binding site of GPIX-specific quinine-dependent antibodies. Blood. 2003;102:1670–7. doi: 10.1182/blood-2002-07-2175. [DOI] [PubMed] [Google Scholar]
- 28.Peterson JA, Nelson TN, Kanack AJ, Aster RH. Fine specificity of drug-dependent antibodies reactive with a restricted domain of platelet GPIIIA. Blood. 2008;111:1234–9. doi: 10.1182/blood-2007-09-112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bougie DW, Wilker PR, Wuitschick ED, Curtis BR, Malik M, Levine S, Lind RN, Pereira J, Aster RH. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100:2071–6. [PubMed] [Google Scholar]
- 30.Aster RH. Immune thrombocytopenia caused by glycoprotein IIb/IIIa inhibitors. Chest. 2005;127:53S–9S. doi: 10.1378/chest.127.2_suppl.53S. [DOI] [PubMed] [Google Scholar]
- 31.Frelinger AL, 3rd, Du XP, Plow EF, Ginsberg MH. Monoclonal antibodies to ligand-occupied conformers of integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem. 1991;266:17106–11. [PubMed] [Google Scholar]
- 32.Honda S, Tomiyama Y, Aoki T, Shiraga M, Kurata Y, Seki J, Matsuzawa Y. Association between ligand-induced conformational changes of integrin IIbbeta3 and IIbbeta3-mediated intracellular Ca2+ signaling. Blood. 1998;92:3675–83. [PubMed] [Google Scholar]
- 33.Artoni A, Li J, Mitchell B, Ruan J, Takagi J, Springer TA, French DL, Coller BS. Integrin beta3 regions controlling binding of murine mAb 7E3: implications for the mechanism of integrin alphaIIbbeta3 activation. Proc Natl Acad Sci U S A. 2004;101:13114–20. doi: 10.1073/pnas.0404201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jubelirer SJ, Koenig BA, Bates MC. Acute profound thrombocytopenia following C7E3 Fab (Abciximab) therapy: case reports, review of the literature and implications for therapy. Am J Hematol. 1999;61:205–8. doi: 10.1002/(sici)1096-8652(199907)61:3<205::aid-ajh8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Dery JP, Braden GA, Lincoff AM, Kereiakes DJ, Browne K, Little T, George BS, Sane DC, Cines DB, Effron MB, Mascelli MA, Langrall MA, Damaraju L, Barnathan ES, Tcheng JE. Final results of the ReoPro readministration registry. Am J Cardiol. 2004;93 :979–84. doi: 10.1016/j.amjcard.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 36.Curtis BR, Swyers J, Divgi A, McFarland JG, Aster RH. Thrombocytopenia after second exposure to abciximab is caused by antibodies that recognize abciximab-coated platelets. Blood. 2002;99:2054–9. doi: 10.1182/blood.v99.6.2054. [DOI] [PubMed] [Google Scholar]
- 37.Curtis BR, Divgi A, Garritty M, Aster RH. Delayed thrombocytopenia after treatment with abciximab: a distinct clinical entity associated with the immune response to the drug. J Thromb Haemost. 2004;2:985–92. doi: 10.1111/j.1538-7836.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- 38.Mascelli MA, Lance ET, Damaraju L, Wagner CL, Weisman HF, Jordan RE. Pharmacodynamic profile of short-term abciximab treatment demonstrates prolonged platelet inhibition with gradual recovery from GP IIb/IIIa receptor blockade. Circulation. 1998;97:1680–8. doi: 10.1161/01.cir.97.17.1680. [DOI] [PubMed] [Google Scholar]
