Abstract
Activation of medium spiny neurons (MSN) of the nucleus accumbens is critical for goal-directed behaviors including cocaine seeking. Studies in cocaine-experienced rodents have revealed three major categories of neuroadaptations that influence the ability of glutamate inputs to activate MSN: changes in synaptic AMPA receptor levels, changes in extracellular non-synaptic glutamate levels, and changes in MSN intrinsic membrane excitability. Most studies have focused on one of these adaptations. Here, we consider the possibility that they are causally related and speculate about how time-dependent changes in their interactions may regulate MSN output during early and late withdrawal from repeated cocaine exposure.
Introduction
The nucleus accumbens (NAc) is a critical brain region for goal-directed behaviors, including behaviors related to drugs of abuse. Medium spiny neurons (MSN), the principal cell type of the NAc, receive glutamate inputs from limbic and cortical regions. These inputs transmit information related to emotional salience (amygdala), context (hippocampus) and executive/motor planning (prefrontal cortex, PFC). The MSN integrate this information and then, through their projections, influence motor regions that execute goal-directed behaviors [1,2]. Accumulating evidence indicates that cocaine-seeking in many animal models of addiction ultimately requires activation of NAc MSN via AMPA-type glutamate receptors (AMPAR) [3,4]. This has triggered tremendous interest in identifying mechanisms that regulate MSN excitability and thereby set the gain on addiction-related behavioral output.
Three categories of cocaine-induced neuroadaptations have been identified that are most directly involved in regulating the ability of glutamate inputs to drive NAc MSN (Figure 1): 1) changes in AMPAR levels, 2) impaired cystine-glutamate exchange, leading to decreased extracellular non-synaptic glutamate levels, and 3) changes in intrinsic membrane excitability of MSN due to alterations in voltage-sensitive conductances. While each adaptation has been well studied, their interactions remain a Bermuda triangle. Except for a few reports [5–7], most studies have stayed in one of the three corners. Here, we will briefly review each of these neuroadaptations and then consider how they may interact to determine the functional output of NAc neurons.
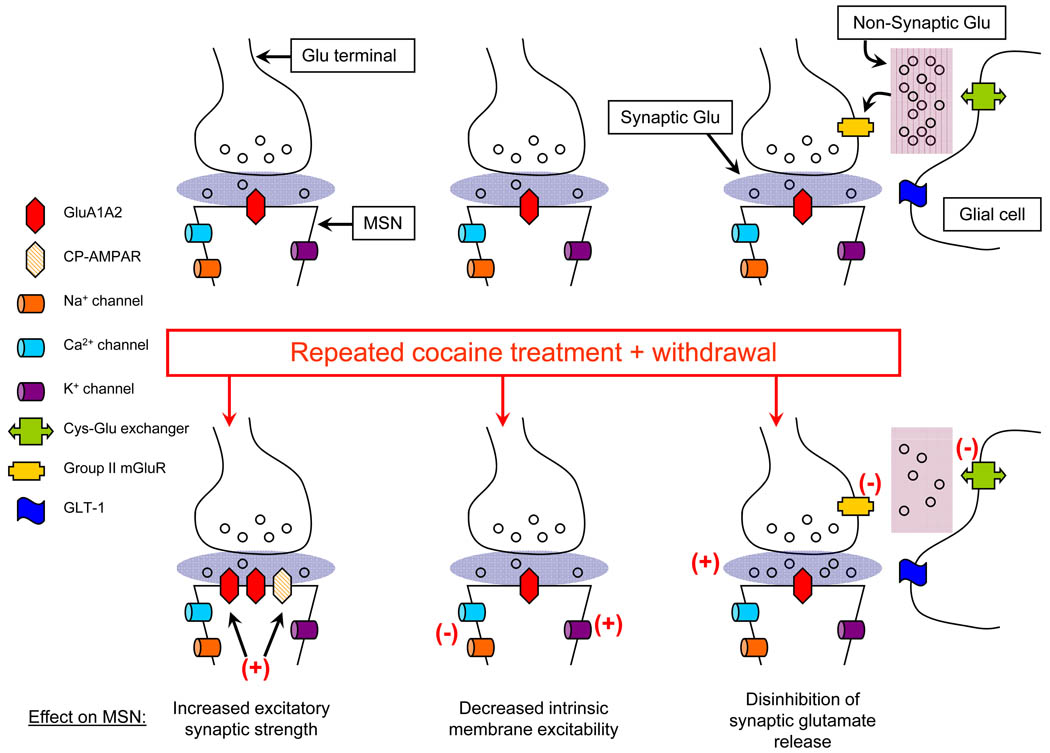
Figure 1. Three major cocaine-induced neuroadaptations that influence the functional output of medium spiny neurons (MSN) of the nucleus accumbens (NAc).
