Abstract
The prenatal testosterone (T)-treated adult female rhesus monkey is one animal model of polycystic ovary syndrome (PCOS) in women, with early prenatal T excess programming a permanent PCOS–like phenotype characterized by luteinizing hormone (LH) hypersecretion from reduced hypothalamic sensitivity to steroid negative feedback and relative insulin excess from increased abdominal adiposity. These combined reproductive and metabolic abnormalities are associated with ovarian hyperandrogenism and follicular arrest in adulthood, as well as premature follicle differentiation and impaired embryo development during gonadotropin therapy for in vitro fertilization (IVF). A second animal model for PCOS, the prenatal T-treated sheep also is characterized by LH hypersecretion from reduced hypothalamic sensitivity to steroid negative feedback, persistent follicles and insulin resistance, but also is associated with intrauterine growth retardation and compensatory growth after birth. The ability of prenatal T excess in both species to alter the developmental trajectory of multiple organ systems in utero provides evidence that the hormonal environment of intrauterine life programs target tissue differentiation, raising the possibility that T excess in human fetal development promotes PCOS in adulthood. Such a hypothesis must include data from clinical studies of PCOS women to clarify the homology between these PCOS-like animal models and PCOS per se in reproductive and metabolic function. Future studies should develop new clinical strategies that improve pregnancy outcome and minimize pregnancy loss in women with disorders of insulin action, including PCOS, obesity and diabetes mellitus as well as minimize transgenerational susceptibility to adult PCOS and its metabolic derangements in male close relatives.
Keywords: Prenatal androgenization, polycystic ovary syndrome, luteinizing hormone, hyperandrogenism, hyperinsulinemia, adiposity
I. Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous syndrome in women characterized by luteinizing hormone (LH) hypersecretion, ovarian hyperandrogenism, hyperinsulinemia from insulin resistance and reduced fecundity. Given a 6.6% estimated prevalence of PCOS in reproductive-aged women in the United States (i.e., at least 4 million affected women), the annual economic burden of PCOS is at least $4.4 billion, of which $1.8, $1.4 and $0.5 billion are for treating type 2 diabetes mellitus, menstrual dysfunction and infertility, respectively. These estimates do not consider the greater frequency of pregnancy-related complications, including gestational diabetes, preeclampsia and miscarriage (1). Based upon the 1990 NIH definition of PCOS as hyperandrogenic chronic anovulation, the consequent annual health-care costs in the United States of evaluating and treating PCOS are at least 3-fold that of hepatitis C and one-third that of morbid obesity (1). These costs undoubtedly underestimate the expense of managing other PCOS phenotypes, defined by the Rotterdam criteria as any two of the three findings: clinical/biochemical hyperandrogenism, ovulatory dysfunction, polycystic ovaries (2).
While its peripubertal onset and familial clustering suggest a heritable etiology for PCOS, several candidate genes, including those regulating insulin action, androgen biosynthesis and gonadal function, have failed to fully explain its prevalence. Emerging data also implicate epigenetic changes in fetal life in the developmental origins of PCOS, with the most notable being the ability of discrete experimentally-induced prenatal testosterone (T) excess to program a permanent PCOS-like phenotype in several species. That T excess in utero programs multiple fetal organ systems agrees with the increased prevalence of PCOS in women with classical congenital adrenal hyperplasia (CAH) from 21 hydroxylase deficiency and with congenital adrenal virilizing tumors (3–6), confirming that the steroid milieu of intrauterine life programs differentiation of fetal target tissue.
In this regard, fetal programming of PCOS traits can be experimentally induced in several species by prenatal T excess, which permanently alters female reproductive and metabolic physiology and provides a means to assess molecular mediators involved in these perturbations. Evidence to date suggests that prenatal T-treated monkeys and sheep, like PCOS patients, manifest anovulatory infertility (7–9), adiposity-dependent compensatory hyperinsulinemia (10, 11), hypergonadotropism (12–15), neuroendocrine feedback defects (11, 13, 14, 16–20, 21), functional hyperandrogenism (22–25) and polycystic ovaries (26, 27). This chapter emphasizes prenatally T-treated monkey and sheep as models of PCOS because follicular differentiation in these species, as in humans and unlike rodents, is completed during fetal life. Data from these studies implicate critical times during fetal development when the steroidal status of the mother permanently alters the physiology of the fetus and modify its genetic susceptibility to disease after birth.
II. Reproductive Defects
II.A. Hyperandrogenism
Ovarian hyperandrogenism is the cardinal feature of PCOS, with in vitro studies of PCOS theca cells showing intrinsically increased androgen biosynthesis and augmented expression of several steroidogenic enzymes, including cytochrome P450 cholesterol side chain cleavage, 17α-hydroxylase/17–20 lyase (P450 c17) and 3β-hydroxysteroid dehydrogenase (28, 29). Hyperandrogenism is widely variable among the various PCOS phenotypes, as defined by Rotterdam criteria, and is more severe in “classic” PCOS (i.e., hyperandrogenic anovulation) than ovulatory PCOS patients (30). A similar hyperandrogenic anovulation can be induced by reprogramming adult ovarian morphology during prenatal development (11, 31). Female rhesus monkeys (32), sheep (7, 8, 33), mice (34) and rats (35) exposed prenatally to excessive levels of T exhibit ovulatory dysfunction in adulthood Table 1). Ovaries are enlarged and polyfollicular in prenatally T-treated monkeys and sheep, and also are hyperandrogenic in prenatally T-treated monkeys and mice (26, 34, 36), while androgen receptor expression is upregulated in ovaries of prenatally T-treated sheep (25).
Table 1.
Reproductive and metabolic PCOS-like abnormalities in prenatally androgenized female rhesus monkeys and sheep. Details of the traits are discussed in the text. ?: trait yet to be assessed.
| PCOS trait1 | Prenatally Androgenized Female Rhesus Monkeys | Prenatally Androgenized Female Sheep | |
|---|---|---|---|
| Early treated | Late treated | ||
| Reproductive | |||
| Ovarian hyperandrogenism | Yes | Yes | Ovarian androgen receptor upregulation |
| Anovulation | Yes | Yes | Yes |
| Enlarged polyfollicular ovaries | Yes | Yes | Yes |
| LH hypersecretion | Yes | No | Yes |
| Reduced steroid negative feedback on LH | Yes | Yes | Yes |
| Impaired embryonic development | Yes | Yes | Impaired fertility |
| Metabolic | |||
| Insulin resistance | Yes | No | Yes |
| Beta cell impairment | Yes | No | ? |
| Hyperglycemia | Yes | Yes | No |
| Increased type 2 diabetes | Yes | No | Unknown |
| Increased abdominal fat | Yes | With increasing BMI | Unknown |
| Hypertension | Unknown | Unknown | Yes |
| Hyperlipidemia | Yes | Unknown | Yes |
Details provided in the text
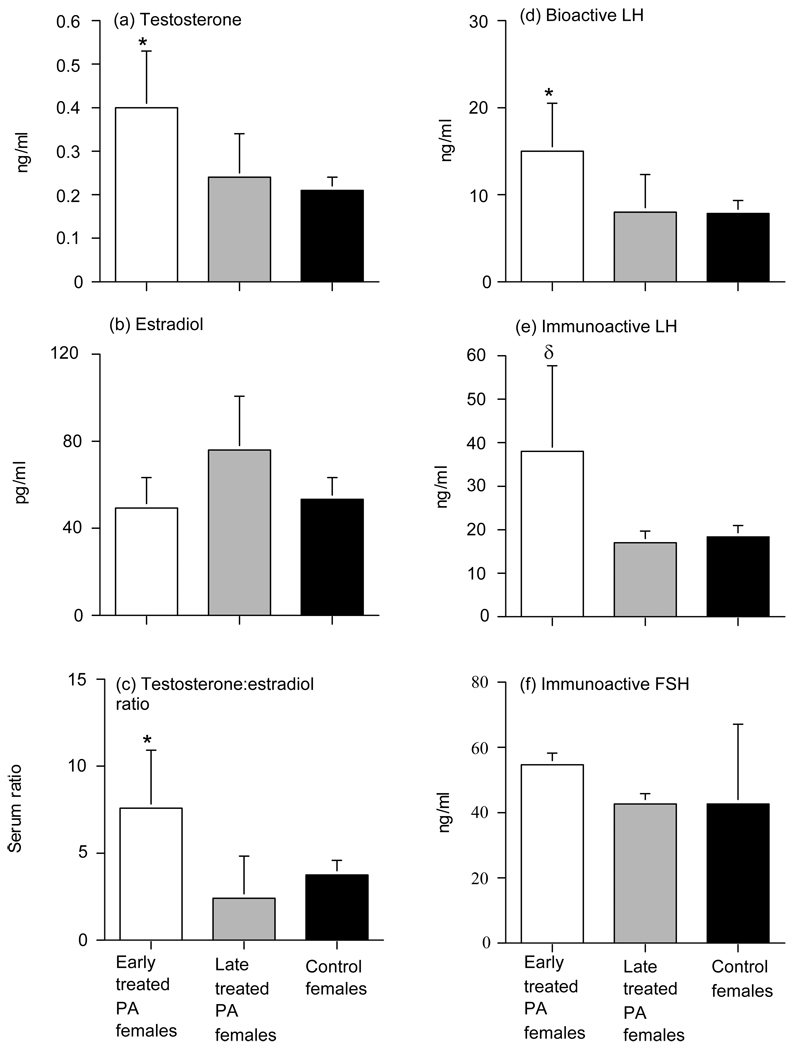
A PCOS–like phenotype can be produced by injecting pregnant rhesus monkeys carrying female fetuses with 10 to 15 mg T propionate (TP) for 15 to 35 days starting on either days 40–60 (early-treated) or days 100–115 (late-treated) postconception (total gestation, 165 days), which elevates circulating T levels in fetal females to those normally found in fetal males (37, 38). These prenatal T treatments coincide with target tissue differentiation and the beginning of neuroendocrine development in early-treated females, and with ovarian follicle development and functional acquisition of hypothalamic sensitivity to hormone negative feedback in late-treated females. Early prenatally T-treated female monkeys exhibit basal hyperandrogenemia (32, 39) (Figure 1), while both early (22) and late (11) prenatally T-treated females demonstrate an exaggerated T response to recombinant human (rh) chorionic gonadotropin (CG) administration. Regardless of the timing of prenatal T treatment, prenatally T-treated female monkeys have a ten-fold increase in the risk of anovulation as adults and have double the normal incidence of polyfollicular ovaries, with 33–50% of anovulatory prenatally T-treated females having such polyfollicular ovaries (36, 39)(Table 1). These traits correspond with the clinical diagnosis of PCOS by either the 1990 NIH or the Rotterdam criteria (1, 2). PCOS-like phenotype also can be produced in sheep by injecting pregnant sheep with 100 mg of T propionate twice per week from days 30–90 of gestation, which exposes fetal females to circulating T levels to those normally found in fetal males. (Padmanabhan V and Abbott DH, unpublished). As in monkeys, this time interval corresponds to when neuroendocrine feedback systems are established and ovarian differentiation occurs (31, 40).
