Abstract
Rationale
Stromal cell–derived factor (SDF)-1/CXCR4 axis has an instrumental role during cardiac development and has been shown to be a potential therapeutic target for optimizing ventricular remodeling after acute myocardial infarction (AMI) and in ischemic cardiomyopathy. Although a therapeutic target, the specific role of cardiac myocyte CXCR4 (CM-CXCR4) expression following cardiogenesis and survival of cardiac myocyte and left ventricular remodeling after AMI is unknown.
Objective
We hypothesized that cardiac myocyte derived CXCR4 is critical for cardiac development, but it may have no role in adulthood secondary to the short transient expression of SDF-1 and the delayed expression of CM-CXCR4 following AMI. To address this issue, we developed congenital and conditional CM-CXCR4−/− mouse models.
Methods and Results
Two strains of CM-CXCR4flox/flox mice were generated by crossing CXCR4flox/flox mice with MCM-Cre+/− mouse and MLC2v-Cre+/− mouse on the C57BL/6J background, yielding CXCR4flox/flox MCM-Cre+/− and CXCR4flox/floxMLC2v-Cre+/− mice. Studies demonstrated recombination in both models congenitally in the MLC2v-Cre+/− mice and following tamoxifen administration in the MCM-Cre+/− mice. Surprisingly the CXCR4flox/floxMLC2v-Cre+/− are viable, had normal cardiac function, and had no evidence of ventricular septal defect. CXCR4flox/floxMCM+/− treated with tamoxifen 2 weeks before AMI demonstrated 90% decrease in cardiac CXCR4 expression 48 hours after AMI. Twenty-one days post AMI, echocardiography revealed no statistically significant difference in the wall thickness, left ventricular dimensions or ejection fraction (40.9±7.5 versus 34.4±2.6%) in CXCR4flox/flox mice versus CM-CXCR4−/− mice regardless of strategy of Cre expression. No differences in vascular density (2369±131 versus 2471±126 vessels/mm2; CXCR4flox/flox versus CM-CXCR4−/− mouse), infarct size, collagen content, or noninfarct zone cardiac myocyte size were observed 21 days after AMI.
Conclusions
We conclude that cardiac myocyte–derived CXCR4 is not essential for cardiac development and, potentially because of the mismatch in timings of peaks of SDF-1 and CXCR4, has no major role in ventricular remodeling after AMI.
Keywords: stem cells, myocardial infarction, cardiogenesis
Stem cell– and gene transfer–based strategies are being pursued in an attempt to decrease infarct size, optimize ventricular remodeling, and prevent the onset of chronic heart failure in patients following acute myocardial infarction (AMI). Although benefits have been demonstrated in several clinical trials and recent metaanalyses,1–3 the mechanism responsible for the benefits seen are under investigation. One potentially important pathway that has been demonstrated to be important by multiple laboratories is the stromal cell–derived factor (SDF)-1/CXCR4 signaling pathway.4–8 This pathway has been implicated in stem cell survival following transplantation, homing of bone marrow–derived and cardiac progenitor stem cells to the infarct zone heart, and cardiac myocyte survival during AMI and chronic heart failure. The SDF-1/CXCR4 axis has also been shown to be critical in cardiac development.9–11 Although SDF-1/CXCR4 axis is a therapeutic target of interest, its natural role in myocardial repair in adulthood is less clear because SDF-1 expression is immediate and transient and cardiac myocyte CXCR4 expression is delayed and persistent following AMI.12,13 More recently, the hypothesis has been forwarded that the basis of benefit associated with stem cell therapy following acute myocardial infarction is the restoration of the temporal alignment of SDF-1 and CXCR4.14,15
CXCR4, a G protein–coupled 7-transmembrane receptor, together with its ligand SDF-1, can play a crucial role during embryonic development and in maintaining the stem cell niche, homing of stem cells at the site of injury, and preservation of the injured tissue. During embryogenesis, CXCR4 expression starts as early as blastocyst formation and is expressed in various different stages of development and a variety of cell types.16 The importance of CXCR4 during development is evident in the CXCR4-null mouse. In these mice, the deficiency of CXCR4 is lethal as the developing embryo acquires various developmental anomalies including defective hematopoiesis (β-lymphopoiesis and myelopoiesis), neurogenesis (abnormal cortex formation), and angiogenesis and cardiogenesis (ventricular septal defect).9–11 Cardiac neural crest participate in the formation of ventricular septum during cardiogenesis and express CXCR417; however, the contribution of cardiac myocyte derived CXCR4 in ventricular formation is not yet recognized.
Following AMI, SDF-1 expression is elevated immediately and peaks within 24 hours. However, cardiac myocyte CXCR4 expression occurs between 48 and 72 hours after AMI.12 This physiological mismatch in the peak of the ligand and its receptor has led various researchers to overexpress SDF-1 in infarcted regions. The prolonged expression of SDF-1 in infarcted tissue leads to reestablishment of stem cell homing, neoangiogenesis, myocardial preservation, and increased ventricular function.18–21 Although SDF-1/CXCR4 axis has been shown to be critical in myocardial reparative process after myocardial infarction (MI), the exact role of CXCR4 derived from cardiac myocytes in ventricular remodeling is not known.
To address this question directly, we have developed a congenital (MLC-2Vcre) and conditional (MCM-cre) deletion of cardiac myocyte CXCR4 using the CXCR4flox/flox mouse. We have characterized these mice before and after AMI.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org and includes detailed information regarding immunostaining, western blotting, echocardiography, left anterior descending artery (LAD) ligation, and cardiac remodeling.