- 39.Aster RH. Can drugs cause autoimmune thrombocytopenic purpura? Semin Hematol. 2000;37:229–38. doi: 10.1016/s0037-1963(00)90101-x. [DOI] [PubMed] [Google Scholar]
- 40.von dem Borne AE, Pegels JG, van der Stadt RJ, van der Plasvan Dalen CM, Helmerhorst FM. Thrombocytopenia associated with gold therapy: a drug-induced autoimmune disease? Br J Haematol. 1986;63:509–16. doi: 10.1111/j.1365-2141.1986.tb07528.x. [DOI] [PubMed] [Google Scholar]
- 41.Vidal F, Fontova R, Richart C. Severe neutropenia and thrombocytopenia associated with infliximab. Ann Intern Med. 2003;139:W–W63. doi: 10.7326/0003-4819-139-3-200308050-00021-w4. [DOI] [PubMed] [Google Scholar]
- 42.Otrock ZK, Mahfouz RA, Oghlakian GO, Salem ZM, Bazarbachi A. Rituximab-induced acute thrombocytopenia: a report of two cases. Haematologica. 2005;90(Suppl):ECR23. [PubMed] [Google Scholar]
- 43.Warkentin TE, Kwon P. Immune thrombocytopenia associated with efalizumab therapy for psoriasis. Ann Intern Med. 2005;143:761–3. doi: 10.7326/0003-4819-143-10-200511150-00028. [DOI] [PubMed] [Google Scholar]
- 44.Pathare SK, Heycock C, Hamilton J. TNFalpha blocker-induced thrombocytopenia. Rheumatology (Oxford) 2006;45:1313–4. doi: 10.1093/rheumatology/kel204. [DOI] [PubMed] [Google Scholar]
- 45.Warkentin TE. Heparin-induced thrombocytopenia. Hematol Oncol Clin North Am. 2007;21:589–607. v. doi: 10.1016/j.hoc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Rauova L, Zhai L, Kowalska MA, Arepally GM, Cines DB, Poncz M. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346–53. doi: 10.1182/blood-2005-08-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warkentin TE, Hayward CP, Boshkov LK, Santos AV, Sheppard JA, Bode AP, Kelton JG. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84:3691–9. [PubMed] [Google Scholar]
- 48.Arepally GM, Mayer IM. Antibodies from patients with heparin-induced thrombocytopenia stimulate monocytic cells to express tissue factor and secrete interleukin-8. Blood. 2001;98:1252–4. doi: 10.1182/blood.v98.4.1252. [DOI] [PubMed] [Google Scholar]
- 49.George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, Vondracek T. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med. 1998;129:886–90. doi: 10.7326/0003-4819-129-11_part_1-199812010-00009. [DOI] [PubMed] [Google Scholar]
- 50.George J, Rizva M. Thrombocytopenia. In: Beutler ELM, Coller B, Kipps T, Seligsohn U, editors. williams Hematology. 6. New York: McGraw Hill; 2005. pp. 1479–94. [Google Scholar]
- 51.Li X, Hunt L, Vesely SK. Drug-induced thrombocytopenia: an updated systematic review. Ann Intern Med. 2005;142:474–5. doi: 10.7326/0003-4819-142-6-200503150-00023. [DOI] [PubMed] [Google Scholar]
- 52.Swisher KK, Li X, Vesely SK, George JN. Drug-induced thrombocytopenia: an updated systematic review, 2008. Drug Saf. 2009;32:85–6. 8 , pii. doi: 10.2165/00002018-200932010-00008. [DOI] [PubMed] [Google Scholar]
- 53.Curtis BR, Kaliszewski J, Marques MB, Saif MW, Nabelle L, Blank J, McFarland JG, Aster RH. Immune-mediated thrombocytopenia resulting from sensitivity to oxaliplatin. Am J Hematol. 2006;81:193–8. doi: 10.1002/ajh.20516. [DOI] [PubMed] [Google Scholar]