Left: AMPA receptor (AMPAR) surface expression (GluA1A2), AMPA/NMDA ratios, and GluA1 and GluA2 levels in synaptic membrane fractions are increased in the NAc of rodents sensitized with non-contingent cocaine injections [9–15]. This occurs during the first week of withdrawal and persists for many weeks. In contrast, Ca2+-permeable AMPARs (CP-AMPARs), which lack the GluA2 subunit [19–21], are added to NAc synapses in association with the incubation of cocaine craving after extended-access cocaine self-administration [18,22,24]. Due to the higher conductance of CP-AMPARs and their ability to couple to Ca2+-dependent signaling pathways, they may enhance MSN output more than upregulation of GluA1A2 receptors. Center: The intrinsic membrane excitability of MSN is decreased after withdrawal from a sensitizing regimen of cocaine [5,7,45–51]. This effect may differ between the core and shell subregions of the NAc [51; Box 1], and its duration is determined by whether cocaine exposure is contingent or non-contingent [7]. Decreased intrinsic excitability is due to decreased Na+ and Ca2+ conductances, and increased K+ conductances [5,45–48,51], secondary to homeostatic synapse-driven membrane plasticity (hSMP) [5] and alterations in protein kinase and phosphatase cascades [45,46,48,49]. Right: Repeated cocaine exposure decreases extracellular non-synaptic glutamate levels in the NAc, altering mechanisms that regulate glutamate synaptic transmission [31]. Synaptically released and extracellular non-synaptic glutamate pools are segregated (e.g., by glutamate transporters that limit diffusion of synaptically released glutamate). Microdialysis samples the extracellular non-synaptic pool (“extracellular glutamate”). This pool is derived mainly (~60%) from the cystine-glutamate (Cys-Glu) exchanger, which operates constitutively to exchange extracellular cystine for intracellular glutamate [36]. It provides glutamate tone on extrasynaptic group II metabotropic glutamate receptors (mGluR) that exert inhibitory control over glutamate neurotransmission [36–38]. Activity of the cystine-glutamate exchanger decreases after cocaine exposure and withdrawal, which decreases extracellular non-synaptic glutamate levels [32–35]. This decreases glutamate tone on group II mGluRs, removing the “brake” on synaptic glutamate release [38]. Other cocaine-induced neuroadaptations, including decreased G protein coupling, also contribute to decreased group II mGluR transmission [31,39]. Manipulating GLT-1, the glial glutamate transporter responsible for the majority of glutamate uptake, can also affect the glutamate transmission required for reinstatement of cocaine-seeking [35,90,91].
One complicating factor is the use of different animal models in different studies (see Glossary). We will focus on rodents exposed to cocaine (either contingent or non-contingent) and then studied after a period of drug withdrawal. Such studies are important because they parallel common human scenarios in which drug use is terminated for a period of time by hospitalization or incarceration [8].
Cocaine-Induced Neuroadaptations
Alterations in postsynaptic AMPAR levels
The effect of cocaine on AMPAR levels in the NAc has been recently reviewed [4]. Most studies have been performed in rodents treated with repeated non-contingent cocaine injections to produce behavioral sensitization. Biochemical and electrophysiological approaches indicate an increase in cell surface and synaptic AMPAR levels that occurs during the first week of withdrawal and then persists for weeks [9–15]. This occurs in both the core and shell subregions of the NAc (Box 1). At first these findings appeared to conflict with prior results demonstrating a long-term depression (LTD)-like state (suggestive of AMPAR downregulation) in the NAc of cocaine-sensitized mice [16]. However, in this earlier study, mice were re-exposed to cocaine (“cocaine challenge”) 24 h before analysis. It turns out that cocaine challenge transiently decreases AMPAR surface expression [10,11,15,17], although AMPARs recover back to upregulated levels within a week [15,17].
Do these adaptations occur in NAc core, shell or both subregions?
Core and shell subregions of the NAc can be distinguished based on their afferent inputs and projection targets, micro-circuitry, and functional role. In general, the core is more involved in executing motor responses to motivationally salient environmental contexts or stimuli, whereas the shell is more involved in unconditioned responses, although these subregions are not independent, as they are embedded within spiraling anatomical pathways that enable information to flow from shell to core to dorsal striatum [81]. However, in considering causal relationships between the cocaine-induced adaptations discussed here, it is important to take into account whether they occur in the core or shell. For example, if we hypothesize that AMPAR upregulation after cocaine withdrawal is a compensatory response to decreased intrinsic membrane excitability, it follows that both phenomenon must occur in the same subregion. Surprisingly, important gaps remain. A brief summary is given below, however, please see Ref. [4] for a more detailed review of these studies.
AMPAR upregulation after withdrawal
Most biochemical studies used mixed core/shell preparations [9,10,12,14,15], while most electrophysiological recordings were performed in the shell [11,22]. However, two studies suggest the phenomenon occurs in both subregions [13,18].
Decreased extracellular glutamate levels
All work has been conducted in the core [31].
Decreased intrinsic membrane excitability
The original studies in rat MSN did not distinguish between core and shell [45–48], but both regions were sampled in these studies (Xiu-Ti Hu, personal communication). The phenomenon was confirmed in rat studies focusing exclusively on MSN in the shell [5,7,50]. However, a recent mouse study [51] found opposite changes in the core compared to the shell. Shell MSN exhibited decreased excitability throughout withdrawal, while core MSN showed increased excitability on WD1–3, which declined to normal levels on WD10–14.
