Abstract
Background
Obesity is a systemic disorder associated with an increase in left ventricular mass and premature death and disability from cardiovascular disease. Although bariatric surgery reverses many of the hormonal and hemodynamic derangements, the long-term collective effects on body composition and left ventricular mass have not been considered before.
Hypothesis
The decline in fat mass and lean mass after weight loss surgery is associated with a decline in left ventricular mass.
Methods
Fifteen severely obese women (mean body mass index or BMI: 46.7 ± 1.7 kg/m2) with medically controlled hypertension underwent bariatric surgery. Left ventricular mass and plasma markers of systemic metabolism, together with BMI, waist and hip circumferences, body composition (fat mass and lean mass), and resting energy expenditure (REE) were measured at 0, 3, 9, 12 and 24 months.
Results
Left ventricular mass continued to decline linearly over the entire period of observation, while rates of weight-loss, loss of lean mass, loss of fat mass, and REE all plateaued at 9 months (p<0.001 for all). Parameters of systemic metabolism normalized by 9 months, and showed no further change at 24 months after surgery.
Conclusions
Even though parameters of obesity, including BMI and body composition, plateau, the benefits of bariatric surgery on systemic metabolism and left ventricular mass are sustained. We propose that the progressive decline of left ventricular mass after weight loss surgery is regulated by neurohumoral factors, and may contribute to improved long-term survival.
Keywords: obesity, weight loss, metabolism, bariatric surgery, left ventricular mass, insulin resistance
INTRODUCTION
Obesity is a systemic disorder associated with an increase in left ventricular mass and premature death and disability from cardiovascular disease.1–3 Although bariatric surgery reverses many of the associated hormonal and hemodynamic changes, the collective effects on body composition and left ventricular mass have not been considered before.
Weight-loss following bariatric surgery is accompanied by significant changes in body composition and resting energy expenditure (REE) as well as rapid reversal of insulin resistance, of hypertension, and of many of the other obesity-related complications.4–9 Weight loss surgery also extends the otherwise shortened life span of patients with severe obesity.3, 10 In spite of significant weight loss, many patients remain clinically obese, although the sequelae of obesity reverse.11, 12 The effects of weight loss surgery on cardiac structure and function have been reported before,11–16 but the long-term effects on left ventricular mass are not known. Because ventricular hypertrophy is of adverse prognostic value for death and disability from cardiovascular disease, 17 we asked whether bariatric surgery affects left ventricular mass in the long term.
The goal of the present study was to compare the changes in body mass index (BMI), waist circumference and hip circumference, fat mass, lean mass, and REE, to the changes in left ventricular mass. We examined the different parameters over a two-year period and found that left ventricular mass decreased linearly while other parameters, including body composition, and metabolic homeostasis, changed in a non-linear fashion or plateaued as early as one month after surgery.
MATERIALS AND METHODS
Subjects
Fifteen consecutive women with clinically severe obesity (BMI 46.7 kg/m2, mean age: 49.1 ± 2.1 years; 10 Caucasian, 4 African-American, 1 Hispanic), underwent bariatric surgery at the University of Texas Medical School at Houston Bariatric Surgery Center. The subjects met inclusion criteria published previously. 14 Exclusion criteria were: patients age less than 18 years, pregnancy, coronary artery disease, ischemic cardiomyopathy, severe peripheral vascular disease, or a current history of smoking. The study was approved by the Committee for the Protection Human Subjects at The University of Texas Health Science Center at Houston. All participants signed a written informed consent. Of the 15 women, 10 underwent laparoscopic small pouch gastric bypass with a Roux-en Y procedure while the remaining 5 underwent laparoscopic adjustable gastric banding procedure. Both procedures were performed as described in the literature. 14, 18, 19 There were no complications with the surgery. There was also no significant difference between groups before and after surgery for all parameters reported here.
Study Protocol
Subjects were analyzed at baseline, one month, three months, six months, nine months, and 24 months post surgery. Individuals were asked to fast 12 hours and abstain from alcohol and exercise for 24 hours prior to evaluation. Each visit consisted of: a standard physical exam, measurements of blood pressure, heart rate, and weight, as well as questions regarding eating habits, activity level, and possible complications related to the surgery. Body composition and resting energy expenditure (REE) were measured, blood was drawn, and an echocardiogram was obtained at each visit.