Mice Generation and In Vivo Analysis of CXCR4 Deletion
Previous studies have reported the generation of mice having CXCR4flox allele, in which exon 2 (2.2 kbp) is flanked by 2 loxP sequences.22 We crossed CXCR4flox/flox mice with the mice bearing a transgene of α-MyHC-MerCreMer (MCM) and MLC2v-Cre. The progeny was crossbred to yield MCM+/−CXCR4flox/flox and MLC2vCRE+CXCR4flox/flox mice. Tamoxifen (40 mg/kg) in corn oil was administered IP in 6-week-old MCM+CXCR4flox/flox male mouse continuously for 5 days. The genomic DNA from the hearts of 8-week-old mice from both types was isolated and subjected to PCR to estimate CXCR4 deletion. The location of the primers used to detect CXCR4 deletion is depicted in Figure 1. The primers used were as follows: forward, 5′-CACTACGCATGACTCGAAATG-3′; reverse, 5′-CCTCGGAATGAAGAGATTATG-3′. All animals were housed in an animal facility of Cleveland Clinic approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, and all animal protocols were approved by Animal Research Committee. All animals had C57Bl6/J background.
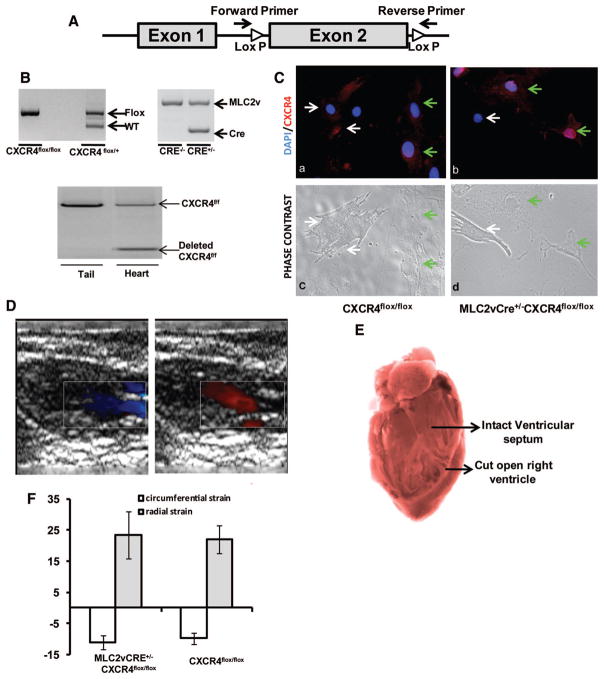
Figure 1. Characterization of MLC2vCRE+/−CXCR4flox/flox mouse.
A, Exon 2 of CXCR4 gene is flanked by loxP sites. The primers bind before and after the loxP sites to detect cleaved CXCR4. B, A PCR analysis on genomic DNA from tail and heart was performed on 8-week-old CXCR4flox/flox, MLC2v-Cre+/−CXCR4flox/flox, and MLC2v-Cre+/−CXCR4flox/+ mice. i and ii show the presence of CXCR4 floxed allele and Cre into the genome, and iii shows deletion of CXCR4 from ventricular homogenates and not from the tail of the same animal. C, CXCR4 staining (red) and phase contrast pictures of neonatal cardiac myocytes from CXCR4flox/flox and MLC2v-Cre+/−CXCR4flox/flox mice shows deletion of CXCR4 from neonatal cardiac myocytes of MLC2v-Cre+/−CXCR4flox/flox mice. White arrows point toward the cardiac myocytes, and the green arrow points toward noncardiac myocyte cells in culture. D, Representative Doppler echocardiography image showing normal outflow and inflow during systole and diastole in MLC2v-Cre+/−CXCR4flox/flox mouse without any evidence of a ventricular septal defect. E, Representative image of view through right ventricle to reveal normal IVS in MLC2v-Cre+/−CXCR4flox/flox mouse. F, Circumferential and radial strain in MLC2v-Cre+/−CXCR4flox/flox and CXCR4flox/flox mice showing no significant difference (n=4 in each group).
SDF-1 mRNA Expression
Three after LAD ligation, the heart were perfused with 10 mL of saline and infarcted left ventricles were cut at a level just below the ligation. The tissue was snap-frozen and stored in −80°C until used. The RNA was harvested using TRIzol and cDNA was synthesized using SuperScript VILO cDNA synthesis kit from Invitrogen. The real-time PCR reaction for SDF-1 and 18S was carried out using TaqMan primers on Applied Biosystems 7500 Real-Time PCR System.
Stem Cell Homing Studies
Mesenchymal stem cells (MSCs) were isolated and prepared as we have previously described.12 The cells were incubated at 37°C. Confluent cells were passaged and plated out at 1:2 to 1:3 dilutions until passage 6. Cells were assayed for their ability to differentiate into the adipogenic, chondrogenic, and osteogenic lineages. Cells were maintained in differentiation media for 2 to 3 weeks. Differentiation was validated by staining the cells with Oil Red (adipogenic lineage), Alcian blue (chondrogenic lineage), or alkaline phosphatase (osteogenic lineage). Cells we induced to express green fluorescent protein (GFP) using a lentiviral construct encoding enhanced green fluorescent protein (EGFP) under the CMV promoter. Seven days after transfection, GFP+ cells were sorted by FACS. Animals were infused via the tail vein with 250 000 GFP+ MSCs 24 hours after LAD ligation. Hearts were harvested 72 hours after MSC infusion and prepared as described above for immunostaining with a primary antibody against GFP. The number of GFP+ cells per high-power fields was quantified in the infarct border zone as described before,12 by blinded observers across a minimum of 4 sections and 12 fields obtained from the mid–left ventricle per animal.