Nearly all AMPARs in the NAc of adult drug-naïve rodents contain the GluA2 subunit [10,11,18], and both biochemical and electrophysiological results indicate that AMPAR upregulation during the first three weeks of withdrawal involves GluA1A2 receptors [9–11,13,15]. Therefore, it was surprising when GluA2-lacking AMPARs (hereafter termed CP-AMPAR, for Ca2+-permeable; see Refs. [19–21]) were found in the NAc of cocaine-sensitized mice on withdrawal day (WD) 35 [22]. This result could reflect the longer period of drug withdrawal. However, mice were very young during cocaine treatment [22] and CP-AMPARs are more prevalent in young NAc neurons [23]. Long withdrawal from non-contingent cocaine treatment in older animals may not result in the same upregulation of CP-AMPARs [24].
What is the functional significance of AMPAR upregulation in the NAc of cocaine-sensitized rats? Although considerable evidence argues that enhanced AMPAR transmission is critical for the expression of locomotor sensitization [e.g., 25,26], the two can apparently be dissociated [9,15,17]. For example, locomotor sensitization can be demonstrated on WD1 whereas AMPAR upregulation has not yet occurred [9]. Once rats have been sensitized by repeated non-contingent stimulant injections, they subsequently show greater motivation in drug self-administration experiments, and there is intriguing evidence that this effect increases with the passage of time in concert with AMPAR upregulation [27]. Perhaps motivation increases because AMPAR upregulation strengthens synaptic connections between NAc MSN and the cortical and limbic glutamate inputs that trigger drug seeking [4].
After extended-access cocaine self-administration, cue-induced cocaine seeking undergoes a progressive, withdrawal-dependent intensification termed incubation [28]. Similar to sensitization, this is associated with enhanced AMPAR transmission in the NAc, but in this case it involves synaptic incorporation of CP-AMPARs [18,22]. Thus, CP-AMPAR levels are very low on WD1, when seeking is low, but contribute significantly to synaptic transmission in the NAc core on WD45, when seeking has intensified or “incubated.” Most importantly, blocking CP-AMPARs in the core inhibits the expression of incubated cue-induced cocaine seeking on WD45, indicating that incubated seeking reflects stronger activation of MSN via high conductance CP-AMPARs [18]. Synaptic incorporation of CP-AMPARs in the mouse NAc shell is similarly linked to enhanced cocaine seeking [22].
Surprisingly, it is not known whether AMPAR upregulation occurs after limited-access cocaine self-administration. However, a recent study found impaired long-term potentiation (LTP) but not LTD of in vivo field potentials in the NAc core on WD21 after limited-access cocaine self-administration ([29]; also see Ref. [6]). This could indicate that the limited-access regimen produced AMPAR upregulation and thus occluded subsequent LTP, although it could also indicate a disabling of LTP mechanisms. A prior study using in vitro whole cell recordings had reported the absence of LTD in the core on WD21 after similar cocaine exposure [30]. The different LTD results might be due to sampling of different populations of excitatory synapses in the two studies [29]. Several other studies have reported changes in the ability to elicit LTP or LTD in the NAc after repeated cocaine exposure, but their experimental design makes them difficult to integrate with the literature on AMPAR upregulation [4].
In summary, AMPARs are upregulated at NAc synapses after withdrawal from both non-contingent and contingent cocaine administration. In both cases, an increase in reactivity of MSN to glutamate afferents is therefore expected. However, increased reactivity may be more pronounced after prolonged withdrawal from extended-access cocaine self-administration due to synaptic incorporation of higher conductance CP-AMPARs.
Decreases in extracellular non-synaptic glutamate levels
Kalivas and colleagues have developed a “glutamate homeostasis” hypothesis of addiction based on studies conducted in the NAc core of cocaine-exposed rats [31]. As depicted in Figure 1, repeated contingent or non-contingent cocaine exposure reduces cystine-glutamate exchange and thus decreases the extracellular non-synaptic glutamate pool in the NAc, as measured by microdialysis [32–35]. This removes glutamate tone from extrasynaptic group II metabotropic glutamate receptors (mGluR) that normally function to limit glutamate release [36–38]. As a result of this decrease in glutamate tone, as well as other adaptations that dampen group II mGluR transmission in the NAc [31,39], a challenge injection of cocaine is able to increase NAc glutamate levels from the reduced level back to the range observed in control rats [25,32–34]. The resulting increase in NAc AMPAR transmission is hypothesized to enable the expression of behavioral sensitization and cocaine-primed reinstatement, both of which involve glutamate projections from medial PFC to NAc core [40,41]. Supporting this hypothesis, glutamate levels in the NAc of cocaine-experienced rats are normalized by acutely activating cystine-glutamate exchange with the cysteine pro-drug N-acetylcysteine. This pharmacological treatment prevents a priming injection of cocaine from increasing extracellular glutamate levels in the NAc and attenuates cocaine-primed reinstatement [32,33,42]. Understanding N-acetylcysteine’s effects on glutamate and dopamine transmission [32,42; also see 33] is important because this drug reduced reactivity to cocaine-related cues in cocaine-dependent human subjects in a double-blind, placebo-controlled clinical trial [44].