Figure 1.
Mean (±SEM) serum values in early (open bars) and late (grey bars) treated prenatally T-treated and control (black bars) adult female rhesus monkeys reflecting (a) testosterone, (b) estradiol, (c) testosterone:estradiol ratio, (d) bioactive LH, (e) immunoactive LH and (f) immunoactive FSH during either the early follicular phase of the menstrual cycle or equivalent time during a 30-day anovulatory period. * p<0.05, versus controls, δ p<0.08 versus controls (Data from reference 39).
II.B. Abnormal follicle development
II.B.1. Increased follicle recruitment
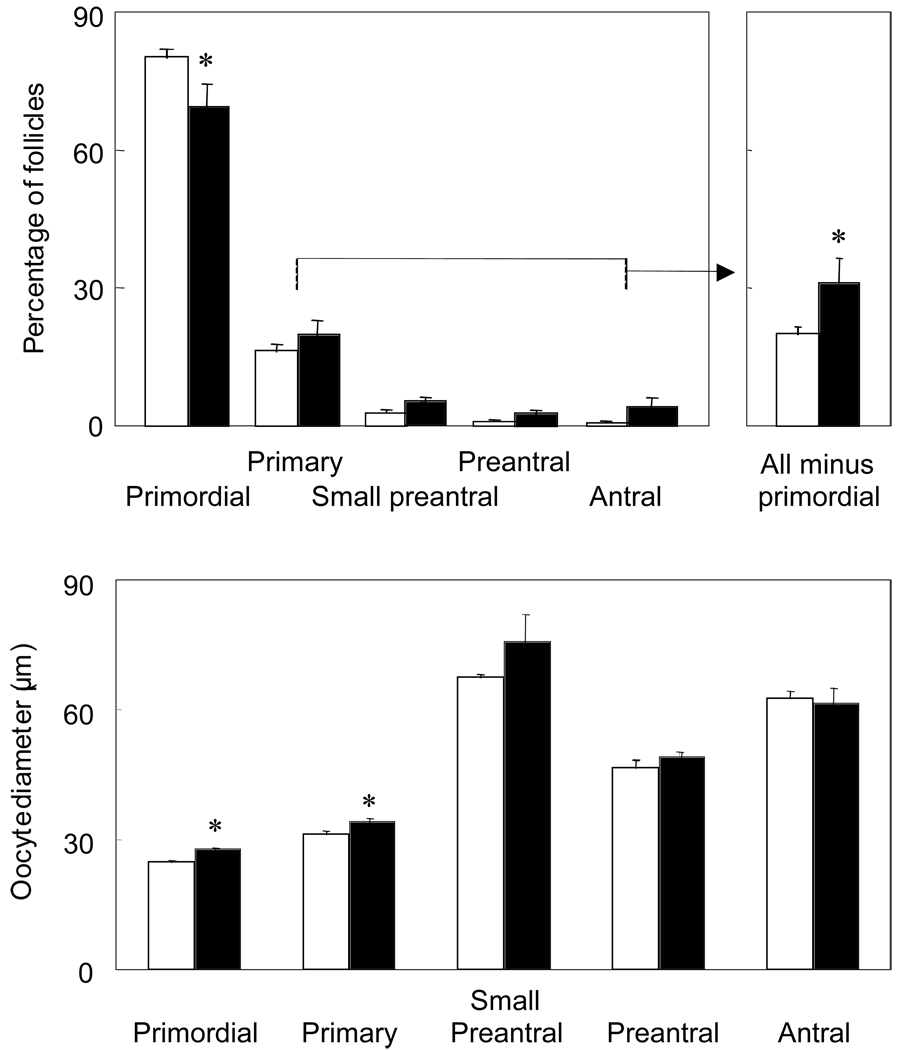
Several morphological findings in PCOS patients implicate increased recruitment of growing follicles from the primordial follicle pool with the development of the polycystic ovaries. One of three histological studies of human ovaries with polycystic morphology shows an increased proportion of primary follicles and a reciprocally decreased proportion of primordial follicles, independent of ovulatory status or atresia (41–43). Experimental evidence for adult hyperandrogenism causing increased recruitment of ovarian follicles comes from T administration to adult female rhesus monkeys increasing the number of primary, growing preantral and small antral follicles and the proliferation of granulosa cells within them (44, 45). Androgen treatment in such adult female monkeys also increases mRNA expression of follicle-stimulating hormone (FSH) receptor, insulin-like growth factor I (IGF-I) and IGF-I receptor in granulosa cells (46, 47), while enhancing IGF-I and its receptor mRNA expression in primordial follicle oocytes (48). Androgens also program enhanced follicle recruitment in utero since prenatal T treatment of sheep from days 30 to 90 of gestation (total gestation, 147 days) increases the proportion of growing follicles (i.e., primary, preantral, and antral follicles combined), decreases the proportion of primordial follicles and induces a polyfollicular phenotype (49) (Figure 2; Table 1).
Figure 2.
Effect of prenatal T treatment from days 30 to 90 of gestation on the distribution of follicles (top panel) and oocyte diameter (bottom panel) in fetal ovine ovaries at 140 d of gestation. Each bar represents mean ± SEM. Asterisks indicate significant differences (P < 0.05) (Data from reference 31).
II.B.2. Impaired follicle growth
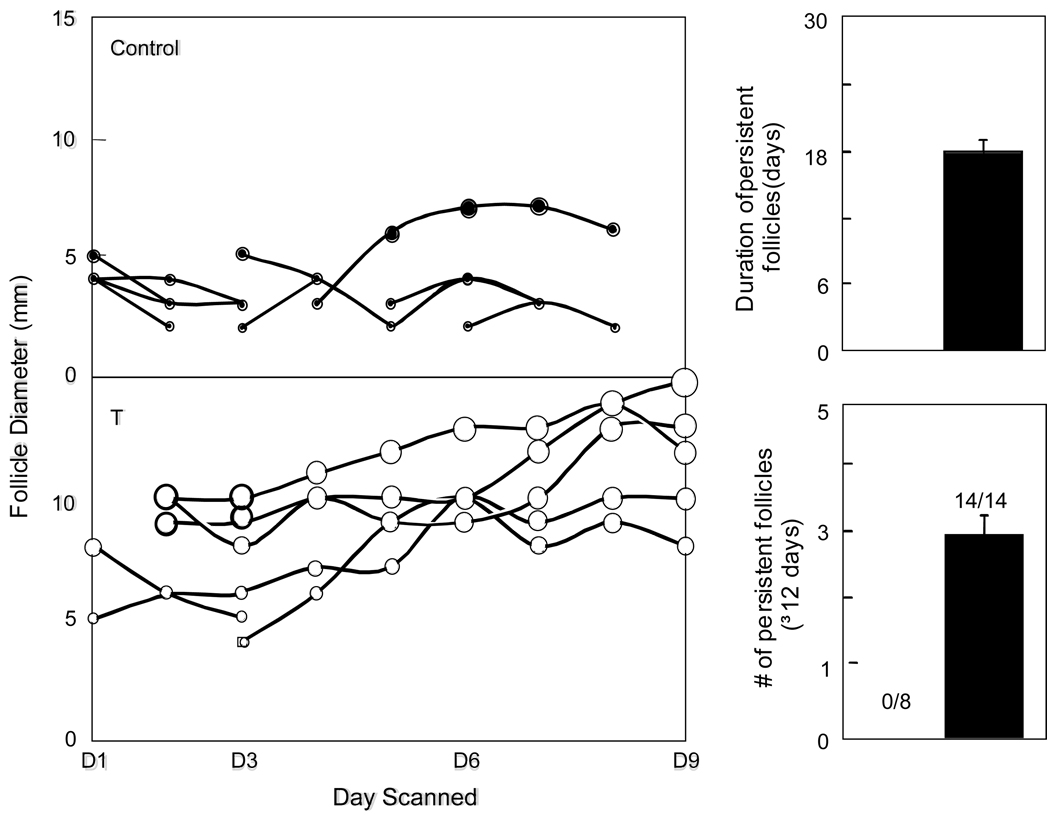
In PCOS, growth of follicles is impaired at the 6–8 mm size when granulosa cells normally begin to express aromatase and convert androgens produced by luteinizing hormone (LH)-stimulated theca cells to estradiol (E2) in the presence of FSH (50, 51). An endogenous inhibitor of estrogen synthesis likely exists in small estrogen-deplete PCOS follicles with sufficient bioactive FSH (52) because cultured granulosa cells from these follicles are hyperresponsive to FSH in vitro (53, 54). New sonographic ovarian studies in PCOS patients show that the number of 2–5 mm follicles positively correlates with serum T levels, while that of 6–9 mm follicles negatively correlates with fasting serum insulin and T levels, as well as body mass index (BMI) (55). Taken together, these findings associate hyperandrogenism with excessive early follicular growth that will not progress to the dominant stage due to androgen excess and/or hyperinsulinemia (55). In this regard, small PCOS follicles have elevated 5α-reductase activity, which increases 5α-reduced androgen levels to concentrations capable of inhibiting aromatase activity in vitro (56, 57). Increased 5α-reductase and decreased aromatase activities also occur in estradiol (E2)-deficient follicles of early prenatally T-treated female rhesus monkeys receiving rhFSH therapy (58). Even in cycling female rhesus monkeys, dihydrotestosterone (DHT) impairs gonadotropin-stimulated E2 secretion (59) and inhibits proliferation of cultured rat granulosa cells (60). Persistent follicular cysts in prenatally T-treated sheep further implicate impaired follicular growth as a contributing factor in developing polycystic ovaries (33) (Figure 3).