Statistical Analysis
Ventricular function was analyzed with one way ANOVA. All values with P<0.05 were considered significant.
Results
Generation of Congenital Cardiac Myocyte–Specific CXCR4-Deficient Mice
CXCR4 deficiency is embryonic lethal and is known to exhibit defective hematopoiesis, neural development, and ventricular septal defects. The ventricular septum begins to form in mouse at day 11 and is complete by day 12.5 postconception (pc).23 MLC2v expression begins at day 9 pc in the primitive heart tube.24 Therefore, to assess the role of cardiac myocyte–specific CXCR4 expression in cardiac development, we generated a mouse with congenital deletion of CXCR4 specifically in cardiac myocytes using the MLC2v-Cre–mediated expression. We successfully crossed MLC2v-Cre+/− (kind gift from Kenneth Chien) with CXCR4flox/flox (kind gift from Yong-Rui Zou) and CXCR4flox/− to generate the MLC2v-Cre+/−CXCR4flox/− mouse and MLC2v-CRE+/− CXCR4flox/flox mouse, respectively. All these mice were viable with litter sizes similar to that observed with MLC2v-Cre+/−. The genotype was determined by genomic PCR from the homogenate obtained from tails (Figure 1A and 1B). The loxP sites surrounds the exon 2 of CXCR4 gene (encodes >90% of protein), and to determine the deletion of exon 2 of CXCR4 gene, we used the primers that bind before and after the loxP sites. Successful recombination was observed from the ventricular homogenate and not from the tail. Immunofluorescence for CXCR4 in neonatal cardiac myocytes in culture showed an absence of CXCR4 staining in MLC2v-Cre+/−CXCR4flox/flox mice. Importantly, noncardiac myocytes, as defined by phase-contrast microscopy from the myocardium of MLC2v-Cre+/−CXCR4flox/flox, remained positive for CXCR4 expression (Figure 1C).
Based on echocardiography and autopsy, no detectable ventricular septal defect or valvular defects were observed (Figure 1D and 1E, respectively). Echocardiography further revealed normal cardiac function in the MLC-2vCre+/−CXCR4flox/flox mice compared to littermates that were CXCR4flox/flox or CXCR4flox/− but lacked MLC2vCre allele. The baseline function was within normal limits and shown in the Table. We also observed no difference in myocardial contractility as measured by myocardial strain imaging (radial strain, 23.4±13.3 versus 21.9±9.8; circumferential strain, −11.1±3.9 versus −9.8±4.1 in the presence and absence of MLC2v-Cre in CXCR4flox/flox or CXCR4flox/− mice (Figure 1F). These data suggest that cardiac myocyte CXCR4 expression is not required for normal heart development.
Table.
Baseline LV Function Obtained by Echocardiographic Parameters of Mice Used in Studies
| Tamoxifen | Congenital Deletion |
Conditional Deletion |
||
|---|---|---|---|---|
| CXCR4flox/flox | MLC2vCre+/− CXCR4flox/flox | MCM+/− CXCR4flox/flox | MCM+/− CXCR4flox/flox | |
| End diastole (mm) | ||||
| IVS | 1.15±0.09 | 1.14±0.06 | 1.23±0.04 | 1.27±0.07 |
| LVPW | 1.12±0.08 | 1.01±0.05 | 1.12±0.06 | 1.08±0.06 |
| LVED | 2.77±0.09 | 2.76±0.15 | 2.70±0.20 | 2.66±0.10 |
| End systole (mm) | ||||
| IVS | 1.82±0.08 | 1.93±0.11 | 1.96±0.07 | 1.71±0.07* |
| LVPW | 1.67±0.05 | 1.65±0.07 | 1.72±0.09 | 1.73±0.04 |
| LVED | 1.37±0.14 | 1.10±0.14 | 1.10±0.22 | 1.41±0.06* |
| Ejection fraction (%) | 87.4±2.5 | 92.1±1.9 | 91.3±2.5 | 83.1±1.5* |
Values are expressed as means±SEM (n=7 in each group).
P<0.05.
IVS indicates interventricular septum; LVPW, left ventricular posterior wall; LVED, left ventricular end dimension.
Generation of Conditionally Regulated Cardiac Myocyte–Specific CXCR4-Deficient Mice
To determine the role of cardiac myocyte–derived CXCR4 in ventricular remodeling after MI, we also generated the conditional cardiac-specific CXCR4-deficient mice. We crossed CXCR4flox/flox and CXCR4flox/− mice with the mercremer (MCM+/−) mice (from The Jackson Laboratories) to generate MCM+/−CXCR4flox/− and MCM+/−CXCR4flox/flox mice, respectively (Figure 2A, i and ii). To verify recombination, we quantified CXCR4 deletion by performing quantitative PCR on the mice treated daily with 40 mg/kg IP of tamoxifen dissolved in corn oil for 2, 4, 6, and 8 days. Using the same PCR strategy described above, we observed no further deletion of CXCR4 following tamoxifen administration beyond 4 days (Figure 2B; Figure 2A, iii).