Results summarized above [32,33] indicate a tight relationship between glutamate levels and cocaine-primed reinstatement. In other cases, however, extracellular glutamate levels did not predict the magnitude of cocaine-primed reinstatement. One study found that extinction training normalized glutamate levels but did not diminish cocaine-primed reinstatement [34], while another study observed that cocaine-primed reinstatement was more robust after extended-access cocaine self-administration than limited-access, even though glutamate efflux during the reinstatement test did not differ between groups [43]. It may be possible to reconcile these results by considering the level of cocaine intake. Total cocaine intake was higher in the studies that found a dissociation between glutamate levels and the magnitude of reinstatement [34,43] compared to studies indicating a tight relationship between these measures [32,33]. Higher cocaine intake may have led to the formation of CP-AMPARs [18,22] and, once in the synapse, CP-AMPARs may persist even if glutamate levels are normalized. This may strengthen the postsynaptic response to the cocaine priming injection in a way that is less dependent on the level of presynaptic glutamate transmission. Dopamine transmission may also contribute to stronger reinstatement after extended-access cocaine self-administration [43].
Decreases in intrinsic membrane excitability
The original studies of cocaine’s effects on intrinsic membrane excitability were conducted in adult rats 3–4 days after discontinuing repeated cocaine injections. NAc MSN in sensitized rats exhibited decreased voltage-sensitive Na+ and Ca2+ currents and increased K+ currents, in association with altered protein kinase and phosphatase signaling in the NAc [45–49]. As would be predicted from these results, MSN in cocaine-sensitized rats exhibited decreased evoked action potential firing on WD3 [50], which was subsequently shown to persist from WD1 through WD21 and to involve changes in multiple K+ currents [5,51]. While it is clear that decreased intrinsic excitability occurs in the NAc shell [5,7,50,51], the core is more controversial (Box 1).
Decreased intrinsic membrane excitability in MSN of the NAc shell may be mediated in part by a novel form of homeostatic plasticity, termed homeostatic synapse-driven membrane plasticity (hSMP), in which persistent changes in the level of synaptic NMDA receptor (NMDAR) transmission lead to compensatory changes in intrinsic excitability [5]. The expression of the hSMP-induced decrease in intrinsic membrane excitability involves SK-type Ca2+-activated K+ channels. When excitatory inputs to MSN are increased, hSMP results in augmentation of the SK channel-mediated afterhyperpolarization potential. This decreases intrinsic membrane excitability, enabling MSN to maintain relatively normal functional output. hSMP could no longer be induced in MSN from cocaine-sensitized rats, either on WD2 or WD21, suggesting it is either disabled or already engaged by repeated cocaine exposure [5]. Thus, alterations in SK channels due to hSMP [5] and in other K+ channels [47,51] may contribute to decreased intrinsic excitability in both early and late cocaine withdrawal, whereas cocaine’s effects on Na+ and Ca2+ currents have only been demonstrated in early withdrawal [45–48], although they may also persist.
What is the behavioral significance of decreased intrinsic excitability? One study showed that reducing the intrinsic excitability of NAc neurons by over-expressing a K+ channel (Kir2.1) produced a phenotype resembling sensitization [50], that is, rats exhibited a stronger locomotor response to cocaine challenge. Furthermore, a comparison of rat strains with low and high levels of MSN intrinsic excitability revealed that the low excitability strain showed a greater locomotor response to cocaine and enhanced cocaine self-administration [7]. These results are striking, but it seems paradoxical that decreasing MSN excitability would enhance cocaine-related behaviors. One explanation, proposed previously (e.g. Ref. [52]), is that decreased intrinsic excitability selectively enhances the signal-to-noise ratio for cocaine-related stimuli. Thus, excitatory inputs carrying many varieties and intensities of stimuli flow into the NAc, but only those that elicit action potentials in MSN may be translated into behavioral output. As a result of the cocaine-induced decrease in intrinsic excitability, MSN may become unresponsive to modest excitatory inputs but remain responsive to stronger inputs. If it is assumed that natural rewards elicit modest excitatory input and highly salient stimuli, like cocaine, elicit strong excitatory input, then the relative behavioral sensitivity to highly salient stimulation would be enhanced in cocaine-sensitized animals. However, an alternative explanation for the results in Ref. 50 is that even short-term changes in excitability (e.g., a few days of decreased excitability during cocaine withdrawal or short-term over-expression of Kir2.1) produce a compensatory increase in synaptic AMPAR levels that is responsible for enhanced responding to cocaine and cocaine-related stimuli. Interestingly, whereas decreased intrinsic excitability persisted from WD2–21 in rats sensitized by non-contingent cocaine injections, it was present on WD2 following limited-access cocaine self-administration, but dissipated by WD21 [7]. Intrinsic excitability has not been studied after extended-access cocaine self-administration.
Time-dependent interactions between excitatory synaptic strength and intrinsic membrane excitability
Excitatory synaptic strength and intrinsic membrane excitability are major determinants of the functional output of NAc MSN. Excitatory synaptic inputs depolarize the membrane potential towards the action potential threshold, whereas intrinsic membrane excitability determines whether and how many action potentials are fired upon membrane depolarization [53]. Therefore, it is important to understand how changes in intrinsic membrane excitability and synaptic strength interact to alter NAc output during cocaine withdrawal. This section will propose a possible scenario based on the studies reviewed above, while the next section will consider the possibility that these phenomena are causally linked via synaptic scaling.