Patient Characteristics, Body Composition and Resting Energy Expenditure
At baseline, one, three, nine and 24 months we measured body weight, BMI, lean mass, fat mass, waist circumference, hip circumference, blood pressure, heart rate and REE. Fat mass and lean mass were determined using a bioimpedence analyzer (RJL Systems, Clinton Twp, MI), indirect calorimetery (Body Gem by Health-e-Tech, Golden, CO) was used to measure REE.
Metabolic Parameters
At baseline, 3, 9 and 24 months, laboratory analyses of plasma were performed to evaluate the plasma markers of obesity (glucose, insulin, tumor necrosis factor alpha [TNF-α], high sensitivity C-reactive protein [hs-CRP], free fatty acids [FFA], adiponectin, and leptin). Insulin resistance was calculated using the Homeostasis Model of Assessment computer model (HOMA2IR). 20 This protocol is further detailed in our previous publication. 14
Echocardiograms
Two-dimensional guided M-mode echocardiographic examinations were performed at baseline, 3, 9 and 24 months using a Sequoia-C-512, (Acuson-Siemens, Malvern, PA). Participants were studied in the left lateral decubitus position and images were acquired from standard parasternal and apical windows. Myocardial contrast agents were utilized to improve left ventricular endocardial delineation as necessary. All studies were read by two echocardiologists blinded to all patient information. The echocardiographic measurements of the LV internal dimension, interventricular septal and posterior wall diastolic thickness were performed off line (Heartlab, Digisonics, Agfa Healthcare, Greenville, SC) on a dedicated echo reading station according to recommendations of the American Society of Echocardiography (ASE). 21
When M-mode measurements could not be obtained optimally, LV internal dimensions and wall thickness measurements were made using the leading edge convention as described by the ASE. LV end-diastolic and end-systolic volumes were calculated using the Teichholz method.22 Relative wall thickness (RWT) was calculated as follows: RWT= [2 × LVPW]/Ded, where LVPW = Left Ventricular posterior wall thickness at end diastole, and Ded=Left Ventricular mid-cavity dimension at end diastole. LV end diastolic dimensions were used to calculate left ventricular mass using a necropsy-validated formula. 23 The ratio of the left ventricular mass over height (LVM/ ht 2.7) was calculated by dividing the left ventricular mass by height in meters raised to the 2.7 power. 21
Statistical Analyses
Statistical analysis was performed with SigmaStat 3.0 (Systat Software, Inc., San Jose, CA). We evaluated all of the study variables for conformation to normality using Q-Q plots, skewness and kurtosis statistics. Significantly non-normal variables were transformed prior to analysis. Paired t-tests were performed to evaluate differences in outcomes between each visit. Repeated-measures ANOVA with post-hoc comparisons and multiple comparison adjustments were made across time point pairs by linear contrasts. Data are expressed as mean values plus or minus the standard error of the mean and as the change in mean values from 0M to 3M, 3M to 9M, and 9M to 24M post surgery with 95% confidence intervals. Pearson correlation coefficients were prepared to evaluate the univariate relationships between variables.