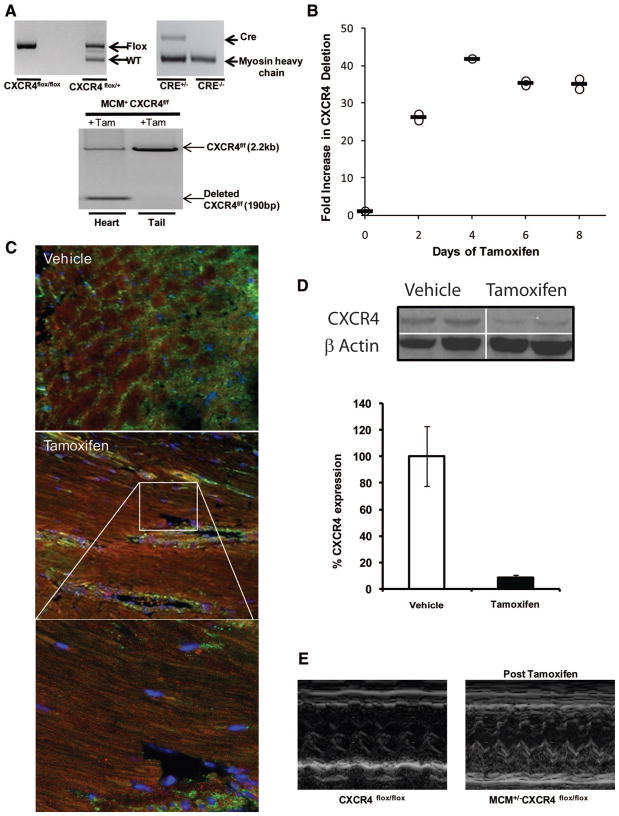
Figure 2. Characterization of MCM+/−CXCR4flox/flox mouse.
A, PCR analysis on genomic DNA from tail and heart was performed on 8-week-old CXCR4flox/flox, MCM+/−CXCR4flox/flox, and MCM+/−CXCR4flox/+ mice. i depicts a wild-type and floxed CXCR4 band; ii depicts CRE band in the genome; and iii depicts deleted band for CXCR4, which was only detected after tamoxifen administration in the ventricular homogenates and not from the tail. B, A dose–response curve for tamoxifen-induced recombination of CXCR4flox/flox based on quantitative PCR shows maximum deletion was achieved after 4 days of tamoxifen administration. C, Representative images of immunofluorescence for CXCR4 48 hours after AMI shows knockdown of CXCR4 in cardiac myocytes of tamoxifen-treated MCM+/−CXCR4flox/flox and endothelial cells positive for CXCR4 (green). Delineated area in CM-CXCR4−/− image is shown below at higher magnificent to aid in identification of nucleated cardiac myocytes devoid of CXCR4 expression. D, Western blot analysis of CXCR4 48 hours after AMI on infarct region shows ≈90% deletion of CXCR4. E, Representative M-mode recording of CXCR4flox/flox and MCM+/−CXCR4flox/flox after tamoxifen administration.
We have previously demonstrated that cardiac myocyte CXCR4 expression is upregulated beginning 48 hours after acute myocardial infarction.12,25 Therefore, to quantify the degree of cardiac myocyte CXCR4 recombination, MCM+/− CXCR4flox/flox and MCM+/−CXCR4flox/− mice were treated with tamoxifen for 5 days, allowed to recover cardiac function for 14 days, and then underwent induction of AMI by LAD ligation. Two days following AMI, immunohistochemistry demonstrated downregulation of cardiac myocyte but not endothelial CXCR4 expression 48 hours after LAD ligation in the tamoxifen-treated group (Figure 2C). Based on Western blots of infarcted heart homogenates, we achieved ≈90% reduction in myocardial CXCR4 protein compared to littermates that did not receive tamoxifen (Figure 2D). The baseline ventricular function before and 14 days after tamoxifen was within normal limits, as shown in the Table. There were modest statistically significant differences in left ventricular end diastolic dimension and ejection fraction in MCM+/−CXCR4flox/flox that received tamoxifen 2 weeks before echocardiography compared to those that did not.