During early withdrawal (WD1–4) from either contingent or non-contingent cocaine, the intrinsic excitability of MSN is decreased [5,7,45–51; Box 1] and AMPAR levels are unchanged or decreased [9,11,13,14,18,22] (see also Refs. [4,54] regarding the interpretation of decreased AMPA/NMDA ratios). Thus, decreased intrinsic excitability is unopposed by AMPAR upregulation. However, NMDAR transmission is enhanced in early withdrawal from non-contingent cocaine due to the formation of silent synapses in NAc neurons during repeated cocaine treatment [54]. By triggering hSMP, this may help explain the decreased intrinsic excitability observed during early withdrawal [5]. At longer withdrawals from non-contingent cocaine (WD10–21), decreased intrinsic excitability persists, at least in the shell [5,7,51; Box 1]. However, AMPARs have now been upregulated at NAc synapses [9–15]. Computer modeling indicates that the AMPAR-related increase in synaptic strength will dominate, resulting in a net increase in MSN output [7]. After limited-access cocaine self-administration, decreased intrinsic excitability in the shell dissipates by WD21, removing this “brake” on MSN output [7]. Thus, if AMPAR upregulation occurs after limited-access cocaine self-administration, it will produce an even stronger enhancement of MSN output, compared to non-contingent cocaine and similar withdrawal times. After extended-access cocaine self-administration, the continuum may be extended towards even greater augmentation of MSN output, due to the addition of high conductance CP-AMPARs [18,22].
Could AMPAR upregulation during cocaine withdrawal result from synaptic scaling?
Synaptic scaling is a form of homeostatic plasticity that enables neurons to stabilize their firing rates by altering synaptic AMPAR levels to compensate for prolonged changes in activity [55,56]. Thus, synaptic AMPAR levels are increased in response to prolonged activity blockade and decreased in response to prolonged increases in activity. Bidirectional synaptic scaling in NAc MSN has recently been demonstrated [23].
How do prolonged changes in activity trigger synaptic scaling? A leading theory is that individual neurons are sensing changes in somatic Ca2+ influx and regulating synaptic strength up or down to compensate [56]. Thus, any change that affects some integrated measure of somatic Ca2+ levels could potentially elicit synaptic scaling. If this theory is correct, it is reasonable to propose that the cocaine-induced decrease in intrinsic membrane excitability may be responsible for AMPAR upregulation in the NAc. Consistent with this idea, decreased intrinsic excitability is observed earlier in withdrawal than AMPAR upregulation (see previous section).
It is also possible that synaptic scaling, leading to AMPAR upregulation, is caused by a drop in excitatory synaptic drive to NAc neurons. Supporting this idea, studies of humans, non-human primates and rats after repeated cocaine exposure have found decreased metabolic activity in cortical areas that send glutamate afferents to the NAc [57–59]. Furthermore, basal firing rates of PFC neurons are decreased by repeated sessions of cocaine self-administration [60]. There are many examples of cocaine-induced neuroadaptations in cortical and limbic regions that could contribute to decreased activity of excitatory projections originating in these regions, including changes in gene expression [61]. On the other hand, two studies observed increased frequency of either miniature or spontaneous AMPAR excitatory postsynaptic currents (EPSCs) in the NAc after cocaine withdrawal [11,18], which appears to argue against a decrease in presynaptic activity. However, increased frequency can theoretically result from an increase in EPSC amplitude, i.e., more events may exceed the detection threshold in cocaine-treated animals due to AMPAR upregulation. Indeed, neither study observed a change in the paired-pulse ratio, a measure of release probability. Thus, rather than reflecting increased presynaptic activity, the increased EPSC frequency may result from postsynaptic enhancement.
It is also possible that increased frequency reflects activity at putative new synaptic contacts that are suggested by increased dendritic spines in the NAc after repeated cocaine treatment [62–64]. Indeed, an important challenge is to understand the relationship between cocaine-induced spine plasticity and the adaptations highlighted in this review. For example, it will be fascinating to determine if AMPAR upregulation occurs at new spines.
Could decreased extracellular non-synaptic glutamate levels represent a trigger for AMPAR upregulation via synaptic scaling?
At first glance, this possibility makes sense and the time-course fits; extracellular non-synaptic glutamate levels are decreased on WD1 [34], before AMPAR upregulation is observed [9,11,13,14,18,22]. At later withdrawal times, these adaptations are often expressed in parallel. For example, both are observed after home-cage withdrawal from either non-contingent [9–15,25,32] or contingent cocaine administration [18,22,33]. Notably, nearly identical cocaine regimens lead to both reduced cystine-glutamate exchange [33], which decreases extracellular glutamate levels, and to synaptic incorporation of CP-AMPARs [18]. Other results suggest that preventing the cocaine-induced decrease in extracellular glutamate levels may interfere with mechanisms underlying incubation of cue-induced cocaine seeking [42], which is dependent on CP-AMPARs [18].