- 54.Lavy R. Thrombocytopenic Purpura Due to Lupinus Termis Bean. J Allergy Clin Immunol. 1964;35:386–9. doi: 10.1016/0021-8707(64)90065-6. [DOI] [PubMed] [Google Scholar]
- 55.Arnold J, Ouwehand WH, Smith GA, Cohen H. A young woman with petechiae. Lancet. 1998;352:618. doi: 10.1016/s0140-6736(98)05194-0. [DOI] [PubMed] [Google Scholar]
- 56.Azuno Y, Yaga K, Sasayama T, Kimoto K. Thrombocytopenia induced by Jui, a traditional Chinese herbal medicine. Lancet. 1999;354:304–5. doi: 10.1016/S0140-6736(99)01336-7. [DOI] [PubMed] [Google Scholar]
- 57.Von Drygalski A, Curtis BR, Bougie DW, McFarland JG, Ahl S, Limbu I, Baker KR, Aster RH. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007;356:904–10. doi: 10.1056/NEJMoa065066. [DOI] [PubMed] [Google Scholar]
- 58.Reddy JC, Shuman MA, Aster RH. Quinine/quinidine-induced thrombocytopenia: a great imitator. Arch Intern Med. 2004;164:218–20. doi: 10.1001/archinte.164.2.218. [DOI] [PubMed] [Google Scholar]
- 59.Schattner A. Quinine hypersensitivity simulating sepsis. Am J Med. 1998;104:488–90. doi: 10.1016/s0002-9343(98)00082-5. [DOI] [PubMed] [Google Scholar]
- 60.Gottschall JL, Elliot W, Lianos E, McFarland JG, Wolfmeyer K, Aster RH. Quinine-induced immune thrombocytopenia associated with hemolytic uremic syndrome: a new clinical entity. Blood. 1991;77:306–10. [PubMed] [Google Scholar]
- 61.Rezkalla SH, Hayes JJ, Curtis BR, Aster RH. Eptifibatide-induced acute profound thrombocytopenia presenting as refractory hypotension. Catheter Cardiovasc Interv. 2003;58:76–9. doi: 10.1002/ccd.10392. [DOI] [PubMed] [Google Scholar]
- 62.Zeigler Z, Shadduck RK, Winkelstein A, Stroupe TK. Immune hemolytic anemia and thrombocytopenia secondary to quinidine: in vitro studies of the quinidine-dependent red cell and platelet antibodies. Blood. 1979;53:396–402. [PubMed] [Google Scholar]
- 63.Stroncek DF, Vercellotti GM, Hammerschmidt DE, Christie DJ, Shankar RA, Jacob HS. Characterization of multiple quinine-dependent antibodies in a patient with episodic hemolytic uremic syndrome and immune agranulocytosis. Blood. 1992;80:241–8. [PubMed] [Google Scholar]
- 64.Kojouri K, Vesely SK, George JN. Quinine-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: frequency, clinical features, and long-term outcomes. Ann Intern Med. 2001;135:1047–51. doi: 10.7326/0003-4819-135-12-200112180-00008. [DOI] [PubMed] [Google Scholar]
- 65.Juang YC, Tsao TC, Chiang YC, Lin JL, Tsai YH. Acute renal failure and severe thrombocytopenia induced by rifampicin: report of a case. J Formos Med Assoc. 1992;91 :475–6. [PubMed] [Google Scholar]
- 66.Dlott JS, Danielson CF, Blue-Hnidy DE, McCarthy LJ. Drug-induced thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: a concise review. Ther Apher Dial. 2004;8:102–11. doi: 10.1111/j.1526-0968.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 67.Freiman JP. Fatal quinine-induced thrombocytopenia. Ann Intern Med. 1990;112:308–9. doi: 10.7326/0003-4819-112-4-308. [DOI] [PubMed] [Google Scholar]
- 68.Fireman Z, Yust I, Abramov AL. Lethal occult pulmonary hemorrhage in drug-induced thrombocytopenia. Chest. 1981;79:358–9. doi: 10.1378/chest.79.3.358. [DOI] [PubMed] [Google Scholar]