Figure 3.
Ovarian follicular dynamics determined by ultrasonography in both ovaries of a representative control and prenatal T-treated sheep are shown in the left panel. Each line represents one follicle. Only follicles that reached a size of 3 mm and persisted for at least 2 days are shown. Note the increase in maximum size and duration of the larger follicles on the ovary in prenatal T-treated sheep. Mean number (bottom right) and duration (top right) of persistent follicles in ovaries of control (n=8) and prenatal T-treated (n=14) sheep. Numbers within bottom histogram indicate number of animals in each group showing persistent follicles (Data from reference 31).
Impaired follicle growth also is associated with hyperinsulinemia from insulin resistance (61). Anovulatory PCOS patients have a greater BMI than their ovulatory sisters despite a similar degree of ovarian hyperandrogenism (62), and weight loss in obese PCOS patients reverses anovulatory infertility (63). Since insulin enhances FSH-induced upregulation of LH receptors in granulosa cells and increases their progesterone (P4) responsiveness to LH (64, 65), hyperinsulinemia presumably induces premature follicle luteinization, which arrests cell proliferation and follicle growth. Consequently small antral PCOS follicles exhibit P4 hypersecretion and overexpress LH receptors (66, 67), causing an exaggerated steroidogenic shift from E2 to P4 production (68). Similarly, exaggerated follicle differentiation occurs in early prenatally T-treated female rhesus monkeys undergoing rhFSH stimulation followed by hCG administration, in which LH hypersecretion and relative insulin excess from increased abdominal adiposity accompany an exaggerated shift in intrafollicular steroidogenesis from androgen and E2 to P4 (69). Conversely, improving hyperinsulinemia in PCOS patients with insulin sensitizing agents lowers serum androgen concentration (70, 71) and restores ovulation in approximately 50% of patients (72), as it does in prenatally T-treated female rhesus monkeys (9, 73).
Also implicated in impaired growth of PCOS follicles are transforming growth factor-β (TGFβ) family members, including activins, inhibins, anti-mullerian hormone, growth differentiation factor 9 (GDF-9) and bone morphogenetic protein 15, which interact with each other to coordinate follicle growth and oocyte development. Activins promote follicular development by enhancing granulosa cell responsiveness to FSH, suppressing androgen synthesis and stimulating oocyte maturation, while inhibins produced by the dominant follicle stimulate theca cell androgen production for E2 synthesis (74, 75). Consequently a shift from an activin-dominant to an inhibin-dominant microenvironment occurs during follicle growth (76), which is impaired in some, but not all, PCOS follicles (77–79). Moreover, low activin A levels and high follistatin levels in the circulation of some PCOS patients (80, 81) correspond with diminished intrafollicular activin in prenatally T-treated sheep (26) and activin β subunit responsiveness to steroid in neonatal mice (82). These findings emphasize the further need to understand how TGFβ family members affect intraovarian paracrine signaling during fetal developmental programming.
II.C. LH hypersecretion
A neuroendocrine hallmark of PCOS is enhanced LH hypersecretion from enhanced gonadotropin-releasing hormone (GnRH) pulsatility. Consequently serum immuno- and bioactive LH levels are increased in about 70% of PCOS patients (83), with elevated LH pulse amplitude and increased LH pulse frequency causing a two- to three-fold elevation in circulating LH versus FSH levels (84). PCOS patients also show an increased LH response to GnRH stimulation (83), along with a sexually dimorphic pattern of exaggerated early LH responsiveness to GnRH analog that more closely resembles that of men and women with congenital adrenal virilizing disorders (e.g., classical CAH and adrenal virilizing carcinoma) than normal women (3, 85). As further evidence of neuoendocrine dysregulation, PCOS patients show reduced hypothalamic sensitivity to P4 negative feedback on LH secretion (86, 87), which can be restored with the androgen receptor blocker, flutamide (88). Moreover, reduced hypothalamic sensitivity to P4 negative feedback on LH secretion in some girls with PCOS during adolescence (87) suggests that prepubertal hyperandrogenism may program reduced hypothalamic feedback inhibition, leading to rapid GnRH pulsatility in early development.
Neuroendocrine dysregulation of LH release also occurs in prenatally T-treated females of several species, including rhesus monkeys (15), sheep (14, 17, 40, 89, 90), hamsters (91) and rodents (35) (Table 1). Early prenatally T-treated female rhesus monkeys exhibit basal LH hypersecretion ((Figure 1), increased pituitary LH responsiveness to GnRH (11, 15) and reduced hypothalamic sensitivity to E2 and P4 negative feedback on LH release (11, 20, 21, 92). Late prenatally T-treated female rhesus monkeys show reduced hypothalamic sensitivity to P4 negative feedback on LH release alone (92). As in PCOS patients, prenatal T-treatment in primates does not abolish the E2-induced LH surge, which nevertheless can be exaggerated and delayed (15, 21). Prenatal T-treatment in sheep also induces LH hypersecretion from reduced hypothalamic sensitivity to E2 and P4 negative feedback (14, 17, 18, 24) as well as increased pituitary responsiveness to GnRH (93) and delays the onset of an otherwise truncated LH surge in Suffolk sheep (13). In Dorsett sheep, E2 fails to generate an LH surge (18, 19). The collective data from both species of prenatally T-treated animals, therefore, demonstrate programming of LH hypersecretion from reduced hypothalamic sensitivity to steroid negative feedback with enhanced GnRH pulsatility (14, 17, 18, 24) and disrupted surge mechanism (13, 18, 19).
II.D. Oocyte developmental competence
Enhanced theca cell androgen biosynthesis (28, 29), increased initiation of primordial follicle growth (43) and exaggerated granulosa cell responsiveness to FSH (54) are features of follicle development in PCOS patients undergoing in vitro fertilization (IVF). With more retrieved oocytes and cleaved embryos available to select for embryo transfer, PCOS patients undergoing IVF often achieve a clinical pregnancy rate comparable to that of similarly-treated normal women (94–96). Nevertheless, such PCOS patients also have increase risks of implantation failure and pregnancy loss (97) as well as impaired oocyte fertilization unrelated to gross chromosomal abnormalities or nuclear maturation (95, 96, 98–100). Moreover, obese PCOS patients experience low oocyte fertilization and failure of embryos to implant in their own uterus or those of their surrogates (101), implicating impaired oocyte developmental competence.
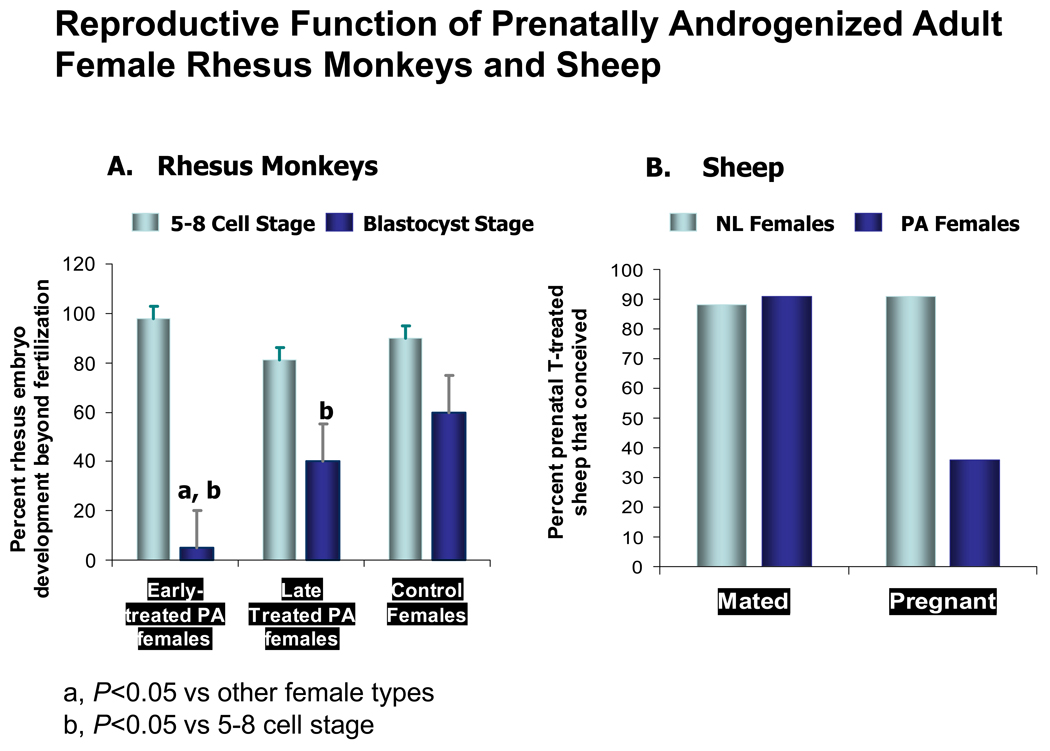
Terminally differentiated follicles of PCOS patients undergoing GnRH analog/rhFSH for IVF are hyperandrogenic with reduced intrafollicular FSH levels; they also contain meiotically-competent (metaphase II) oocytes with abnormal gene expression profiles (102, 103). Based upon timing of the oocyte to T programming in utero, prenatally T-treated female rhesus monkeys undergoing gonadotropin stimulation for IVF also experience abnormal follicle development and impaired oocyte development (20, 58). All prenatally T-treated female monkeys show abnormal intrafollicular steroidogenesis with reduced blastocyst formation (20, 58), neither of which can be predicted by circulating hormone levels, or from number and maturity of oocytes collected (Figure 4). Differing from the hormonal profile of PCOS follicles, low intrafollicular E2 and androstenedione (A4) levels in late prenatally T-treated female monkeys receiving rhFSH therapy alone (58) are accompanied by a subtle impairment of blastocyst development after combined rhFSH/hCG therapy (20), consistent with E2-enhanced oocyte development in primates (104, 105).
Figure 4.