However, there is a problem with the idea that a cocaine-induced decrease in extracellular non-synaptic glutamate levels triggers synaptic scaling and thus explains AMPAR upregulation. Given the segregation between synaptic and non-synaptic glutamate pools (Figure 1), it is not necessarily the case that the cocaine-induced decrease in extracellular glutamate levels would lead to decreased synaptic glutamate transmission. In fact, the opposite would be predicted, based on evidence that decreasing extracellular glutamate levels removes glutamate tone on extrasynaptic group II mGluRs that suppress synaptic glutamate release [38]. On the other hand, the functional impact of the group II mGluR mechanism depends on whether synaptic transmission is active. This mechanism could be “silent” during cocaine withdrawal if synaptic glutamate release is already low due to potentially depressed firing of excitatory afferents to the NAc (see previous section, and Ref. [23]). However, in this case, although AMPAR scaling-up could occur during withdrawal, it would be attributable to decreased presynaptic glutamate transmission rather than decreased extracellular glutamate levels. When presynaptic glutamate transmission is activated by a priming injection of cocaine, the absence of group II mGluR tone may become functionally significant and help enable cocaine-primed reinstatement [31].
Is there an alternative way to link decreased extracellular glutamate levels to AMPAR upregulation? As in other brain regions, group I mGluR activation in the NAc leads to AMPAR internalization [65]. One possibility is that decreased group I mGluR transmission during cocaine withdrawal, due to receptor downregulation or decreased Homer signaling [66–70], permits CP-AMPARs to accumulate at NAc synapses. Indeed, tonic levels of group I mGluR transmission normally downregulate CP-AMPARs at parallel fiber-stellate cell synapses in the cerebellum [71]. Although we are proposing a mechanism that operates locally within the NAc, disrupting group I mGluR function in the ventral tegmental area (VTA) has been shown to remove a “brake” on cocaine-induced synaptic plasticity in the VTA and thus facilitate the addition of CP-AMPARs to NAc synapses in young cocaine-sensitized mice [22].
Concluding remarks
Here, we have suggested possible interactions that may occur over the course of cocaine withdrawal between adaptations in synaptic AMPAR levels, intrinsic membrane excitability, and extracellular glutamate levels. Putting NAc core and shell differences aside, these three adaptations are likely to affect the majority of MSN. Therefore in discussing their interactions, MSN were treated as a homogeneous population, and an increase in their activation was equated with greater cocaine seeking. Obviously, this is a major oversimplification. MSN participate in functional ensembles [72] that exhibit discrete and non-overlapping patterns of activity during electrophysiological recordings in awake rats [73,74]. Gating interactions among presynaptic inputs probably select the ensembles that are activated in a particular situation. For example, activation of dopamine inputs, leading to D1 dopamine receptor stimulation, sustains the depolarized upstate in which MSN can be activated by glutamate inputs [75], which may relate to a dual requirement for dopamine and glutamate transmission in certain cocaine-related behaviors (e.g., Refs. [43,76,77]). Furthermore, synaptic plasticity may affect the ability of different presynaptic inputs to “compete” for influence over MSN output [78]. Once these presynaptic variables conspire to select an ensemble, relatively global postsynaptic adaptations (e.g., AMPAR upregulation) may determine the strength of ensemble activation. For example, AMPAR upregulation could explain stronger MSN activation in association with the incubation of cocaine craving [79,80].
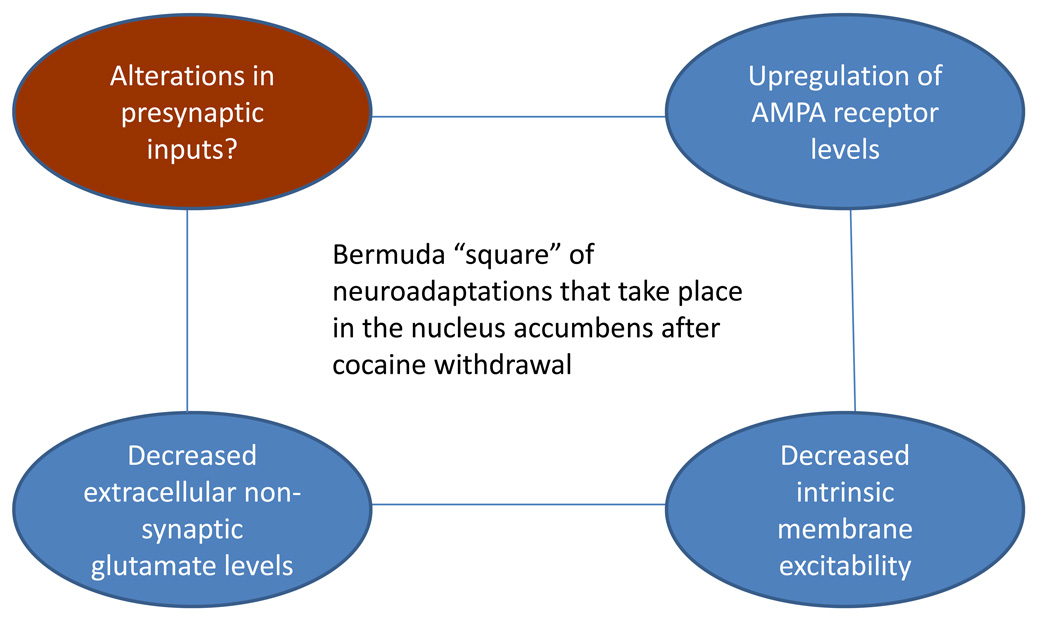
Despite a growing understanding of the neuroadaptations highlighted here that occur after cocaine withdrawal, we are still far from understanding the sequence of cellular events by which cocaine experience causes the NAc to enter a new state in which cocaine-related signals have acquired more influence. As the preceding paragraph makes clear, one of the important missing pieces of the puzzle is the effect of cocaine withdrawal on the level of presynaptic activity in particular pathways that form synaptic connections with NAc MSN. For example, information about presynaptic activity after cocaine withdrawal is necessary to evaluate hypotheses about homeostatic plasticity. Therefore, the Bermuda triangle is really a square (Figure 2) and the fourth corner may prove the most complicated to map.
