Autotaxin (ATX), a pyrophosphatase/phosphodiesterase enzyme, is a promising drug target for many indications and is only distantly related to enzymes of previously determined structure. Here, the cloning, expression, purification, crystallization and preliminary diffraction analysis of ATX are reported.
Keywords: autotaxin, (ecto)nucleotide pyrophosphatase/phosphodiesterase 2, lysophosphatidylcholine, lysophosphatidylcholine
Abstract
Rat autotaxin has been cloned, expressed, purified to homogeneity and crystallized via hanging-drop vapour diffusion using PEG 3350 as precipitant and ammonium iodide and sodium thiocyanate as salts. The crystals diffracted to a maximum resolution of 2.05 Å and belonged to space group P1, with unit-cell parameters a = 53.8, b = 63.3, c = 70.5 Å, α = 98.8, β = 106.2, γ = 99.8°. Preliminary X-ray diffraction analysis indicated the presence of one molecule per asymmetric unit, with a solvent content of 47%.
1. Introduction
Autotaxin (ATX), which is also known as (ecto)nucleotide pyrophosphatase/phosphodiesterase 2 (ectoNPP2), functions as an extracellular phosphodiesterase/pyrophosphatase that mediates the conversion of lysophosphatidylcholine (LPC) to lysophosphatidic acid (LPA) (Umezu-Goto et al., 2002 ▶). Mature ATX consists of two somatomedin domains, an NPP domain and a MORPHO (modulator of oligodendrocyte remodelling and focal adhesion organization) domain (Yuelling & Fuss, 2008 ▶). It is approximately 98 kDa in size. It is a glycoprotein and it has been shown that only one of the four ATX glycosylation sites is required for functional activity (Jansen et al., 2007 ▶).
ATX plays a role in a wide range of functions in vivo, from the conversion of LPC to LPA and the differentiation of myelinating oligodendrocytes (Yuelling & Fuss, 2008 ▶) to lymphocyte entry into lymphoid organs (Kanda et al., 2008 ▶). ATX has been proposed to be an important central nervous system, gastrointestinal, oncology, inflammation and immunology target (Khurana et al., 2008 ▶; Inoue et al., 2008 ▶; Prestwich et al., 2008 ▶; Kehlen et al., 2001 ▶). Medicinal chemistry efforts have identified several classes of ATX inhibitors (Prestwich et al., 2008 ▶; van Meeteren et al., 2008 ▶); Cui et al., 2007 ▶; Saunders et al., 2008 ▶; Ferry et al., 2008 ▶; Parrill et al., 2007 ▶). As there are no publicly disclosed structures of closely related enzymes, an ATX structure would deepen our understanding of its basic biological roles as well as accelerate the design and development of promising drug candidates.
The laboratory of Anastassis Perrakis at the Netherlands Cancer Institute (NKI) have designed a mutant rat ATX (rATX) construct that yielded a complete data set to 2.4 Å resolution (Hausmann et al., 2010 ▶). In the process of reproducing and optimizing crystallization with this construct, we found a new crystallization condition that resulted in larger, more mechanically robust and less radiation-sensitive crystals. Here, we describe the results obtained for the optimized crystallization of rATX and its X-ray diffraction data to 2.05 Å resolution.
2. Experimental procedure
2.1. Expression and purification of rat autotaxin
The rATX mutant cell line (Hausmann et al., 2010 ▶) was thawed into a T75 flask, incubated at 5% CO2 and 310 K and expanded into T175 flasks upon confluency. The growth medium was DMEM supplemented with 10% FBS, penicillin/streptomycin, 2 mM glutamine (final concentration) and 100 mg l−1 hygromycin (Invitrogen). Two confluent T175 flasks were used to seed each roller bottle (Greiner Bio One) containing 800 ml growth medium. The roller bottles were maintained at 5% CO2 and 310 K. When the cells began to reach confluency, the medium was harvested semi-weekly and replaced with the same growth medium containing 5% FBS. The roller bottles were maintained until the cells began to fall off the surface.