- 69.Brasic JR. Quinine-induced thrombocytopenia in a 64-year-old man who consumed tonic water to relieve nocturnal leg cramps. Mayo Clin Proc. 2001;76:863–4. doi: 10.1016/s0025-6196(11)63235-7. [DOI] [PubMed] [Google Scholar]
- 70.Kojouri K, Perdue JJ, Medina PJ, George JN. Occult quinine-induced thrombocytopenia. J Okla State Med Assoc. 2000;93:519–21. [PubMed] [Google Scholar]
- 71.Reid DM, Shulman NR. Drug purpura due to surreptitious quinidine intake. Ann Intern Med. 1988;108:206–8. doi: 10.7326/0003-4819-108-2-206. [DOI] [PubMed] [Google Scholar]
- 72.Curtis BR, McFarland JG, Wu GG, Visentin GP, Aster RH. Antibodies in sulfonamide-induced immune thrombocytopenia recognize calcium-dependent epitopes on the glycoprotein IIb/IIIa complex. Blood. 1994;84:176–83. [PubMed] [Google Scholar]
- 73.Cimo PL, Pisciotta AV, Desai RG, Pino JL, Aster RH. Detection of drug-dependent antibodies by the 51Cr platelet lysis test: documentation of immune thrombocytopenia induced by diphenylhydantoin, diazepam, and sulfisoxazole. Am J Hematol. 1977;2:65–72. doi: 10.1002/ajh.2830020109. [DOI] [PubMed] [Google Scholar]
- 74.Karpatkin M, Siskind GW, Karpatkin S. The platelet factor 3 immunoinjury technique re-evaluated. Development of a rapid test for antiplatelet antibody. Detection in various clinical disorders, including immunologic drug-induced and neonatal thrombocytopenias. J Lab Clin Med. 1977;89:400–8. [PubMed] [Google Scholar]
- 75.Leach MF, Cooper LK, AuBuchon JP. Detection of drug-dependent, platelet-reactive antibodies by solid-phase red cell adherence assays. Br J Haematol. 1997;97:755–61. doi: 10.1046/j.1365-2141.1997.1472960.x. [DOI] [PubMed] [Google Scholar]
- 76.Bougie D, Aster R. Immune thrombocytopenia resulting from sensitivity to metabolites of naproxen and acetaminophen. Blood. 2001;97:3846–50. doi: 10.1182/blood.v97.12.3846. [DOI] [PubMed] [Google Scholar]
- 77.Eisner EV, Shahidi NT. Immune thrombocytopenia due to a drug metabolite. N Engl J Med. 1972;287:376–81. doi: 10.1056/NEJM197208242870803. [DOI] [PubMed] [Google Scholar]
- 78.Bougie DW, Benito AI, Sanchez-Abarca LI, Torres R, Birenbaum J, Aster RH. Acute thrombocytopenia caused by sensitivity to the glucuronide conjugate of acetaminophen. Blood. 2007;109:3608–9. doi: 10.1182/blood-2006-12-063941. [DOI] [PubMed] [Google Scholar]
- 79.Schmitt SK, Tomford JW. Quinine-induced pancytopenia and coagulopathy. Ann Intern Med. 1994;120:90–1. doi: 10.7326/0003-4819-120-1-199401010-00025. [DOI] [PubMed] [Google Scholar]
- 80.Crosby WH. Editorial: Wet purpura, dry purpura. JAMA. 1975;232:744–5. doi: 10.1001/jama.232.7.744. [DOI] [PubMed] [Google Scholar]
- 81.Ray JB, Brereton WF, Nullet FR. Intravenous immune globulin for the treatment of presumed quinidine-induced thrombocytopenia. DICP. 1990;24:693–5. doi: 10.1177/106002809002400706. [DOI] [PubMed] [Google Scholar]
- 82.Herrington A, Mahmood A, Berger R. Treatment options in sulfamethoxazole-trimethoprim-induced thrombocytopenic purpura. South Med J. 1994;87:948–50. doi: 10.1097/00007611-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 83.Pourrat O. Treatment of drug-related diseases by plasma exchanges. Ann Med Interne (Paris) 1994;145:357–60. [PubMed] [Google Scholar]