A). Mean (upper 95% confidence limit) percentage of zygotes developing to the 5–8 cell (open bars) and blastocyst (solid bars) stages in 5 early prenatally T-treated, 5 late prenatally T-treated and 5 control adult female rhesus monkeys following ovarian hyperstimulation for IVF. a: p<0.05 versus control and late prenatally T-treated females at the same stage; b: p<0.05 versus 5–8 cell stage (Data modified from reference 20). B) Histograms on the right shows percentage of prenatal T-treated sheep (n=12) that successfully mated or conceived following estrus synchronization with two injections of PGF2α administered 11 days apart. To overcome mating preference, ram access was limited to only prenatal T-treated females. First service mating and pregnancy results for the breeding herd (n=109; hatched bar) bred during the same time are provided for comparison (Data modified from reference 112).
In early prenatally T-treated female monkeys, low follicle fluid E2 and A4 levels after both stimulation protocols are accompanied by an elevated P4/E2 ratio and a profound impairment of blastocyst development following combined rhFSH/hCG therapy. These findings suggest that as in humans both the E2 concentration and the P4/E2 ratio in the follicle affects oocyte development (104, 106) (Figure 4). Equally important, early, but not late, prenatally T-treated female monkeys undergoing rhFSH therapy for IVF show LH hypersecretion ((Figure 1, Table 1) and relative hyperinsulinemia at oocyte retrieval (20), an important finding since insulin together with FSH upregulates LH receptor expression in cultured murine cumulus-oocyte complexes and reduces blastocyst development (107).
Interestingly, reduced follicle fluid E2 and A4 levels in early prenatally T-treated rhesus monkeys undergoing rhFSH/hCG therapy for IVF (20) resemble those of IVF patients with diminished ovarian reserve (108) more than those of similarly-treated PCOS patients (102) and probably represent paracrine dysregulation of thecal cell P450 c17 activity (109). Nevertheless, prenatal T treatment appears to perturb follicle growth and oocyte development by limiting the production of E2 or its action in the presence of androgen (20, 58, 110, 111). These findings in concert with the observation that prenatally T-treated sheep also are subfertile (112) raises concern that the effects of prenatal T treatment on oocyte development ((Figure 2) might have transgenerational consequences for female offspring ((Figure 4).
III. Metabolic Defects
As major risk factors for type 2 diabetes mellitus and atherosclerosis (113), PCOS and obesity have independent and additive adverse effects on insulin action, with PCOS patients being more insulin resistant than weight-matched normal women (114). These defects appear to stem from intrinsic abnormalities of post-receptor insulin signaling (e.g. excess serine phosphorylation), abnormal insulin secretion (114, 115), or polymorphic genes controlling insulin action (116–118). While several factors influence insulin sensitivity, including ethnicity, history of diabetes mellitus and BMI (114, 119–121), increased abdominal adiposity is a common feature of PCOS that impairs insulin sensitivity and it is largely responsible for the increased insulin resistance observed in obese PCOS patients versus BMI-matched normal women (120). Moreover, increased abdominal adiposity is central to metabolic syndrome, a constellation of cardiovascular risk factors also including dyslipidemia, hyperglycemia and hypertension that is highly prevalent in adolescent PCOS patients (122).
Like humans, rhesus monkeys are susceptible to obesity and its glucoregulatory impairments (123). Prenatally T-treated female rhesus monkeys selectively deposit fat intra-abdominally and exhibit impaired insulin secretion or action in ways that closely resemble those of PCOS women, depending on whether the androgen excess occurred during early or late gestation (124–126) (Table 2). Detailed measures of body composition using computerized tomography with dual X-ray absorptiometry show that early T-treated females have increased visceral fat compared to control females, even when corrected for BMI and total body fat (125). Late T-treated females have increased total body and non-visceral abdominal fat compared to control females (126). Interestingly, both early and late T-treated PA females preferentially accumulate visceral fat with increasing BMI, while normal females preferentially accumulate non-visceral fat (126). Metabolic studies further show that early T-treated females have impaired insulin secretion, liberate more fatty acids than control females during a frequently sampled intravenous glucose tolerance test (73, 127) and exhibit basal serum insulin levels that are positively correlated with the amounts of total body, total abdominal and visceral fat stores (126). Late T-treated females show decrements in insulin sensitivity with increasing BMI, with preservation of insulin secretory function (14). The resulting metabolic abnormalities from adiposity-related insulin resistance in prenatally T-treated female monkeys contribute to an increased risk of diabetes mellitus (27.3% and 11.1% in early-treated and late-treated females, respectively). Moreover, prenatally T-treated sheep develop impaired insulin sensitivity in early postnatal life (10), together with hypertension and hypercholesterolemia after puberty (128) as additional components of the metabolic syndrome as seen in PCOS patients (Table 1 and Table 2).
Table 2.
Prediction of abnormal traits expected in prenatally androgenized female rhesus monkeys and sheep from the Barker hypothesis. Details of the traits are discussed in the text. +: trait present, −: trait absent, ?: trait yet to be assessed.
| Abnormal traits | Barker hypothesis prediction | Early treated PA female observation | Late treated PA female observation | PA female sheep observation |
|---|---|---|---|---|
| Low birthweight | + | − | − | + |
| Catch-up growth | + | − | ? | + |
| Visceral obesity | + | + | + with high BMI | ? |
| Insulin resistance | + | + | − | + |
| Beta cell impairment | + | + | − | ? |
| Glucose intolerance | + | + | + | ? |
| Hypertension | + | ? | ? | + |
Ameliorating impaired insulin action has beneficial glucoregulatory effects in both PCOS patients and prenatally T-treated female monkeys, as evidenced by the abilities of metformin and thiazolidinediones to improve insulin action in PCOS patients (70, 129, 130) and of pioglitazone to improve insulin action in both early and late prenatally T-treated monkeys (73). Such parallels in metabolic dysfunction between PCOS patients and prenatally T-treated female monkeys as well as sheep provide additional evidence of fetal androgen excess programming of metabolic function. Studies in sheep suggest programming of insulin resistance is facilitated by androgenic action of T (131).
IV. Barker Hypothesis
According to the developmental origins of adult disease hypothesis (i.e., the Barker hypothesis), adverse influences in early development lead to permanent changes in physiology and metabolism, resulting in increased disease risk in adulthood (Table 2). Original observations that regions of England having the highest rates of infant mortality in the early 20th century also had the highest rates of mortality from coronary heart disease decades later have been further supported by subsequent studies showing an association between low birth weight and adult development of cardiovascular disease (CVD), hypertension, insulin resistance and type 2 diabetes mellitus (132). Teleologically, fetal undernutrition would favor genes important for energy conservation (i.e., thrifty genotype), which would be beneficial in times of food scarcity, but would lead to obesity and diabetes when food becomes abundant later in life (133). Alternatively, fetal undernutrition might lead to an organized process in which fetal brain development is spared to the detriment of other organ systems (i.e., thrifty phenotype), perhaps as an adaptive response for postnatal survival in a nutrient-deplete environment (134). Evidence for such a phenomenon in PCOS can be found in poor intrauterine growth and low birth weight accompanying precocious puberty and PCOS in northern Spanish women (135) and PCOS pregnancies in Chilean women (136), but not in larger groups of Finnish (137) and Dutch individuals (138). Theoretically, maternal T excess could reduce fetal growth and birth weight through impaired placental function since experimentally-induced maternal T excess decreases rodent and sheep offspring birthweight (24, 139, 140); impaired placental aromatization in women also accompanies diminished uteroplacental perfusion and low infant birthweight (141, 142). Advanced placental differentiation to reduce intrauterine growth restriction also is a feature of T-treated pregnant sheep (143).
While prenatally T-treated female sheep (49, 140, 144) and rats (145) exhibit intrauterine growth retardation (IUGR) and low birth weight, prenatal T-treated rhesus monkeys do not (39, 146) (Table 2). Furthermore the IUGR in prenatally T-treated fetal sheep near term is characterized by an increased head to fetal weight ratio (49) corresponding with a brain-sparing effect (147) and is followed by postnatal weight gain (or catch-up growth) (140) and with insulin resistance in adulthood (24) (Table 2). Early prenatally T-treated female rhesus monkeys (39), on the other hand, show an increase in body weight during early infancy (Abbott and Tarantal, unpublished results), as well as during late adolescence/early adulthood and undergo delayed puberty in a manner similar to that of male puberty. Therefore, prenatally T-treated sheep and rats may be suitable models for PCOS with placental insufficiency, particularly since the former have enlarged left cardiac ventricles, kidneys and adrenals suggestive of CVD (24), while the latter have increased mortality (148).
V. Adrenal
Resembling the 25–60% prevalence of adrenal hyperandrogenism in PCOS (149, 150), early prenatally T-treated female monkeys show adrenal hyperandrogenism, presumably from enhanced 17α-hydroxylase/17,20 lyase activity of the zona reticularis in adulthood (23). Basal and ACTH-stimulated cortisol levels, however, are normal in prenatally T-treated monkeys. Basal and ACTH-stimulated cortisol levels are also normal in prenatally T-treated adult sheep (Padmanabhan, unpublished). Moreover, prenatally T-treated sheep demonstrate a proportionate increase in fetal adrenal weight with IUGR near term, demonstrating an altered trajectory of adrenal development accompanying fetal growth retardation (49).
VI. Alternate mechanisms of developmental programming
During the second trimester of human development, serum T levels are elevated into the male range in 40% of female fetuses (151). Therefore the wide variation in androgenic exposure that normally occurs during human development could certainly influence developmental programming of the fetus. Mechanisms beyond T-induced developmental programming, however, also may exist since close male relatives of PCOS patients exhibit metabolic dysfunction similar to that of their female kin (152–154). In support of this, prenatally T-treated male monkeys exhibit insulin resistance and diminished insulin response to glucose in adulthood (155), despite normal male levels of circulating T during fetal life (38). Therefore the combination of steroid and metabolic abnormalities in utero might perturb development of several fetal organ systems and increase the risk of developing reproductive and metabolic diseases in later life. Consistent with this hypothesis, genes for receptors to insulin, IGF-I and IGF-II and protein for P450c17 enzyme exist in second trimester human fetal ovaries (156, 157). In addition, female stillbirth offspring of diabetic mothers have increased birth weight and pancreatic beta cell hyperplasia with hirsutism, ovarian theca-lutein cysts and thecal cell hyperplasia (158, 159), while elevated amniotic fluid levels of the β-hCG and T occur in pregnant diabetic mothers receiving insulin (160).