Figure 2. Bermuda “square” depicting the three neuroadaptations that are highlighted in this review (blue ovals) and a relatively unexplored area (brown oval).
An important challenge is to understand how time-dependent changes in their interactions regulate the functional output of nucleus accumbens (NAc) medium spiny neurons during cocaine withdrawal, causing the NAc to enter a new state in which cocaine-related signals have acquired more influence. Lines between ovals are not meant to indicate direct causal relationships.
Acknowledgements
I thank past and present members of my laboratory who contributed to the ideas presented in this review and the National Institute on Drug Abuse for supporting our research (DA09621, DA015835, DA000453 and DA029099).
GLOSSARY
- Non-contingent vs. contingent drug exposure
The former refers to drug administration that does not depend on the animal’s behavior, such as experimenter-administered injections. Contingent drug administration depends on the animal’s response, as in drug self-administration experiments.
- Behavioral sensitization
Describes the progressive enhancement of behavioral responses to a drug that occurs during repeated exposure and then persists for weeks to months [82]. It can be induced by both non-contingent and contingent drug exposure. Sensitization of locomotion and stereotyped behaviors are commonly studied, but sensitization also occurs to the incentive-motivational properties of drugs and cues paired with drugs. For example, rats that are pretreated with psychostimulant drugs, resulting in sensitization, will acquire psychostimulant self-administration more readily and work harder to obtain drug under a progressive ratio schedule [83].
- Self-administration
Animals are trained to lever press or nose poke to receive an intravenous infusion of drug. Self-administration procedures may differ in many ways, including whether training to respond for food proceeds drug self-administration, the dose of drug available, the duration of access to drug, the number of responses required to obtain drug, whether cues are paired with drug delivery, or whether the number of attainable infusions is limited. These differences complicate the integration of findings from different labs. However, self-administration procedures can be generally divided into limited-access or extended-access procedures.
- Limited-access vs. extended-access cocaine self-administration
In limited-access procedures, drug is generally available for 1–3 h per session for a week or two. Extended-access procedures utilize longer sessions (e.g., 6 h/day) or more days. Extended-access cocaine self-administration produces different or enhanced behaviors compared to limited-access cocaine self-administration (e.g., escalation of cocaine intake, increased motivation for cocaine, and enhanced pursuit of cocaine despite adverse consequences), suggesting that the former more closely models the compulsive drug seeking and taking characteristic of addiction [84–86; but see 87].
- Extinction vs. withdrawal
After self-administration is established, animals typically undergo either extinction training or withdrawal. During extinction training, responding on the previously active lever no longer results in drug delivery. This leads to a decrease (extinction) of the behavioral response (e.g., lever pressing) that originally delivered the drug. Withdrawal usually occurs in home cages. This is sometimes referred to as forced abstinence, but we use the term withdrawal because abstinence implies voluntary restraint. Extinction is itself a form of learning and is associated with its own effects on glutamate transmission that are distinct from those produced by drug withdrawal [29,68,88,89]. It is complex to dissect apart the subsets of neuroadaptations produced by cocaine itself, the withdrawal period, and extinction training [4]. Thus, studies employing extinction vs. withdrawal cannot be “lumped together”.
- Tests for drug seeking after extinction or withdrawal
These are employed to study mechanisms of relapse. After extinction training, the renewal or reinstatement of responding is induced by one of the same stimuli that often triggers relapse in humans: a stressor, a cue previously paired with drug (cue-induced reinstatement), or non-contingent re-exposure to the drug itself (drug-primed reinstatement). After withdrawal, drug seeking behavior is usually assessed by re-exposing animals to the self-administration chamber in the presence or absence of drug-paired cues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The author has nothing to disclose.