RESULTS
Clinical Data
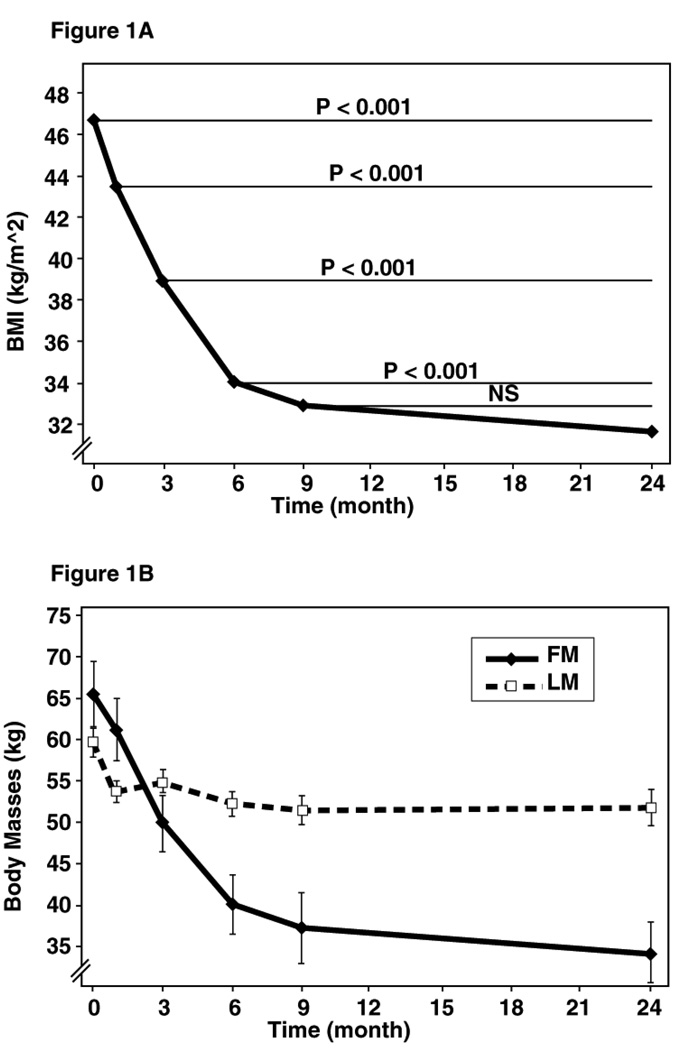
Significant physical and metabolic changes were observed as early as 1 month post surgery (Figure 1A and B) and continued to be present throughout the entire two-year period. The clinical, hemodynamic and metabolic data are summarized in Table 1.
Figure 1.
Figure 1A: Decline of the BMI over time. The rate of decline of BMI was greatest in the initial 6 months following surgery. Thereafter, the BMI continues to decline at a slower rate.
Figure 1B: Comparison of the Body Masses (fat mass and lean mass) after surgery. The decrease in lean mass and fat mass was greatest during the first month after surgery. Lean mass plateaued after 1 month, while fat mass continued to decrease sharply until 6 months and then slowed over the subsequent months.
Table 1.
Clinical, hemodynamic, and metabolic changes after surgery
| 0 to 3 months Mean Difference |
Significance (p value)† |
3 to 9 months Mean Differences |
Significance (p valued)† |
9 to 24 months Mean Difference |
Significance (p value)† |
|
|---|---|---|---|---|---|---|
| Clinical Data | ||||||
| Weight (kg) | −21.9 | <0.001 | −16.3 | <0.001 | −2.3 | NS |
| Excess Weight (%) | −31.5 | <0.001 | −24.3 | <0.001 | 1.9 | NS |
| Waist Circ. (cm) | −13.9 | <0.001 | −14.2 | <0.001 | −4.6 | NS |
| Hip Circ. (cm) | −15.6 | <0.001 | −10.4 | <0.001 | −0.6 | NS |
| WHR | −0.003 | NS | −0.04 | NS | −0.04 | NS |
| REE (Cal) | −384.1 | <0.001 | −222.7 | <0.001 | 36.9 | NS |
| REE/LM (Cal/kg) | −4.0 | NS | −2.0 | NS | −0.8 | NS |
| LVM/LM (g/kg) | 0 | NS | −0.06 | NS | −2.8 | NS |
| Hemodynamic Data | ||||||
| SBP (mm Hg) | −2.6 | NS | −3.0 | NS | 6.5 | NS |
| DBP (mm Hg) | −3.3 | NS | −3.6 | NS | 7.6 | NS |
| HR (bpm) | 9.9 | NS | 6.1 | NS | 2.6 | NS |
| Metabolic Data | ||||||
| Glucose (mg/dL) | −22.1 | NS | −7.7 | NS | 8.8 | NS |
| Insulin (µU/mL) | −11.7 | <0.003 | −2.3 | NS | 1.2 | NS |
| HOMA2-IR | −4.9 | NS | 0.3 | NS | ||
| Adiponectin (µg/mL) | −1.2 | NS | −0.7 | NS | 9.2 | <0.0001 |
| Leptin (ng/mL) | −24.6 | <0.001 | 17.3 | NS | 7.7 | NS |
| TNF-α (pg/mL) | −0.8 | NS | −2.4 | NS | 3.3 | NS |
| hs-CRP (mg/dL) | −0.3 | <0.003 | 0.2 | NS | 0.01 | NS |
| FFA (mmol/L) | −0.2 | NS | 0.3 | 0.01 | 0.006 | NS |
All values are the mean differences of 15 patients.