Moreover, while direct androgen action in the female fetus may account for some aspects of adult reproductive function, some elements of fetal developmental programming may be mediated by conversion of T to E2 through placental or fetal gonadal aromatization. Cancer and infertility have long been recognized in women exposed prenatally to diethylstilbestrol (DES) (161, 162), as have paraovarian cysts and infertility in rodents exposed perinatally to DES, allyl E2, or E2-17β or E2 benzoate (163–165). More recently, persistent follicular cysts have been noted in sheep exposed prenatally to T, but not to DHT (33, 166), while IUGR and LH surge defects occur in similarly-treated sheep exposed to bisphenol, an estrogenic endocrine-disrupting compound (167). At the ovarian level, reduced primordial follicle numbers occur in ovaries of late gestational fetal baboons following maternal exposure to an aromatase inhibitor (168), while decreased oocyte-granulosa cell microvilli, and presumably perturbed oocyte-granulosa cell signaling, characterize the maturing fetal ovary following diminished estrogen exposure (169, 170).
Conclusions
Prenatal T-treatment in monkeys and sheep programs a permanent PCOS-like phenotype characterized by LH hypersecretion from reduced hypothalamic sensitivity to sex steroid negative feedback, functional hyperandrogenism, ovulatory dysfunction, polycystic ovaries and impaired glucose-insulin homeostasis.
Prenatal T-treatment in both species induces female subfertility, which in part represents the impaired developmental competence of primate oocytes.
Mechanisms beyond T-induced developmental programming likely exist since exposure of monkeys to prenatal T excess also impairs glucose-insulin homeostasis without affecting body weight in both adult sexes, while similarly-treated fetal sheep show intrauterine growth retardation with compensatory growth after birth.
Critical times exist during fetal development when the steroidal status of the mother permanently alter the physiology of the fetus and modify its susceptibility to disease after birth.
Optimizing the effects of the maternal diet and hormonal environment on fetal growth and development might minimize transgenerational susceptibility to PCOS and to its metabolic derangements in male close relatives and could improve the fertility of PCOS women while reducing their risk of pregnancy-related complications.
Key unanswered questions
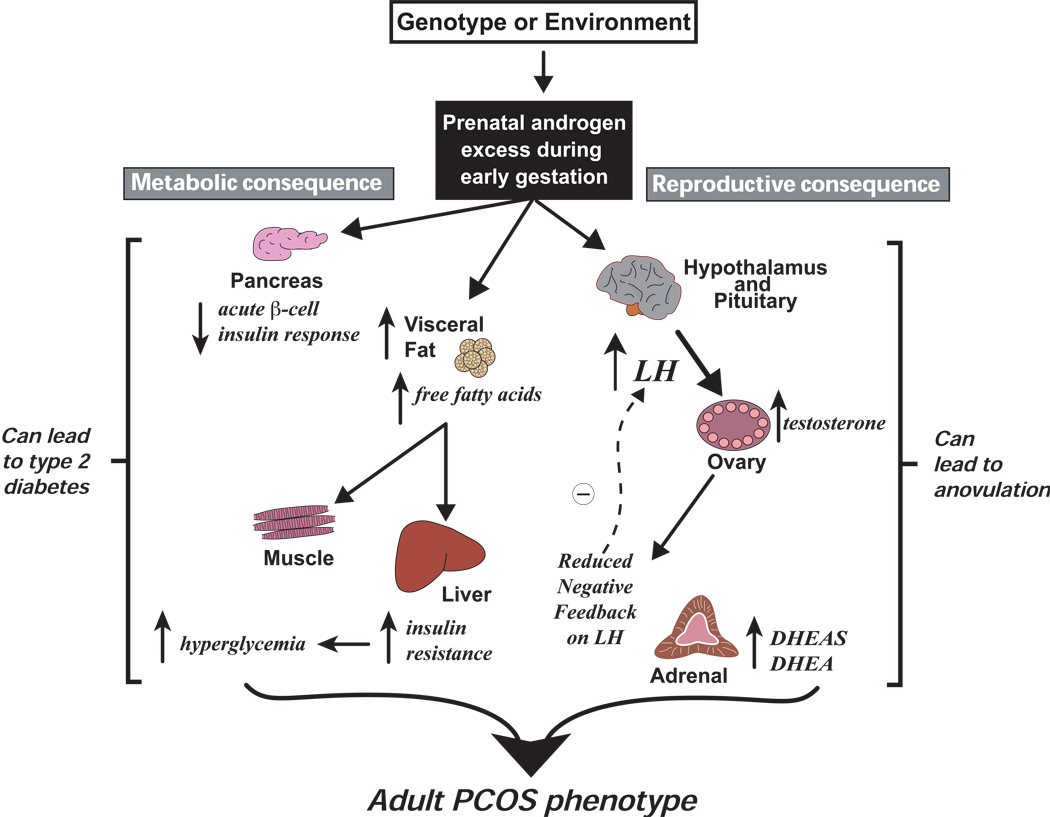
The prenatally T-treated animal models of PCOS implicate hyperandrogenism or hyperestrogenism during critical times of fetal development in the pathogenesis of PCOS and of its metabolic derangements in male close relatives. They agree with the increased prevalence of PCOS in women exposed to fetal T excess, including CAH from 21-hydroxylase deficiency and congenital adrenal virilizing tumors (3–6) and of insulin resistance in men with 21-hydroxylase deficiency (171). As such, T excess programming probably leads to at least two abnormalities, namely reproductive and metabolic, which may interact to increase susceptibility to an adult PCOS phenotype (Figure 5). Equally important and evident in prenatally T-treated animal models of PCOS, variation in gestational timing of T excess programming in utero, along with differences in target tissue sensitivity to steroid action, also may contribute to heterogeneity in the adult phenotype.
Figure 5.
Diagrammatic representation of possible early gestation, fetal androgen excess programming of adult PCOS traits. Genetic or environmental mechanisms induce fetal hyperandrogenism causing permanent changes in reproductive and metabolic function. Reproductive consequences include: (1) altered hypothalamic-pituitary function causing LH hypersecretion, (2) ovarian hyperandrogenism with or without LH hypersecretion, (3) reduced steroid hormone negative feedback regulation of LH, (4) adrenal hyperandrogenism, and (5) ovulatory dysfunction. Metabolic consequences include: (1) increased abdominal adiposity with elevated circulating total free fatty acid levels, (2) impaired pancreatic insulin secretory response to glucose, (3) impaired insulin action and compensatory hyperinsulinemia, (4) hyperglycemia, and (5) increased incidence of type 2 diabetes. Insulin resistance and compensatory hyperinsulinemia may be functionally implicated in the anovulatory mechanism (Data from reference 39).
Unfortunately, experimental constraints on the use of human fetal tissue for biomedical research limit our knowledge of the relationships between the human fetus and its maternal environment. Consequently, understanding how developmental programming affects human growth and development continues to require animal models to pioneer the probable fetal origins of adult disease. In doing so, future animal studies need to clarify the neuroendocrine mechanisms governing hypothalamic sensitivity to hormone negative feedback and the endocrine/paracrine signaling and their effects on follicle growth and oocyte development. At the ovarian level, knowledge of how developmentally relevant endocrine/paracrine factors and genes interact to promote optimal gene expression in the fetal oocyte for later fertilization and successful preimplantation embryogenesis also is necessary. With such information, new clinical strategies targeting long-term correction of follicle growth and development could improve fertility, optimize ovarian responsiveness to gonadotropin therapy and enhance pregnancy outcome by IVF, thereby promoting the transfer of fewer embryos into the uterus and decreasing the risk of multiple gestation and its adverse consequences on maternal-fetal health.
Also important is recognizing how the maternal environment affects fetal growth and development. With obesity the fastest-growing medical problem in America and two-thirds of American adults being overweight, impaired glucose-insulin homeostasis in pregnancy from insulin-resistance diseases, including obesity, PCOS and diabetes mellitus, also have implications on fetal developmental programming. Whether such programming events are secondary to altered abdominal adiposity or additional pancreatic or insulin receptor-mediated events, the implications are that genetically-determined hyperandrogenism can be modified by both maternal and environmental factors to program an adult PCOS phenotype and its male equivalent. In support of this, nutrient-deficient diets also can adversely affect long-term physiology of the offspring (172, 173) and alter DNA methylation in the human placenta (174), suggesting detrimental outcomes from epigenetic and metabolic abnormalities. Therefore additional clinical strategies that optimize the effects of the maternal diet and environment on fetal growth and development may be able to minimize transgenerational susceptibility to acquiring the adult PCOS phenotype and its metabolic derangements in male close relatives.
Acknowledgments
Supported by USPHS grants P01 HD44232 and HD 41098 to VP, U01 HD 044650 to DAD, R01 RR013635 to DHA, P50 HD044405 and P51 RR 000167 to the National Primate Research Center, University of Wisconsin, Madison and Organon USA, Inc. This work was partially supported by the NICHD National Cooperative Program on Female Health and Egg Quality under cooperative agreement U01 HD044650.
Contributor Information
Daniel A. Dumesic, Email: danieldumesic@aol.com.
David H. Abbott, Email: abbott@primate.wisc.edu.
Vasantha Padmanabhan, Email: vasantha@umich.edu.