References
- 1.Groenewegen HJ, et al. Convergence and segregation of ventral striatal inputs and outputs. Ann. N. Y. Acad. Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 2.Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann. N.Y. Acad. Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 4.Wolf ME, Ferrario CR. AMPA receptor plasticity after cocaine withdrawal. Neurosci. Biobehav. Rev. 2010 doi: 10.1016/j.neubiorev.2010.01.013. doi:10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa M, et al. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J. Neurosci. 2009;29:5820–5831. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moussawi K, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mu P, et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J. Neurosci. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr. Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudreau AC, et al. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourrich S, et al. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudreau AC, et al. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J. Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghasemzadeh MB, et al. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neurosci. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J. Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrario CR, et al. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacol. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas MJ, et al. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 17.Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J. Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cull-Candy S, et al. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr. Opin. Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Isaac JT, et al. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Wolf ME. Nucleus accumbens neurons exhibit synaptic scaling that is occluded by repeated dopamine pre-exposure. Eur. J. Neurosci. 2009;30:539–550. doi: 10.1111/j.1460-9568.2009.06852.x. [DOI] [PubMed] [Google Scholar]
- 24.McCutcheon JE, et al. Insertion of GluA2-lacking AMPA receptors after long withdrawal from cocaine self-administration but not experimenter-administered cocaine. Soc. Neurosci. Abstr. 2010;36 doi: 10.1523/JNEUROSCI.0350-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce RC, et al. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacol. (Berl) 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- 27.Suto N, et al. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacol. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- 28.Lu L, et al. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47 Suppl 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Knackstedt LA, et al. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine-seeking. J. Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin M, et al. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 31.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 32.Baker DA, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 33.Madayag A, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J. Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miguéns M, et al. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacol. 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 35.Knackstedt LA, et al. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psych. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker DA, et al. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xi Z-X, et al. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J. Pharmacol. Exp. Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 38.Moran MM, et al. Cystine-glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J. Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xi Z-X, et al. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J. Pharmacol. Exp. Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 40.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt HD, et al. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur. J. Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 42.Kau KS, et al. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neurosci. 2007;155:530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madayag A, et al. Drug-induced plasticity contributing to heightened relapse susceptibility: neurochemical changes and augmented reinstatement in high-intake rats. J. Neurosci. 2010;30:210–217. doi: 10.1523/JNEUROSCI.1342-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaRowe SD, et al. Is cocaine desire reduced by N-acetylcysteine? Am. J. Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XF, et al. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J. Neurosci. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XF, et al. Repeated cocaine treatment decreases whole-cell calcium current in rat nucleus accumbens neurons. J. Pharmacol. Exp. Ther. 2002;301:1119–1125. doi: 10.1124/jpet.301.3.1119. [DOI] [PubMed] [Google Scholar]
- 47.Hu XT, et al. Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity of K+ channels in rat nucleus accumbens neurons. J. Neurophysiol. 2004;92:1597–1607. doi: 10.1152/jn.00217.2004. [DOI] [PubMed] [Google Scholar]
- 48.Hu XT, et al. Repeated cocaine administration decreases calcineurin (PP2B) but enhances DARPP-32 modulation of sodium currents in rat nucleus accumbens neurons. Neuropsychopharmacol. 2005;30:916–926. doi: 10.1038/sj.npp.1300654. [DOI] [PubMed] [Google Scholar]
- 49.Hu XT. Cocaine withdrawal and neuro-adaptations in ion channel function. Mol. Neurobiol. 2007;35:95–112. doi: 10.1007/BF02700626. [DOI] [PubMed] [Google Scholar]
- 50.Dong Y, et al. CREB modulates excitability of nucleus accumbens neurons. Nat. Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- 51.Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J. Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu XT, Kalivas PW. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29:610–616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Hille B. Ion channels of excitable membranes. Sinauer Associates, Inc.; 2001. [Google Scholar]
- 54.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 56.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porrino LJ, et al. The effects of cocaine: a shifting target over the course of addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammer RP, Jr, Cooke ES. Gradual tolerance of metabolic activity is produced in mesolimbic regions by chronic cocaine treatment, while subsequent cocaine challenge activates extrapyramidal regions of rat brain. J. Neurosci. 1994;14:4289–4296. doi: 10.1523/JNEUROSCI.14-07-04289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J. Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winstanley CA, et al. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J. Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacol. 2004;47 Suppl. 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 63.Shen HW, et al. Altered dendritic spine plasticity in cocaine-withdrawn rats. J. Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangiavacchi S, Wolf ME. Stimulation of N-methyl-D-aspartate receptors, AMPA receptors or metabotropic glutamate receptors leads to rapid internalization of AMPA receptors in cultured nucleus accumbens neurons. Eur. J. Neurosci. 2004;20:649–657. doi: 10.1111/j.1460-9568.2004.03511.x. [DOI] [PubMed] [Google Scholar]
- 66.Swanson CJ, et al. Repeated cocaine administration attenuates group I metabotropic glutamate receptor- mediated glutamate release and behavioral activation: A potential role for Homer. J. Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Shahar O, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghasemzadeh MB, et al. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci. Lett. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hao Y, et al. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol. Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.02.011. doi:10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szumlinski KK, et al. Homers regulate drug-induced neuroplasticity: Implications for addiction. Biochem. Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly L, et al. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat. Neurosci. 2009;12:593–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- 72.Pennartz CM, et al. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 73.Peoples LL, et al. The role of accumbal hypoactivity in cocaine addiction. ScientificWorldJournal. 2007;7:22–45. doi: 10.1100/tsw.2007.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacol. 2009;56 Suppl. 1:149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Donnell P. Dopamine gating of forebrain neural ensembles. Eur. J. Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- 76.Anderson SM, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat. Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 77.Owesson-White CA, et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur. J. Neurosci. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacol. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- 80.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J. Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meredith GE, et al. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct. Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set-point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 85.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 86.Ben-Shahar O, et al. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oleson EB, Roberts DCS. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;32:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sutton MA, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 89.Ghasemzadeh MB, et al. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 90.Sari Y, et al. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J. Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pendyam S, et al. Computational model of extracellular glutamate in the nucleus accumbens incorporates neuroadaptations by chronic cocaine. Neurosci. 2009;158:1266–1276. doi: 10.1016/j.neuroscience.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]