Effect of CXCR4 on Ventricular Remodeling and Function Before and After MI
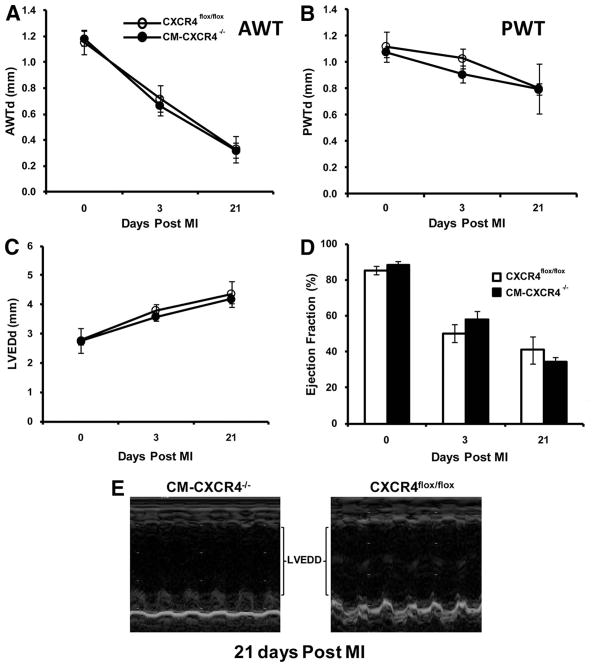
MCM+/−CXCR4flox/flox mice were treated with tamoxifen (40 mg/kg, IP) for 5 days at 6 weeks of age. As recently reported in studies with the MCM mouse, following tamoxifen administration, we observed a transient decrease in cardiac function that resolved 10 to 14 days later26; therefore, we did not perform any procedures on the MCM mice until 14 days after tamoxifen administration. Mice that did (MCM+/−CXCR4flox/flox) or did not receive tamoxifen and (MLC2vCRE+/−CXCR4flox/flox and CXCR4flox/flox) underwent LAD ligation to induce AMI, and their cardiac function was monitored by echocardiography. We hypothesized that because SDF-1 expression is transiently expressed for a short period of time immediately after AMI and begins to return to baseline by the time cardiac myocyte CXCR4 expression is significantly upregulated, the deletion of cardiac myocyte CXCR4 expression would not alter cardiac function or cardiac remodeling. Consistent with our hypothesis, 3 days after AMI, the ejection fraction, anterior wall thickness, and posterior wall thickness of the cardiac myocyte–specific CXCR4−/− mice (MCM+/−CXCR4flox/flox and MLC2vCRE+/−CXCR4flox/flox) were: ejection fraction, 57.8±5.0%; anterior wall thickness, 0.71±0.07 mm; and PW thickness, 0.92±0.10 mm, respectively; there were not statistically different from mice that did not receive tamoxifen (ejection fraction, 50.1±5.0%; anterior wall thickness, 0.73±0.08 mm; and PW thickness, 1.04±0.11 mm, respectively) (Figure 3A through 3F). Twenty-one days after AMI, the ventricular function and remodeling was not statistically different between the absence and presence of cardiac myocyte CXCR4 expression: ejection fraction, 34.4±2.6% versus 40.9±7.5%; anterior wall thickness, 0.36±0.12 versus 0.32±0.11 mm; and PWT, 0.81±0.21 versus 0.83±0.04 mm, respectively (Figure 3A through 3E).
Figure 3. Ventricular function assessment of cardiac myocyte–specific CXCR4-null hearts after AMI.
Echocardiographic analysis of 8- to 10-week-old CXCR4flox/flox and cardiac-specific CXCR4−/− mice as a function of time after AMI. A, Anterior wall thickness (AWT) (mm). B through D, Posterior wall thickness (PWT) (mm) (B), LV end-diastolic dimension (LVEDd) (mm) (C), and ejection fraction (%) (D) at 0, 3, and 21 days after AMI (n=7 for CXCR4flox/flox and n=12 for CM-CXCR4−/− at each time point). There was no significant difference between the measured parameters at days 3 and 21 after AMI. Data represent means±SEM. E, Representative M-mode recording of CXCR4flox/flox and cardiac-specific CXCR4−/− mice 21 days after AMI.
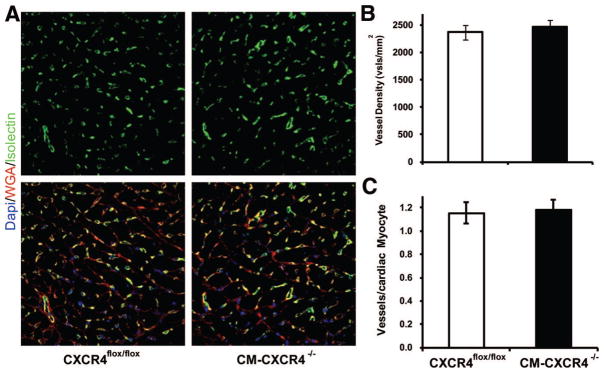
We evaluated vascular density 21 days after AMI to determine whether cardiac myocyte CXCR4 may have had any effect on vascular density. Using isolectin staining (Figure 4), we measured vascular density in the infarct borderline zone and found similar levels of vascular density in the presence and absence of cardiac myocyte CXCR4 (CM-CXCR4) expression (2471±126 versus 2369±131 vessels/mm2, respectively; Figure 4A). We further quantified vascular density as vessels per cardiac myocyte in the noninfarct zone 21 days after AMI (Figure 4B). We observed no difference in vessels per myocyte between groups (vessels/cardiac myocyte: 1.15±0.09 versus 1.18±0.09, CXCR4f/f versus CM-CXCR4−/−, n=5 animals per group, P=0.61)
Figure 4.
A, Representative photomicrographs of vascular density from CXCR4flox/flox (left) and CM-CXCR4−/− (right) mice 21 days after MI (green, isolectin; red, wheat germ agglutinin; blue, DAPI). Vascular density calculated as vessels/mm2 (B) and vessels/cardiac myocyte (C) in untreated CM-specific CXCR4flox/flox (n=4) and treated CM-specific CXCR4−/− (n=6) mice showed no statistically significant difference among the groups; P>0.05.