Abbreviations: BMI- body mass index; Waist Circ- waist circumference; Hip Circ- hip circumference; WHR- waisthip ratio; REE-resting energy expenditure; LM- lean mass; LVM- left ventricular mass; ht- height; SBP- systolic blood pressure; DBP- diastolic blood pressure; HR- heart rate; HOMA- Homeostatic Model for Assessment for insulin; TNF-α- tumor necrosis factor alpha; hs-CRP-hormone sensitive C reactive protein; FFA – free fatty acids; NS- not significant.
Significance for a difference at alpha <0.05.
All patients were severely obese at the start of the study (BMI 46.7 kg/m2) and experienced significant weight reduction (from 126.7kg to 86.8 kg), yet remained clinically obese at 24 months following surgery (BMI 32.4 kg/m2) (Figure 1A). More specifically, the BMI decreased by almost 8 kg/m2 in the initial 3 months post-operatively and 6.1 kg/m2 in the subsequent 6 months (p<0.001) (Figure 1A). The rate of decline of the BMI in the 9- to 24-month period slowed to just 0.9 kg/m2. Excess weight decreased by 31.5% in the first 3months and by another 24.3% in the subsequent six months (p<0.001). There was no further decrease thereafter (Table 1).
Waist circumference decreased by 13.9 cm in the first 3 months, and another 14.2 cm in the subsequent 6 months (p<0.001). Hip circumference decreased in the first 9 months (26 cm, p<0.001) while only nominally decreasing in the 9- to 24-month period (Table 1).
Fat mass decreased significantly in the first 3 months and insignificantly in the subsequent 6 months (15.5 kg and 12.1 kg, p<0.001) (Figure 1B). There was no further change subsequently. Lean mass decreased in the first 3 months and once more in the subsequent 6 months (−4.9 kg and −3.3 kg, respectively, P <.001, P= .002).
Hemodynamic Parameters
Systolic and diastolic blood pressures did not change over the 24-month period (Table 1). However, before surgery all patients required antihypertensive medications. These medications included thiazide diuretics, beta blockers, ACE inhibitors, calcium channel blockers and angiotensin receptor blockers. Following surgery, the requirements for antihypertensive medications decreased dramatically, with 13/15 patients off all antihypertensive medications at 3 months. There was a trend for the heart rate to decrease in the initial 3 months (from 79 to 69 beats per minute) and in the subsequent 6 months from 69 to 63 beats per minute, suggesting a decrease in sympathetic tone.
Metabolism
Resting energy expenditure decreased in the first 3 months as well as the subsequent 6 months (−374 and −228 Cal/kg p<0.001) while increasing nominally in the 9- to 24-month period (Table 1).
Serum glucose levels fell from 110 ± 41 to 88 ± 40 mg/dL at 3 months. At 24 months the average fasting glucose was 90 ± 18 mg/dL. Insulin levels declined most dramatically in the initial three months (from 22.3 to 10.2 µU/mL), with a further decline over the subsequent six months. HOMA2 IR indices declined most dramatically in the initial 3 month period (from 7.5 to 2.6).
Adiponectin levels rose throughout the entire postoperative period with the most dramatic increase between 9 and 24 months. In contrast, leptin levels declined most dramatically in the first 3 months (from 53 to 29 ng/mL). The levels continued to decline over the subsequent 6 months but rose in the final 24-month period (from 17.3 to 26.3ng/mL). TNF alpha levels increased in the first 3 months after surgery (from 7.9 to 8.6 pg/mL), most likely the result of the surgical trauma. Thereafter, the levels declined in the subsequent study periods (to 6.5 and 2.9 pg/mL, respectively). Levels of hs-CRP declined in the initial 9 months of the study (from 0.6 to 0.4 to 0.2 mg/dL) and remained unchanged in the subsequent 15 months. FFA levels increased in the initial 3 months (from 0.8 ± 0.2 to 1.0 ± 0.3 mmol/L), suggesting enhanced rates of lipolysis. Thereafter, levels declined to 0.7 mmol/L and remained unchanged for the subsequent 15 months.