References
- 1.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–4658. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 2.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 3.Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 4.Merke DP, Cutler GB., Jr New ideas for medical treatment of congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30:121–135. doi: 10.1016/s0889-8529(08)70022-7. [DOI] [PubMed] [Google Scholar]
- 5.Phocas I, Chryssikopoulos A, Sarandakou A, Rizos D, Trakakis E. A contribution to the classification of cases of non-classic 21-hydroxylase-deficient congenital adrenal hyperplasia. Gynecol Endocrinol. 1995;9:229–238. doi: 10.3109/09513599509160451. [DOI] [PubMed] [Google Scholar]
- 6.Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ. Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Obstet Gynecol Surv. 2003;58:275–284. doi: 10.1097/01.OGX.0000062966.93819.5B. [DOI] [PubMed] [Google Scholar]
- 7.Clarke IJ, Scaramuzzi RJ, Short RV. Ovulation in prenatally androgenized ewes. J Endocrinol. 1977;73:385–389. doi: 10.1677/joe.0.0730385. [DOI] [PubMed] [Google Scholar]
- 8.Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- 9.Abbott DH, Foong SC, Barnett DK, Dumesic DA. Nonhuman primates contribute unique understanding to anovulatory infertility in women. ILAR. 2004;45:116–131. doi: 10.1093/ilar.45.2.116. [DOI] [PubMed] [Google Scholar]
- 10.Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol. 2005;289:E801–E806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- 11.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 12.Fabre-Nys C, Venier G. Sexual differentiation of sexual behavior and preovulatory LH surge in ewes. Psychoneuroendocr. 1991;16:383–396. doi: 10.1016/0306-4530(91)90003-c. [DOI] [PubMed] [Google Scholar]
- 13.Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- 14.Sarma HN, Manikkam M, Herkimer C, Dell’Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4491. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- 15.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67:155–163. doi: 10.1016/s0015-0282(97)81873-0. [DOI] [PubMed] [Google Scholar]
- 16.Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep Rev Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
- 17.Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–5805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JE, Birch RA, Foster DL, Padmanabhan V. Prenatal exposure of the ovine fetus to androgens sexually differentiates the steroid feedback mechanisms that control gonadotropin releasing hormone secretion and disrupts ovarian cycles. Arch Sex Behav. 2002;31:35–41. doi: 10.1023/a:1014075016956. [DOI] [PubMed] [Google Scholar]
- 19.Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol Reprod. 2005;72:619–627. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- 20.Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- 21.Steiner RA, Clifton DK, Spies HG, Resko JA. Sexual differentiation and feedback control of luteinizing hormone secretion in the rhesus monkey. Biol Reprod. 1976;15:206–212. doi: 10.1095/biolreprod15.2.206. [DOI] [PubMed] [Google Scholar]
- 22.Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–172. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- 23.Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol. 2006;246:165–174. doi: 10.1016/j.mce.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Manikkam M, Steckler T, Padmanabhan V. Developmental programming: prenatal testosterone excess increases ovarian androgen receptors in fetal sheep. 40th Annual Meeting of the Society for the Study of Reproduction; July 22–25 2007; San Antonio, Tx. [Google Scholar]
- 26.West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol. 2001;185:51–59. doi: 10.1016/s0303-7207(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 27.Abbott DH, Colman RJ, Kemnitz JW, Eisner JR, Dumesic DA. Prenatal androgen excess programs for polycystic ovarian syndrome in female rhesus monkeys. In: Chang J, Heindel JJ, Dunaif A, editors. Polycystic Ovary Syndrome. New York: Marcel Dekker, Inc, New York; 2002A. pp. 119–133. [Google Scholar]
- 28.Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 29.Nelson VL, Qin K, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF, III, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 30.Carmina E, Chu MC, Longo RA, Rini GB, Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. 2005;90:2545–2549. doi: 10.1210/jc.2004-2279. [DOI] [PubMed] [Google Scholar]
- 31.Padmanabhan V, Veiga-Lopez A, Abbott DH, Dumesic DA. Developmental programming of ovarian disruption. In: Gonzalez-Bulnes A, editor. Novel Concepts in Ovarian Endocrinology. India: Research Signpost; 2007. in press. [Google Scholar]
- 32.Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–67. doi: 10.1016/s1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 33.Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects. Partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101:7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foecking EM, Szabo M, Schwartz NB, Levine JF. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72:1475–1483. doi: 10.1095/biolreprod.105.039800. [DOI] [PubMed] [Google Scholar]
- 36.Abbott DH, Dumesic DA, Eisner JR, Kemnitz JW, Goy RW. The prenatally androgenized female rhesus monkey as a model for PCOS. In: Azziz R, Nestler JE, Dewailly D, editors. Androgen Excess Disorders in Women. Philadelphia: Lippincott-Raven; 1997. pp. 369–382. [Google Scholar]
- 37.Resko JA, Ellinwood WE. Sexual differentiation of the brain of primates. In: Serio M, Motta M, Zanisi M, Martini L, editors. Sexual Differentiation: Basic and Clinical Aspects. New York: Raven Press; 1984. pp. 169–181. [Google Scholar]
- 38.Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biol Reprod. 1987;37:1185–1191. doi: 10.1095/biolreprod37.5.1185. [DOI] [PubMed] [Google Scholar]
- 39.Abbott DH, Bruns CM, Barnett DK, Dumesic DA. Fetal programming of polycystic ovary syndrome. In: Kovacs G, Norman R, editors. Polycystic Ovary Syndrome. 2nd Edition. Cambridge: Cambridge University Press; 2007. pp. 262–287. [Google Scholar]
- 40.Foster DL, Jackson LM, Padmanabhan V. Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinology. 2006;254–255:109–119. doi: 10.1016/j.mce.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis.”. Obstet Gynecol Survey. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson G. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5321–5327. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- 43.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 44.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicle growth in the primate ovarian. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–2485. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- 46.Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- 47.Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod. 1999A;14:2328–2332. doi: 10.1093/humrep/14.9.2328. [DOI] [PubMed] [Google Scholar]
- 48.Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999B;61:353–357. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- 49.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- 50.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypothesis. Endo Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 51.Jakimiuk AJ, Weitsman SR, Brzechffa PR, Magoffin DA. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod. 1998;4:1–8. doi: 10.1093/molehr/4.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Padmanabhan V, Christman GM, Randolph JF, Kelch RP, Marshall JC, Beitins IZ. Dynamics of bioactive FSH secretion in women with polycystic ovarian syndrome (PCOS): effects of estradiol and progesterone. Fertility and Sterility. 2001;75:881–888. doi: 10.1016/s0015-0282(01)01694-6. [DOI] [PubMed] [Google Scholar]
- 53.Erickson GF, Magoffin DA, Garzo VG, Cheung AP, Chang RJ. Granulosa cells of polycystic ovaries: are they normal or abnormal? Hum Reprod. 1992;7:293–299. doi: 10.1093/oxfordjournals.humrep.a137638. [DOI] [PubMed] [Google Scholar]
- 54.Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab. 1994;79:1355–1360. doi: 10.1210/jcem.79.5.7962330. [DOI] [PubMed] [Google Scholar]
- 55.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 56.Jakimiuk AJ, Weitsman SR, Magoffin DA. 5α-Reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:2414–2418. doi: 10.1210/jcem.84.7.5863. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal SK, Judd HL, Magoffin DA. A mechanism for the suppression of estrogen production in polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:3686–3691. doi: 10.1210/jcem.81.10.8855823. [DOI] [PubMed] [Google Scholar]
- 58.Dumesic DA, Schramm RD, Bird IM, Peterson E, Paprocki AM, Zhou R, Abbott DH. Reduced intrafollicular androstenedione and estradiol levels in early-treated prenatally androgenized female rhesus monkeys receiving FSH therapy for in vitro fertilization. Biol Reprod. 2003;69 doi: 10.1095/biolreprod.102.015164. 1213–121. [DOI] [PubMed] [Google Scholar]
- 59.Zeleznik AJ, Little-Ihrig L, Ramasawamy S. Administration of dihydrotestosterone to rhesus monkeys inhibits gonadotropin-stimulated ovarian steroidogenesis. J Clin Endocrinol Metab. 2004;89:860–866. doi: 10.1210/jc.2003-031292. [DOI] [PubMed] [Google Scholar]
- 60.Pradeep PK, Li X, Peegel H, Menon KMJ. Dihydrotestosterone inhibits granulosa cell proliferation by decreasing the cyclin D2 mRNA expression and cell cycle arrest at G1 phase. Endocrinology. 2002;143:2930–2935. doi: 10.1210/endo.143.8.8961. [DOI] [PubMed] [Google Scholar]
- 61.Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999;28:361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 62.Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87:2128–2133. doi: 10.1210/jcem.87.5.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502–1505. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 64.Willis D, Franks S. Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-1 insulin-like growth factor receptor. J Clin Endocrinol Metab. 1995;80:3788–3790. doi: 10.1210/jcem.80.12.8530637. [DOI] [PubMed] [Google Scholar]
- 65.Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81:302–309. doi: 10.1210/jcem.81.1.8550768. [DOI] [PubMed] [Google Scholar]
- 66.Willis D, Watson H, Mason H, Galea R, Brincat M, Franks S. Premature response to LH of granulosa cells from anovulatory women with polycystic ovaries: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 67.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overproduced in thecal and granulosa cells from polycystic ovaries. J Clin Metab Endocrinol. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 68.Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163:49–52. doi: 10.1016/s0303-7207(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 69.Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- 70.Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- 71.Norman RJ, Kidson WJ, Cuneo RC, Zacharin MR. Metformin and intervention in polycystic ovary syndrome. MJA. 2001;174:580–583. doi: 10.5694/j.1326-5377.2001.tb143439.x. [DOI] [PubMed] [Google Scholar]