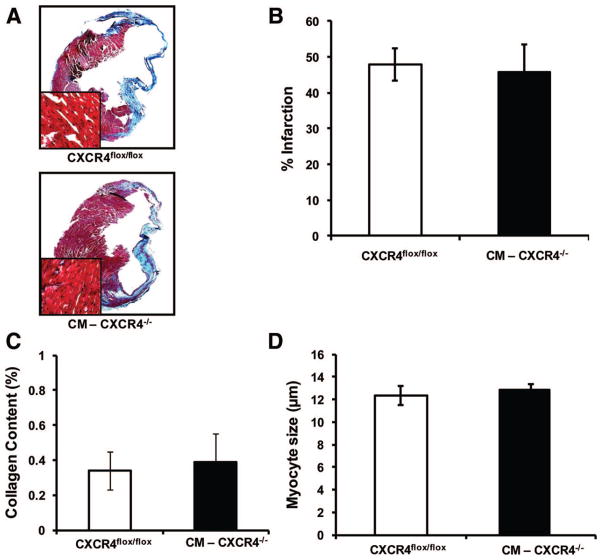
To further characterize the cardiac remodeling 21 days after MI (Figure 5A through 5D), we measured infarct size 21 days after LAD ligation using Masson’s trichrome stain (Figure 5B). We found no statistically significant difference in the infarct size of CXCR4flox/flox animals (47.93±4.42% of the left ventricular [LV] area, n=3) compared to CM-CXCR4−/− animals (45.89±7.61% of the LV area, n=3).
Figure 5.
A, Masson’s trichrome staining of CXCR4flox/flox and CM-CXCR4−/− on mice paraffin-embedded heart sections 21 days after MI. B, Infarct size assessment in mice 21 days after LAD ligation. C, Interstitial fibrosis in viable myocardium 21 days after MI. D, Cardiac myocyte diameter (microns) in posterior wall. Data represent means±SD (n=4 per group).
We further characterized the response to MI in the noninfarct zone to determine whether cardiac myocyte CXCR4 had a role in remodeling in the noninfarct zone. The interstitial fibrosis within the noninfarct zone of the myocardium 21 days after MI (Figure 5C) was 0.34±0.11% versus 0.38±0.16% of the LV area and cardiac myocyte diameter in the posterior wall remote from the infarct zone (Figure 5D) was 12.34±0.80 versus 12.89±0.46 μm in CXCR4flox/flox (n=4) compared to CM-CXCR4−/− animals (n=4).
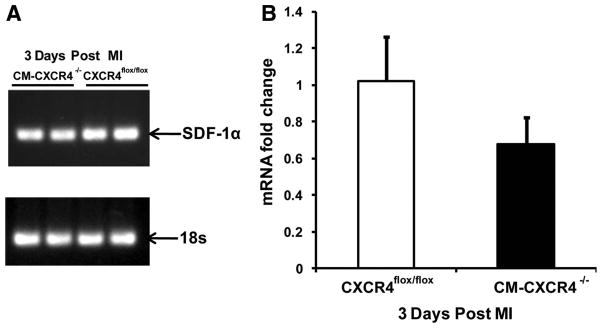
SDF-1α mRNA levels were analyzed 3 days after LAD ligation by real-time PCR. We observed by real-time PCR that there was a 33% decrease (P=0.08, n=4 per group) in SDF-1 mRNA expression in animals that were CM-CXCR4−/− compared to CM-CXCR4+/+ (Figure 6). To determine whether this decrease in SDF-1 expression significantly altered stem cell homing, we infused 250 000 GFP+ MSCs via tail vein 1 day after AMI and euthanized animals 4 days after AMI (3 days after MSC infusion).12 Immunofluorescence was used to quantify the number of MSCs per millimeter squared of tissue and revealed no significant differences in MSCs present (CXCR4flox/flox: 17.4±7.33 cells/mm2 versus CM-CXCR4−/−: 17.2±3.1 cells/mm2; P=0.99, n=5 per group), suggesting that the degree of change in SDF-1 expression in the CM-CXCR4−/− is not sufficient to alter stem cell homing to the heart.
Figure 6.
A, Representative SDF-1α mRNA expression by real-time PCR reaction at 3 days after MI. B, Quantification of quantitative PCR of myocardial SDF-1 expression 3 days after MI. Data represent means±SD (n=3 to 4 animals in each group).
Discussion
Because significant body of literature has been developed that has demonstrated a critical role for the SDF-1/CXCR4 axis in cardiac development10,27 and myocardial response to cell therapy12,14,28,29 the goal of this study was to analyze the importance of cardiac myocyte derived CXCR4 expression during development and repair following AMI. To address this question we generated a congenital cardiac deletion of CXCR4 mouse and a conditional tamoxifen inducible cardiac myocytes specific CXCR4 deletion mouse. We characterized the baseline function of these animals and then studied the myocardial response to injury in these mice.
Cardiac Myocyte–Derived CXCR4 in Cardiac Development
SDF-1 and CXCR4 are ubiquitously expressed during embryogenesis. However, the timing and the stage specific expression of the cytokine and ligand orchestrate the migration of stem cells and development of various organs. CXCR4- and SDF-1–deficient mice present with similar phenotypes and exhibits ventricular septal defect in the membranous part of the septum. Studies have demonstrated that CXCR4 is expressed in the outflow tract and descending part of bulbus cordis by cardiac myocytes, endothelial cells and cardiac neural crest cells during septum formation.30,31 However, the contribution of specific cell type expression of CXCR4 during septum formation is not known. MLC2v is expressed in the outflow tract at day 9 pc, before formation of the septum which starts at or near day 11 pc. We chose MLC2v as a promoter to regulate cre expression to specifically remove cardiac myocyte CXCR4 expression before formation of the septum to determine whether the lack of cardiac myocyte CXCR4 expression would alter cell patterning leading to the development of ventricular septal defect formation. Our results demonstrate that the CXCR4 derived from cardiac myocytes does not play a role in the formation of ventricular septal defects observed in CXCR4−/− mice. This finding suggests that CXCR4 expression is necessary for the patterning of cells for the heart and that this patterning occurs before expression of proteins in the endocardial cushions and muscular septum32 that define cardiac myocytes.