Left Ventricular Mass
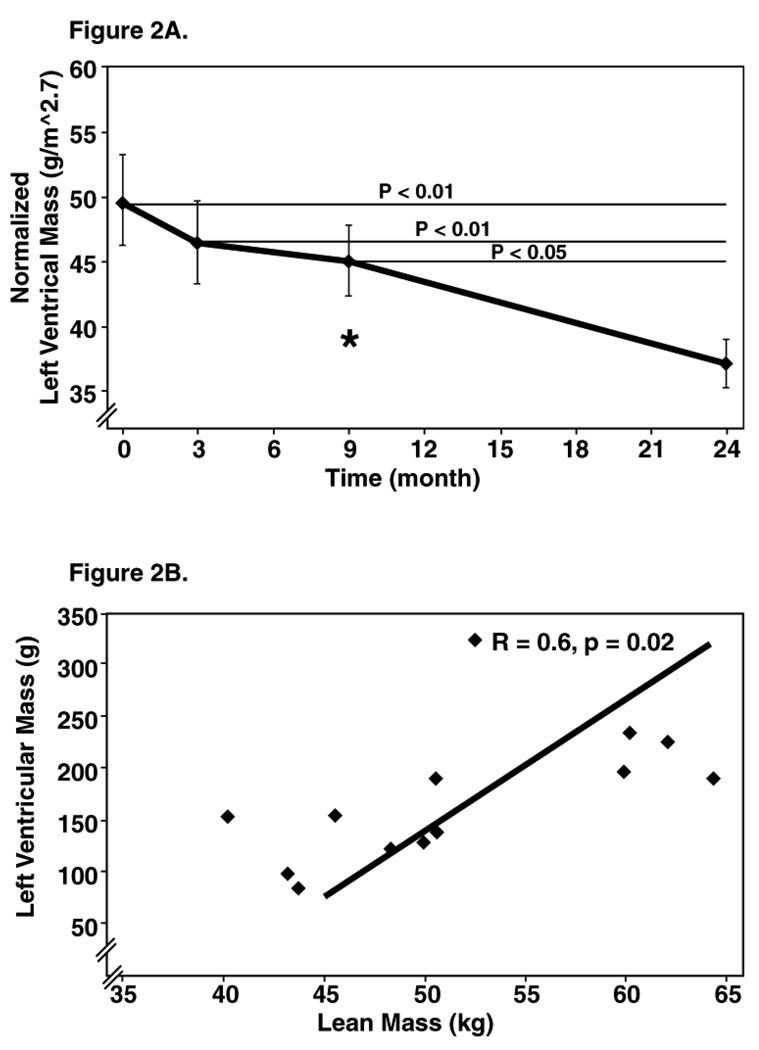
Figure 2A shows the decline of left ventricular mass as a function of time after surgery. The greatest rate of decline was during the first 3 months. The rate of decline slowed in the 3 to 9 and accelerated again between 9 to 24 month s. Thus, in contrast to BMI, lean mass and fat mass, left ventricular mass continued to decline, in contrast to BMI lean mass and fat mass. Figure 2B shows left ventricular mass as a function of lean mass at 9 months after surgery. There was a significant correlation between the two parameters at this time point, suggesting that bigger people have bigger hearts. However, at the other time points no such correlation existed (data not shown).
Figure 2.
Figure 2A: Progressive decline in left ventricular mass (LVM): Left ventricular mass declined progressively and normalized by 9 months (normal cut-off for women is 46.7 g/m2.7).
Figure 2B: Correlation of left ventricular mass to lean mass at 9 months. Pearson’s correlation indicated a strong correlation of left ventricular mass and lean mass at 9 months, but not at other time period.