- 72.Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:1–6. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DA. Pioglitazone improves insulin action and normalizes menstrual cycles in a nonhuman primate model of polycystic ovary syndrome. Reprod Toxicol. 2007;23:438–448. doi: 10.1016/j.reprotox.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadatsuki M, Tsutsumi O, Yamada R, Muramatsu M, Taketani Y. Local regulatory effects of activin A and follistatin on meiotic maturation of rat oocytes. Biochem Biophys Res Commun. 1993;196:388–395. doi: 10.1006/bbrc.1993.2261. [DOI] [PubMed] [Google Scholar]
- 75.Knight PG, Glister C. Potential local regulatory functions of inhibins, activins and follistatin in the ovary. Reproduction. 2001;121:503–512. doi: 10.1530/rep.0.1210503. [DOI] [PubMed] [Google Scholar]
- 76.Schneyer AL, Fujiwara T, Fox J, Welt CK, Adams J, Messerlian GM, Taylor AE. Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: relationship to circulating hormone levels. J Clin Endocrinol Metab. 2000;85:3319–3330. doi: 10.1210/jcem.85.9.6767. [DOI] [PubMed] [Google Scholar]
- 77.Lambert-Messerlian G, Taylor A, Leykin L, Isaacson K, Toth T, Chang Y, Schneyer A. Characterization of intrafollicular steroid hormones, inhibin, and follistatin in women with and without polycystic ovarian syndrome following gonadotropin stimulation. Biol Reprod. 1997;57:1211–1216. doi: 10.1095/biolreprod57.5.1211. [DOI] [PubMed] [Google Scholar]
- 78.Magoffin DA, Jakimiuk AJ, Inhibin A. inhibin B and activin concentrations in follicular fluid from women with polycystic ovary syndrome. Hum Reprod. 1998;13:2693–2698. doi: 10.1093/humrep/13.10.2693. [DOI] [PubMed] [Google Scholar]
- 79.Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab. 2005;90:5582–5587. doi: 10.1210/jc.2005-0695. [DOI] [PubMed] [Google Scholar]
- 80.Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentrations are higher and activin levels are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16:668–672. doi: 10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- 81.Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homberg R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552–2556. doi: 10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]
- 82.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148:1968–1976. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- 83.Lobo RA. The syndrome of hyperandrogenic chronic anovulation. In: Mishell DR Jr, Davajan V, Lobo RA, editors. Infertility, Contraception and Reproductive Endocrinology. Third Edition. Cambridge: Blackwell Scientific Publications; 1991. pp. 447–487. [Google Scholar]
- 84.Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF., Jr Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165–172. doi: 10.1210/jcem-66-1-165. [DOI] [PubMed] [Google Scholar]
- 85.Rosenfield RL, Barnes RB, Cara JF, Lucky AW. Dysregulation of cytochrome P450c17a as the cause of polycystic ovarian syndrome. Fertil Steril. 1990;53:785–791. [PubMed] [Google Scholar]
- 86.Marshall J, Eagleson C, McCartney C. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:295–324. doi: 10.1016/s0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]
- 87.Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenic adolescent girls. J Clin Endocrinol Metab. 2005;90:2810–2815. doi: 10.1210/jc.2004-2359. [DOI] [PubMed] [Google Scholar]
- 88.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 89.Wood RI, Mehta V, Herbosa CG, Foster DL. Prenatal testosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in the developing sheep. Neuroendocrinology. 1995;62:238–247. doi: 10.1159/000127010. [DOI] [PubMed] [Google Scholar]
- 90.Foster DL, Padmanabhan V, Wood RI, Robinson JE. Sexual differentiation of the neuroendocrine control of gonadotrophin secretion: concepts derived from sheep models. Reprod Suppl. 2002;59:83–99. [PubMed] [Google Scholar]
- 91.Buhl AE, Norman RL, Resko JA. Sex differences in estrogen-induced gonadotropin release in hamsters. Biol Reprod. 1978;18:592–597. doi: 10.1095/biolreprod18.4.592. [DOI] [PubMed] [Google Scholar]
- 92.Levine JE, Terasawa E, Hoffman SM, Dobbert MJW, Foecking EM, Abbott DH. Luteinizing hormone (LH) hypersecretion and diminished LH responses to RU486 in a nonhuman primate model for polycystic ovary syndrome (PCOS) Abstract P1-85. 87th Annual Meeting of the Endocrine Society. 2005:4–7. San Diego, CA, June. [Google Scholar]
- 93.Flak J, Herkimer C, Han D, Padmanabhan V. Fetal programming: Prenatal testosterone treatment, by its androgenic action, programs adult hypergonadotropism partly by increasing pituitary sensitivity to GnRH. Abstract. 2005;143:113. Biol Rep Special Issue. [Google Scholar]
- 94.Esinler I, Bayar U, Bozdag G, Yarali H. Outcome of intracytoplasmic sperm injection in patients with polycystic ovary syndrome or isolated polycystic ovaries. Fertil Steril. 2005;84:932–937. doi: 10.1016/j.fertnstert.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 95.Heijnen EMEW, Eijkemans MJC, Hughes EG, Laven JSE, Macklon NS, Fauser BCJM. A meta-analysis of outcomes of conventional IVF in women with polycstic ovary syndrome. Human Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 96.Lu XE, Yang XF, Li MG, Zhou FZ, Zhu YM, Huang HF. Outcome of in vitro fertilization-embryo transfer in treatment of polycystic ovarian syndrome. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;35:319–322. doi: 10.3785/j.issn.1008-9292.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 97.Ludwig M, Finas DF, Al-Hasani S, Diedrich K, Ortmann O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14:354–358. doi: 10.1093/humrep/14.2.354. [DOI] [PubMed] [Google Scholar]
- 98.Hwang JL, Seow KM, Lin YH, Hsieh BC, Huang LW, Chen HJ, Huang SC, Chen CY, Chen PH, Tzeng CR. IVF versus ICSI in sibling oocytes from patients with polycystic ovarian syndrome: a randomized controlled trial. Hum Reprod. 2005;20:1261–1265. doi: 10.1093/humrep/deh786. [DOI] [PubMed] [Google Scholar]
- 99.Sengoku K, Tamate K, Takuma N, Yoshida T, Goishi K, Ishikawa M. The chromosomal normality of unfertilized oocytes from patients with polycystic ovarian syndrome. Hum Reprod. 1997;12:474–477. doi: 10.1093/humrep/12.3.474. [DOI] [PubMed] [Google Scholar]
- 100.Kodama H, Fukuda J, Karube H, Matsui T, Shimizu Y, Tanaka T. High incidence of embryo transfer cancellations in patients with polycystic ovary syndrome. Hum Reprod. 1995;10:1962–1967. doi: 10.1093/oxfordjournals.humrep.a136217. [DOI] [PubMed] [Google Scholar]
- 101.Cano F, Garcia-Velasco JA, Millet A, Remohi J, Simon C, Pellicer A. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet. 1997;14:254–260. doi: 10.1007/BF02765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome (PCOS) patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–2333. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- 103.Wood JR, Dumesic DA, Abbott DH, Strauss JF. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 104.Tesarik J, Mendoza C. Nongenomic effects of 17β -estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 105.Zheng P, Wei S, Bavister BD, Yang J, Ding C, Ji W. 17β-estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod. 2003;18:2137–2144. doi: 10.1093/humrep/deg410. [DOI] [PubMed] [Google Scholar]
- 106.Kreiner D, Liu HC, Itskovitz J, Veeck L, Rosenwaks Z. Follicular fluid estradiol and progesterone are markers of preovulatory oocyte quality. Fertil Steril. 1987;48:991–994. [PubMed] [Google Scholar]
- 107.Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- 108.Foong SC, Abbott DH, Lesnick TG, Session DR, Walker DL, Dumesic DA. Diminished intrafollicular estradiol (E2) levels in women with reduced ovarian responsiveness to recombinant human follicle stimulating hormone (FSH) therapy for in vitro fertilization (IVF) Fertil Steril. 2005;83:1377–1383. doi: 10.1016/j.fertnstert.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 109.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotropin’ model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 110.Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum Reprod Update. 1997;3:95–100. doi: 10.1093/humupd/3.2.95. [DOI] [PubMed] [Google Scholar]
- 111.Yding Andersen C. Characteristics of human follicular fluid associated with successful conception after in vitro fertilization. J Clin Endocrinol Metab. 1993;77:1227–1234. doi: 10.1210/jcem.77.5.7521343. [DOI] [PubMed] [Google Scholar]
- 112.Steckler TL, Robertts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007;67:459–467. doi: 10.1016/j.theriogenology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Wild RA. Hyperandrogenism: Implications for cardiovascular, endometrial, and breast disease. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery, and Technology’. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 1617–1634. [Google Scholar]
- 114.Dunaif A. Insulin resistance and the polycystic ovarian syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 115.Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80:2586–2593. doi: 10.1210/jcem.80.9.7673399. [DOI] [PubMed] [Google Scholar]
- 116.Waterworth DM, Bennett ST, Gharani N, McCarthy M, Hague S, Batty S, Conway GS, White DW, Todd JA, Franks S, Williamson R. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet. 1997;349:986–990. doi: 10.1016/S0140-6736(96)08368-7. [DOI] [PubMed] [Google Scholar]
- 117.Urbanek M, Legro RS, Driscoll D, Strauss JF, Dunaif A, Spielman RS. Searching for the polycystic ovary syndrome genes. J Pediatr Endocrinol. 2000;13 Suppl.5:1311–1313. [PubMed] [Google Scholar]
- 118.Tucci S, Futterweit W, Concepcion ES, Greenberg DA, Villanueva R, Davies TF, Tomer Y. Evidence for association of polycystic ovary syndrome in Caucasian women with a marker at the insulin receptor gene locus. J Clin Endocrinol Metab. 2001;86:446–449. doi: 10.1210/jcem.86.1.7274. [DOI] [PubMed] [Google Scholar]
- 119.Diamanti-Kandarakis E, Mitrakou A, Hennes MMI, Platanissiotis D, Kaklas N, Spina J, Georgiadou E, Hoffmann RG, Kissebah AH, Raptis S. Insulin sensitivity and antiandrogen therapy in women with polycystic ovary syndrome. Metabolism. 1995;44:525–531. doi: 10.1016/0026-0495(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 120.Holte J, Bergh T, Berne CH, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;78:1052–1058. doi: 10.1210/jcem.78.5.8175959. [DOI] [PubMed] [Google Scholar]
- 121.Vrbikova J, Cibula D, Dvorakova K, Stanicka S, Sindelka G, Hill M, Fanta M, Vondra K, Skrha J. Insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:2942–2945. doi: 10.1210/jc.2003-031378. [DOI] [PubMed] [Google Scholar]