Cardiac Myocyte–Derived CXCR4 in Myocardial Repair
We generated the conditional deletion of CXCR4 mouse because we initially hypothesized that the congenital deletion of cardiac myocyte CXCR4 would potentially be lethal or result in cardiac anomalies or dysfunction. Therefore, we wanted to allow normal cardiac development in the presence of cardiac myocyte CXCR4 and then conditionally delete cardiac myocyte CXCR4 following the administration of tamoxifen.
Of note, as has been recently reported,26 tamoxifen administration results in a transient decrease in cardiac function in the MCM mice. Consistent with these observations, we observed significant degrees of cardiac dysfunction following the administration of tamoxifen to the mercremer mouse that resolved 14 days after the final dose. Thus, all our studies commenced 14 days after the final dose of tamoxifen and in animals with recovered cardiac function.
Our initial hypotheses were that the lack of cardiac myocyte CXCR4 would prove lethal secondary to abnormal cardiogenesis and at the very least ventricular septal defect formation. Our findings with the MLV2v-Cre mouse would suggest this hypothesis was false. We further hypothesized that the absence of cardiac myocyte CXCR4 would not adversely affect left ventricular remodeling or function following AMI because SDF-1 is rapidly and transiently expressed following AMI before cardiac myocyte CXCR4 is upregulated. This hypothesis proved to be true.
Recent studies have demonstrated that MSCs with or without SDF-1 overexpression have beneficial effects on ventricular function after MI.16 Various mechanisms including stem cell homing, neoangiogenesis, and decreased myocytes death have been suggested. Studies have also demonstrated that SDF-1 overexpression initiates CXCR4 signaling in hypoxic cardiac myocytes and induce antiapoptotic pathway by Akt phosphorylation.12 CXCR4 following AMI is also involved in the recruitment of endothelial progenitor cells and MSCs to the site of injury.28,33–35 Furthermore, the role of SDF-1/CXCR4 signaling is questionable in recruitment and differentiation of cardiac stem cells into cardiac myocytes.29
It is interesting that we observed a decrease in myocardial SDF-1 expression in the CM-CXCR4−/− mice. A recent study in which adenovirus encoding CXCR4 was used to constitutively overexpress CXCR4 in the myocardium before inducing myocardial infarction demonstrated an increase in cardiac SDF-1 expression.36 Thus, it would appear, based on this study and our findings, that cardiac CXCR4 expression is correlated with SDF-1 expression in response to AMI. Importantly, with respect to our findings, we did not observe a significant difference in stem cell homing between hearts that had cardiac myocyte CXCR4 expression and those that did not. However, it is yet undefined and awaits future studies to determine whether the myocardial response to stem cell engraftment will be blunted in the absence of cardiac myocyte CXCR4 expression.
The MCM+/−CXCR4flox/flox mouse developed for these studies will serve as a tool to dissect out the mechanisms responsible for the beneficial effects associated with the administration of SDF-1. In particular, because baseline and post-AMI cardiac function is the same in the presence and absence of cardiac myocyte CXCR4 expression, differences in response to stem cell therapy will be able to be attributed to the absence of cardiac myocyte CXCR4. We will further be able to determine the importance and relative contribution of CXCR4 on strategies associated with enhancing cardiac myocyte survival such as ischemic preconditioning and growth factor delivery.15,37 In conclusion, deletion of cardiac myocyte CXCR4 does not alter cardiac development or function. Similarly, deletion of cardiac myocyte CXCR4 before AMI does not alter myocardial response to injury. These data, taken together, would suggest no role for cardiac myocyte CXCR4 expression in development, normal physiology, or response to injury. Future studies will need to determine whether there is a role for cardiac myocyte CXCR4 expression in the modulation of the myocardial response to injury mediated by SDF-1 and/or stem cell therapy.
Novelty and Significance.
What Is Known?
SDF-1/CXCR4 axis is important in stem cell–based myocardial repair.
Myocardial SDF-1 expression begins to decline before cardiac myocyte CXCR4 expression is upregulated after acute myocardial infarction (MI).
CXCR4-deficient mice die in utero with various birth defects including ventricular septal defect.
What New Information Does This Contribute?
Cardiac myocyte CXCR4 expression is not essential for heart development.
CXCR4 deletion from adult cardiac myocytes does not affect ventricular remodeling after MI in the absence of stem cell treatment.
The SDF-1α/CXCR4 axis plays a major role in the recruitment of bone marrow–derived and cardiac stem cells to the infarct zone after acute myocardial infarction. Recent data suggest that cardiac myocytes upregulate CXCR4 after MI and that extended SDF-1 expression may increase cardiac myocyte survival. Assessing these effects is complicated by the fact that CXCR4-deficient mice die of various birth defects in utero. Thus, it becomes critical to dissect out the role cardiac myocyte–derived CXCR4 (CM-CXCR4) plays after MI and during heart development. To investigate these issues, we generated congenital and conditional cardiac myocyte–specific CXCR4-deficient mouse models. We found that congenital CM-CXCR4 deletion is not essential for heart development and ventricular remodeling after AMI. These are novel findings and have been reported for the first time in literature. Moreover, the mouse models we have generated in our laboratory will be useful for investigating the contribution of CM-CXCR4 to improved ventricular remodeling observed in response to stem cells delivery.