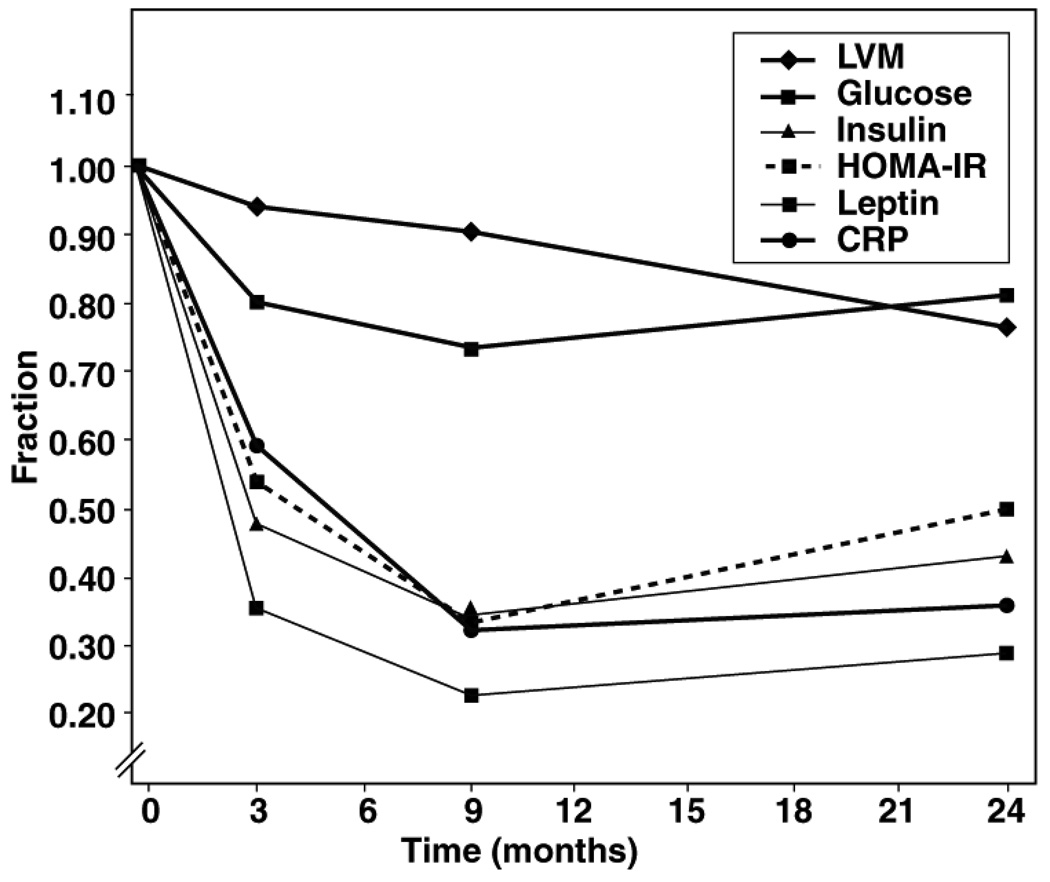
In order to assess the relationship between humoral factors and left ventricular mass, we examined the fractional decline of left ventricular mass and the plasma markers of obesity after surgery (Figure 3). Where left ventricular mass declines linearly, plasma markers change more in a parabolic manner. Glucose, insulin, HOMA2-IR, leptin and hs-CRP all fell sharply from their baseline in the first 3 months following surgery, plateau by 9 months and thereafter begin to rise, but did not reach the pre-operative levels by 24 months.
Figure 3.
Fractional Change form Baseline of left ventricular mass and Plasma Markers of Obesity after Bariatric Surgery: Left ventricular mass declines linearly over the 24-month time period while plasma markers of obesity decline sharply in the initial 3 months and nadir at 9 months. Thereafter the plasma markers begin to increase towards, but not to, the pre-operative levels.
DISCUSSION
We made the following observations: 1) weight decreased early after weight loss surgery, but plateaued when patients were still obese; 2) left ventricular mass declined progressively, and continued to decline at a linear rate for at least 24 months after weight loss surgery; 3) left ventricular mass was overall insensitive to body composition, suggesting that the heart continued to remodel even after body weight has reached a new plateau; and 4) systemic metabolism normalized by 3 months, and remained so 24 months, after surgery.
Decrease in Left Ventricular Mass
The most striking finding in this study is the unexpected dissociation of the left ventricular mass, BMI, fat mass and lean mass. Estimation of left ventricular mass offers prognositic information beyond that of traditional cardiovascular risk factors.17, 24 In our cohort left ventricular mass decreased in an almost linear fashion compared to the sharper “step” of the lean mass at 1 month and of the fat mass at 6 months. However, the initial decline in left ventricular mass (0 to 9 months) seemed less pronounced than the decline in BMI, fat mass, and lean mass (Figure 1 and Figure 2). It is of interest that the first 3 months after surgery are also the period of significant decline for glucose, insulin, HOMA2IR and hs-CRP. The rates of decline for these plasma markers nadir by 9 months post surgery and begin to increase, while the left ventricular mass deceases linearly over the entire 24-month period (Figure 3). In other words, the heart continues to remodel long after the metabolic imbalances have been corrected.