- 122.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 123.Kemnitz JW, Goy RW, Flitsch TJ, Lohmiller JJ, Robinson JA. Obesity in male and female rhesus monkeys: fat distribution, glucoregulation, and serum androgen levels. J Clin Endocrinol Metab. 1989;69:287–293. doi: 10.1210/jcem-69-2-287. [DOI] [PubMed] [Google Scholar]
- 124.Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- 125.Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003;11:279–286. doi: 10.1038/oby.2003.42. [DOI] [PubMed] [Google Scholar]
- 126.Bruns CM, Baum ST, Colman RJ, Dumesic DA, Eisner JR, Jensen MD, Whigham LD, Abbott DH. Prenatal androgen excess negatively impacts body fat distribution in a nonhuman primate model of polycystic ovary syndrome (PCOS) Int J of Obesity. in press doi: 10.1038/sj.ijo.0803638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abbott DH, Eisner JR, Goodfriend T, Medley RD, Peterson EJ, Colman RJ, Kemnitz JW, Dumesic DA. Leptin and total free fatty acids are elevated in the circulation of prenatally androgenized female rhesus monkeys. 84rd Annual Meeting of The Endocrine Society; June 19–22, 2002B; San Francisco, CA. pp. 19–22. Abstract P2-329. [Google Scholar]
- 128.King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am J Physiol Endocrinol Metab. 2007 Feb 27; doi: 10.1152/ajpendo.00668.2006. [Epub ahead of print] PMID: 17327368. [DOI] [PubMed] [Google Scholar]
- 129.Brettenthaler N, De Geyter C, Huber PR, Keller U. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:3835–3840. doi: 10.1210/jc.2003-031737. [DOI] [PubMed] [Google Scholar]
- 130.Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril. 2004;82:893–902. doi: 10.1016/j.fertnstert.2004.02.127. [DOI] [PubMed] [Google Scholar]
- 131.Lee JS, Aizenberg E, Djoumbi D, Foster DL, Padmanabhan V. Fetal programming of the postnatal responsiveness of LH to estradiol negative feedback in sheep: time and duration of exposure and quality of prenatal steroid. Abstract. 2005;660(Biol Rep Special Issue):227. [Google Scholar]
- 132.Barker DJP. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 133.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 134.De Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 135.Ibanez L, Potau N, Zampolli M, Prat N, Virdis R, Vicens-Calvet E, Carrascosa A. Hyperinsulinemia in postpubertal girls with a history of premature pubarche and functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1996;81:1237–1243. doi: 10.1210/jcem.81.3.8772605. [DOI] [PubMed] [Google Scholar]
- 136.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburu B, Gazitua R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- 137.Laitinen J, Taponen S, Martikainen H, Pouta A, Millwood I, Hartikainen AL, Ruokonen A, Sovio U, McCarthy MI, Franks S, Jarvelin MR. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 2003;27:710–715. doi: 10.1038/sj.ijo.0802301. [DOI] [PubMed] [Google Scholar]
- 138.Sadrzadeh S, Klip WA, Broekmans FJ, Schats R, Willemsen WN, Burger CW, Van Leeuwen FE, Lambalk CB. OMEGA Project group: Birth weight and age at menarche in patients with polycystic ovary syndrome or diminished ovarian reserve, in a retrospective cohort. Hum Reprod. 2003;18:2225–2230. doi: 10.1093/humrep/deg409. [DOI] [PubMed] [Google Scholar]
- 139.McGivern RF. Low birthweight in rats induced by prenatal exposure to testosterone combined with alcohol, pair-feeding, or stress. Teratology. 1989;40:335–338. doi: 10.1002/tera.1420400405. [DOI] [PubMed] [Google Scholar]
- 140.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 141.Tanguy G, Thoumsin HJ, Zorn JR, Cedard L. DHEA-S-loading test in cases of intrauterine growth retardation: relationship between the pattern of the maternal plasma metabolites and the fetoplacental dysfunction. Gynecol Obstet Invest. 1981;12:305–316. doi: 10.1159/000299660. [DOI] [PubMed] [Google Scholar]
- 142.Thoumsin HJ, Alsat E, Cedard L. In vitro aromatization of androgens into estrogens in placental insufficiency. Gynecol Obstet Invest. 1982;13:37–43. doi: 10.1159/000299482. [DOI] [PubMed] [Google Scholar]
- 143.Astapova O, Steckler TS, Lee TM, Jackson LM, Padmanabhan V. Fetal programming: Advanced placentome differentiation in testosterone-treated sheep is facilitated by androgenic actions of testosterone. Abstract. 2005;579(Biol Rep Special Issue):209. [Google Scholar]
- 144.Crespi EJ, Steckler T, Mohankumar PS, Padmanabhan V. Prenatal testosterone excess and IGF bioavailability during intrauterine growth retardation and postnatal catch-up growth in sheep. J Physiology. 2006;572:119–130. doi: 10.1113/jphysiol.2005.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Slob AK, den Hamer R, Woutersen PJ, van der Werff ten Bosch JJ. Prenatal testosterone propionate and postnatal ovarian activity in the rat. Acta Endocrinol (Copenh) 1983;103:420–427. doi: 10.1530/acta.0.1030420. [DOI] [PubMed] [Google Scholar]
- 146.Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal exposure: anatomical and endocrine effects on junvenile male and female rhesus monkeys. Horm Behav. 2000;38:52–66. doi: 10.1006/hbeh.2000.1608. [DOI] [PubMed] [Google Scholar]
- 147.Desai M, Hales CN. Role of fetal and infant growth in programming metabolism in later life. Biol Rev. 1997;72:329–348. doi: 10.1017/s0006323196005026. [DOI] [PubMed] [Google Scholar]
- 148.Wolf CJ, LeBlanc GA, Gray LE., Jr Interactive effects of vinclozolin and testosterone propionate on pregnancy and sexual differentiation of the male and female SD rat. Toxicol Sci. 2004;78:135–143. doi: 10.1093/toxsci/kfh018. [DOI] [PubMed] [Google Scholar]
- 149.Moran C, Azziz R. The role of the adrenal cortex in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:63–75. doi: 10.1016/s0889-8545(05)70185-6. [DOI] [PubMed] [Google Scholar]
- 150.Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol. 1992;167:1807–1812. doi: 10.1016/0002-9378(92)91779-a. [DOI] [PubMed] [Google Scholar]
- 151.Beck Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73:525–532. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- 152.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 153.Fox R. Prevalence of a positive family history of type 2 diabetes in women with polycystic ovarian disease. Gynecol Endocrinol. 1999;13:390–393. doi: 10.3109/09513599909167585. [DOI] [PubMed] [Google Scholar]
- 154.Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Perez-Bravo F. Prevalence of Type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–964. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- 155.Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab. 2004;89:6218–6223. doi: 10.1210/jc.2004-0918. [DOI] [PubMed] [Google Scholar]
- 156.Shifren JL, Osathanondh R, Yeh J. Human fetal ovaries and uteri: developmental expression of genes encoding the insulin, insulin-like growth factor I, and insulin-like growth factor II receptors. Fertil Steril. 1993;59:1036–1040. doi: 10.1016/s0015-0282(16)55924-x. [DOI] [PubMed] [Google Scholar]
- 157.Cole B, Hensinger K, Maciel GAR, Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab. 2006;91:3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- 158.Driscoll SG, Benirschke K, Curtis GW. Neonatal deaths among infants of diabetic mothers. Postmortem findings in ninety-five infants. Am J Dis Child. 1960;100:818–835. doi: 10.1001/archpedi.1960.04020040820004. [DOI] [PubMed] [Google Scholar]
- 159.Hultquist GT, Olding LB. Endocrine pathology of infants of diabetic mothers. A quantitative morphological analysis including a comparison with infants of iso-immunized and of non-diabetic mothers. Acta Endocrinol (Copenh) 1981;241 Suppl:1–202. [PubMed] [Google Scholar]
- 160.Barbieri RL, Saltzman DH, Torday JS, Randall RW, Frigoletto FD, Ryan KJ. Elevated concentrations of the β-subunit of human chorionic gonadotropin and testosterone in the amniotic fluid of gestations of diabetic mothers. Am J Obstet Gynecol. 1986;154:1039–1043. doi: 10.1016/0002-9378(86)90746-5. [DOI] [PubMed] [Google Scholar]
- 161.Senekjian EK, Potkul RK, Frey K, Herbst AL. Infertility among daughters either exposed or not exposed to diethylstilbestrol. Am J Obstet Gynecol. 1988;158:493–498. doi: 10.1016/0002-9378(88)90012-9. [DOI] [PubMed] [Google Scholar]
- 162.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 163.Tchernitchin AN, Tchernitchin N. Imprinting of paths of heterodifferentiation by prenatal or neonatal exposure to hormone, pharmaceuticals, pollutants and other agents or conditions. Med Sci Res. 1992;20:391–397. [Google Scholar]
- 164.Tchernitchin AN, Tchernitchin NN, Mena MA, Unda C, Soto J. Imprinting: perinatal exposures cause the development of diseases during the adult age. Acta Biol Hung. 1999;50:425–440. [PubMed] [Google Scholar]
- 165.Newbold RR, Bullock BC, McLachlan JA. Exposure to diethylstilbestrol during pregnancy permanently alters the ovary and oviduct. Biol Reprod. 1983;28:735–744. doi: 10.1095/biolreprod28.3.735. [DOI] [PubMed] [Google Scholar]
- 166.Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental Programming: Follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. in press doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- 167.Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- 168.Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Developmental regulation of baboon fetal ovarian maturation by estrogen. Biol Reprod. 2002;67:1148–1156. doi: 10.1095/biolreprod67.4.1148. [DOI] [PubMed] [Google Scholar]
- 169.Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Regulation of oocyte microvilli development in the baboon fetal ovary by estrogen. Endocrinology. 2004;145:959–966. doi: 10.1210/en.2003-1078. [DOI] [PubMed] [Google Scholar]
- 170.Cecconi S, Ciccarelli C, Barberi M, Macchiarelli G, Canipari R. Granulosa cell-oocyte interactions. Eur J Obstet Gynecol Reprod Biol. 2004;115 Suppl 1:S19–S22. doi: 10.1016/j.ejogrb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 171.Hautanen A, Raikkonen K, Adlercreutz H. Associations between pituitary-adrenocortical function and abdominal obesity, hyperinsulinaemia and dyslipidaemia in normotensive males. J Intern Med. 1997;241:451–461. doi: 10.1111/j.1365-2796.1997.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 172.Rees WD, Wilson FA, Maloney CA. Sulfur amino acid metabolism in pregnancy: the impact of methionine in the maternal diet. J Nutr. 2006;136 Suppl 6:1701S–1705S. doi: 10.1093/jn/136.6.1701S. [DOI] [PubMed] [Google Scholar]
- 173.Kwong WY, Miller DJ, Wilkins AP, Dear MS, Wright JN, Osmond C, Zhang J, Fleming TP. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol Reprod Dev. 2007;74:48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]
- 174.Park BH, Kim YJ, Park JS, Lee HY, Ha EH, Min JW, Park HS. Folate and homocysteine levels during pregnancy affect DNA methylation in human placenta. J Prev Med Pub Health. 2005;38:437–442. [PubMed] [Google Scholar]