Acknowledgments
Sources of Funding
This work was funded by the Skirball Foundation. W.G. was the recipient of funding from the Fuad Jubran Center for Middle East Medical Education.
Non-standard Abbreviations and Acronyms
- AMI
acute myocardial infarction
- CXCR4
CXC chemokine receptor-4
- CM-CXCR4
cardiac myocyte–specific CXCR4
- EGFP
enhanced green fluorescent protein
- GFP
green fluorescent protein
- LAD
left anterior descending artery
- LV
left ventricular
- LVED
left ventricular end dimension
- MCM
MerCreMer, tamoxifen-induced α-myosin heavy chain driven Cre
- MI
myocardial infarction
- MLC2v
ventricular myosin regulatory light chain-2
- MSC
mesenchymal stem cell
- pc
postconception
- SDF
stromal cell–derived factor
Footnotes
Disclosures
None.
References
- 1.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 2.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 3.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 4.Brunner S, Winogradow J, Huber BC, Zaruba MM, Fischer R, David R, Assmann G, Herbach N, Wanke R, Mueller-Hoecker J, Franz WM. Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis. FASEB J. 2009;23:351–361. doi: 10.1096/fj.08-109462. [DOI] [PubMed] [Google Scholar]
- 5.Askari A, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor-1 on stem cell homing and tissue regeneration in ischemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 6.Czarnowska E, Gajerska-Dzieciatkowska M, Kusmierski K, Lichomski J, Machaj EK, Pojda Z, Brudek M, Beresewicz A. Expression of SDF-1-CXCR4 axis and an anti-remodelling effectiveness of foetal-liver stem cell transplantation in the infarcted rat heart. J Physiol Pharmacol. 2007;58:729–744. [PubMed] [Google Scholar]
- 7.Klopsch C, Furlani D, Gabel R, Li W, Pittermann E, Ugurlucan M, Kundt G, Zingler C, Titze U, Wang W, Ong LL, Wagner K, Li RK, Ma N, Steinhoff G. Intracardiac injection of erythropoietin induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model. J Cell Mol Med. 2009;13:664–679. doi: 10.1111/j.1582-4934.2008.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misao Y, Arai M, Ohno T, Ushikoshi H, Onogi H, Kobayashi H, Takemura G, Minatoguchi S, Fujiwara T, Fujiwara H. Modification of post-myocardial infarction granulocyte-colony stimulating factor therapy with myelo-suppressives. Circ J. 2007;71:580–590. doi: 10.1253/circj.71.580. [DOI] [PubMed] [Google Scholar]
- 9.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in C. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 11.Odemis V, Lamp E, Pezeshki G, Moepps B, Schilling K, Gierschik P, Littman DR, Engele J. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30:494–505. doi: 10.1016/j.mcn.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Mal N, kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 13.Damas JK, Eiken HG, Oie E, Bjerkeli V, Yndestad A, Ueland T, Tonnessen T, Geiran OR, Aass H, Simonsen S, Christensen G, Froland SS, Attramadal H, Gullestad L, Aukrust P. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000;47:778–787. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 14.Penn MS. Importance of the SDF-1/CXCR4 axis in myocardial repair. Circ Res. 2009;104:1133–1135. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penn MS, Agarwal U. IGF-1 and mechanisms of myocardial repair. Int J Cardiol. 2010;138:1–2. doi: 10.1016/j.ijcard.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 16.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 17.Miller RJ, Banisadr G, Bhattacharyya BJ. CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol. 2008;198:31–38. doi: 10.1016/j.jneuroim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18:1240–1242. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 19.De FE, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 20.Lima e Silva, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara MC, Lauer T, Aslam S, Gong YY, Xiao WH, Khu NH, Thut C, Campochiaro PA. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 21.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 22.Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossant J. Mouse mutants and cardiac development: new molecular insights into cardiogenesis. Circ Res. 1996;78:349–353. doi: 10.1161/01.res.78.3.349. [DOI] [PubMed] [Google Scholar]
- 24.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 25.Deglurkar I, Mal N, Mills WR, Popovic ZB, McCarthy P, Blackstone EH, laurita KR, Penn MS. Mechanical and electrical effects of cell-based gene therapy for ischemic cardiomyopathy are independent. Hum Gene Ther. 2006;17:1144–1151. doi: 10.1089/hum.2006.17.1144. [DOI] [PubMed] [Google Scholar]
- 26.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson TJ, Faustino RS, Chiriac A, Crespo-Diaz R, Behfar A, Terzic A. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells. 2008;26:1464–1473. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 29.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor-cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, Koenen TB, Krajnc-Franken MA, Gossen JA. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46:235–245. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 31.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez A, MacKay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien TX, Lee KJ, Chien KR. Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proc Natl Acad Sci U S A. 1993;90:5157–5161. doi: 10.1073/pnas.90.11.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz de AC, Luttun A, Carmeliet P. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell. 2006;124:18–21. doi: 10.1016/j.cell.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, Zhang L, Huang Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36:644–650. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]