In a short-term follow-up study severely obese adolescent patients with cardiac abnormalities remained clinically obese after weight-loss surgery, but demonstrated a significant reversal of their cardiac abnormalities. 11 There has also been a case report of a heart failure patient who underwent weight-loss surgery was removed from the heart transplant list and remained clinically stable for at least eight years after the weight-loss surgery.13 However, none of the studies examined the long-term effect of forced weight loss on left ventricular mass.
The benefits of weight-loss surgery on cardiac function are evident, but understanding the pattern of change and the mechanisms behind reverse left ventricular remodeling remains elusive. While the association between LVH, hypertension and increased afterload is well established, only one study has shown that left ventricular mass significantly decreases in normotensive obese patients, who lost weight and remained normotensive. 25 Our data concur with this study. Neurohumoral determinants of left ventricular mass may be suspected in patients who lose weight after bariatric surgery because hemodynamic changes alone cannot account for the observed decreases in left ventricular mass. Although not investigated by us, it seems reasonable to assume that an improvement in sleep disordered breathing may be responsible for the decline in left ventricular mass. Evidence for the role of neurohumoral effect on left ventricular mass can be found in the obstructive sleep apnea patients’ response to continuous positive airway pressure (CPAP).26 Here regression of LVH and improved left ventricular ejection fraction occurred as a result of abolition of cyclical surges in left ventricular wall tension during sleep and chronic downward resetting of sympathetic outflow and peripheral resistance secondary to the abolition of obstructive apnea.27, 28 It is well possible that the patients we followed in this study experienced a decrease in frequency and severity of sleep apnea.
Changes in Body Composition
Another major observation of this study is that after a rapid decline of lean mass by one month after surgery, the lean mass and fat mass no longer move in parallel. Previous long-term studies on the efficacy of weight-loss surgeries have reported weight regain and a return of co-morbid conditions, such as diabetes, hypertension, and glucolipotoxicity.5, 10 We have observed earlier that metabolic improvements are less pronounced in lap band patients compared to Roux-en-Y patients.14 We and others also have observed that weight-relapse begins as early as 2 years post-surgery depending on the type of weight loss surgery.29 Others have also shown that overall mortality improves from obesity related complications, even while there is a regaining of weight along with the return of obesity related co-morbidities.2, 3, 30, 31 While the exact mechanisms behind the weight relapse are not known, some factors thought to play a role include: a gradual dilatation of the gastric pouch in laparoscopic adjustable gastric banding,32 increase of energy intake,10 and decreased physical activity over time.33, 34 We did not observe weight regain and return of comorbid conditions in our cohort.
Systemic Metabolism
Metabolic dysregulation of obesity is accompanied by both adaptive and maladaptive responses in the heart.35 Elevated FFA levels in patients with glucose intolerance or type 2 diabetes have been associated with structural and functional changes in the heart.28, 36 The improvement in FFA and adiponectin levels, as well as the normalization of glucose, insulin and leptin levels in our cohort may play a role in the continued reduction of left ventricular mass, even at 24 months, despite no further weight loss. This issue requires further investigation.
CONCLUSIONS
Even though parameters of obesity, including BMI and body composition, plateau, the benefits of bariatric surgery on metabolism and left ventricular mass are sustained. We propose that the decline of left ventricular mass after weight loss surgery is regulated by neurohumoral factors. No matter what the mechanism, the decline in left ventricular mass is likely to contribute to the improved long-term survival of patients following weight loss surgery.
Acknowledgements
This study was supported by The National Heart, Lung, and Blood Institute of the US Public Health Service (R01HL073162), and Grant # M01RR002558 for the Center for Clinical Research, University of Texas Medical School at Houston. We wish to thank Dr. Eddie Barasch for his insightful editorial review, and Mrs. Roxy Tate for her expert editorial assistance.
Source of Support: The National Heart, Lung, and Blood Institute of the US Public Health Service (R01HL073162). Grant # M01RR002558 for the Center for Clinical Research, University of Texas Medical School at Houston
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Verification: None of the authors of this work have any financial conflicts of interest to disclose. All authors had access to the data and a role in writing the manuscript.
REFERENCES
- 1.Mokdad AH, Marks JS, Stroup D, Gerberding J. Actual Causes of Death in the United States, 2000. J Am Med Assoc. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.Brolin RE. Bariatric surgery and long-term control of morbid obesity. J Am Med Assoc. 2002;288:2793–2796. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 5.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Lee YC, Ser KH, Chen JC, Chen SC. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–1125. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 8.Strauss BJ, Marks SJ, Growcott JP, et al. Body composition changes following laparoscopic gastric banding for morbid obesity. Acta Diabetol. 2003;40 Suppl 1:S266–S269. doi: 10.1007/s00592-003-0083-1. [DOI] [PubMed] [Google Scholar]
- 9.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–559. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 11.Ippisch HM, Inge TH, Daniels SR, et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008;51:1342–1348. doi: 10.1016/j.jacc.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Leichman JG, Wilson EB, Scarborough T, et al. Dramatic reversal of derangements in muscle metabolism and diastolic left ventricular function after bariatric surgery. Am J Med. 2008;121:966–973. doi: 10.1016/j.amjmed.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyengar S, Leier CV. Rescue bariatric surgery for obesity-induced cardiomyopathy. Am J Med. 2006;119:e5–e6. doi: 10.1016/j.amjmed.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 14.Leichman JG, Aguilar D, King TM, et al. Improvements in systemic metabolism, anthropometrics, and left ventricular geometry 3 months after bariatric surgery. Surg Obes Relat Dis. 2006;2:592–599. doi: 10.1016/j.soard.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198–202. doi: 10.1016/j.cardfail.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Mathier MA, Ramanathan RC. Impact of obesity and bariatric surgery on cardiovascular disease. Med Clin North Am. 2007;91:415–431. doi: 10.1016/j.mcna.2007.02.002. x–xi. [DOI] [PubMed] [Google Scholar]
- 17.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 18.Fielding GA, Allen JW. A step-by-step guide to placement of the LAP-BAND adjustable gastric banding system. Am J Surg. 2002;184 doi: 10.1016/s0002-9610(02)01176-5. 26S–30S. [DOI] [PubMed] [Google Scholar]
- 19.Higa KD, Boone KB, Ho T, Davies OG. Laparoscopic Roux-en-Y gastric bypass for morbid obesity: technique and preliminary results of our first 400 patients. Arch Surg. 2000;135:1029–1033. doi: 10.1001/archsurg.135.9.1029. discussion 1033–4. [DOI] [PubMed] [Google Scholar]
- 20.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 21.Committee A. Recommendations for Chamber Quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee. J Am Soc Echocardiogr. 2005;18:1434–1464. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 24.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 25.Himeno E, Nishino K, Nakashima Y, Kuroiwa A, Ikeda M. Weight reduction regresses left ventricular mass regardless of blood pressure level in obese subjects. Am Heart J. 1996;131:313–319. doi: 10.1016/s0002-8703(96)90360-9. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 27.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waradekar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996;153:1333–1338. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- 29.Trakhtenbroit MA, Leichman JG, Algahim MF, et al. Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med. 2009;122:435–442. doi: 10.1016/j.amjmed.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 31.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller MK, Wildi S, Scholz T, Clavien PA, Weber M. Laparoscopic pouch resizing and redo of gastro-jejunal anastomosis for pouch dilatation following gastric bypass. Obes Surg. 2005;15:1089–1095. doi: 10.1381/0960892055002257. [DOI] [PubMed] [Google Scholar]
- 33.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 34.Cook CM, Edwards C. Success habits of long-term gastric bypass patients. Obes Surg. 1999;9:80–82. doi: 10.1381/096089299765553872. [DOI] [PubMed] [Google Scholar]
- 35.Harmancey R, Wilson CR, Taegtmeyer H. Adaptation and maladaptation of the heart in obesity. Hypertension. 2008;52:181–187. doi: 10.1161/HYPERTENSIONAHA.108.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]