rATX was purified using an adaptation of the procedure described by Hausmann et al. (2010 ▶). All purification procedures were performed at 278 K unless otherwise noted. 50 mM Tris–HCl pH 8.0 and 300 mM NaCl were added to 1 l HEK media. 5 ml Ni2+–NTA Superflow resin was added and the protein was allowed to batch bind overnight. The resin slurry was poured into an XK26 column and the flowthrough was collected. 20 column volumes of buffer were used to wash the resin, followed by another four column volumes of buffer containing 20 mM imidazole. The protein was eluted using four column volumes of buffer containing 150 mM imidazole. The eluate was diluted threefold with 50 mM Tris pH 8.0 to lower the conductivity to below 7 mS and the protein was batch bound for 1 h to an optional 5 ml Q-Sepharose FF resin. The resin slurry was poured into an XK26 column and washed with a step gradient using three column volumes of 50 mM Tris pH 8.0 containing 75 mM NaCl followed by three column volumes of buffer containing 150 mM NaCl. Samples containing autotaxin with a purity greater than 85% as determined by SDS–PAGE were concentrated to less than 5 ml using an Amicon-10 and loaded onto a Superdex 200 (16/60) column equilibrated with 50 mM Tris pH 8.0, 300 mM NaCl. Fractions containing autotaxin with a purity greater than 95% were pooled, concentrated to approximately 10 mg ml−1 and subsequently diluted to 5 mg ml−1 with 50 mM Tris pH 8.0. The purified and concentrated autotaxin was then aliquoted, flash-frozen on dry ice and stored at 193 K. Protein concentrations were determined from the A 280nm using an extinction coefficient of 1.0 AU ml mg−1.
2.2. Crystallization
Initial crystallization screening was carried out at 295 K using Art Robbins CrystalMation Intelli-Plate 96-3 sitting-drop vapor-diffusion trays (catalog No. HR3-118 from Hampton Research) by mixing 300 nl protein solution with 300 nl precipitant solution and equilibrating against 85 µl well solution. A Mosquito robot (TTP LabTech) was used to set up multiple crystallization screens using rATX at a concentration of 3.5–5.0 mg ml−1 in 50 mM Tris pH 8.0 (277 K) and 150 mM NaCl. The original crystallization condition (condition No. 12 from the PEG/Ion crystallization screen; Hampton Research) was further optimized by the addition of 0.24–0.34 M sodium thiocyanate to yield a final well volume of 500 µl in an SBS footprint 24-well hanging-drop vapor-diffusion tray. These results were not reproducible with alternate sources of ammonium iodide other than the well solution purchased from Hampton Research. The best crystals were obtained using 3 µl of a spin-filtered protein solution and 1.5 µl well solution. A simple substitution of seed stock (prepared by crushing a single crystal in 50 µl precipitant solution) for well solution in crystallization drops dramatically increased the number of crystals formed.
2.3. X-ray data collection and processing
All crystals were soaked for 2 s in a cryoprotective solution consisting of crystallization solution supplemented with 15% glycerol and an additional 15% PEG 3350 prior to flash-freezing to 100 K by plunging the crystals into liquid nitrogen. A native data set was collected at 50 eV above the zinc edge on beamline 23-ID-D at APS, which is equipped with a MAR Mosaic 300 detector. A complete data set was obtained using several wedges of data from a single crystal with multiple translations to alleviate the effects of radiation damage. The data were processed using DENZO and SCALEPACK from the HKL-2000 package (Otwinowski & Minor, 1997 ▶); statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics for rATX crystals.
Values in parentheses are for the highest resolution shell.
| Crystal data | |
| No. of crystals | 1 |
| Beamline | APS 23-ID-D |
| Detector | MAR Mosaic 300 |
| Total rotation/rotation per frame (°) | 460/1 |
| Space group | P1 |
| Unit-cell parameters (Å, °) | a = 53.8, b = 63.3, c = 70.5, α = 98.8, β = 106.2, γ = 99.8 |
| Data collection | |
| Wavelength (Å) | 1.28299 |
| Mosaicity (°) | 0.4 |
| Resolution (Å) | 20.0–2.05 (2.12–2.05) |
| Total No. of reflections† | 511856 |
| No. of unique data | 52153 |
| Multiplicity | 4.8 (3.0) |
| Data completeness (%) | 96.8 (93.9) |
| Average I/σ(I) | 13.9 (2.49) |
| Rmerge‡ | 0.097 (0.378) |
| Molecules per asymmetric unit | 1 |
| Matthews coefficient (Å3 Da−1) | 2.3 |
| Solvent content (%) | 47 |
This value includes measurements of partial reflections.
R
merge = 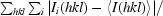
 .
.
3. Results
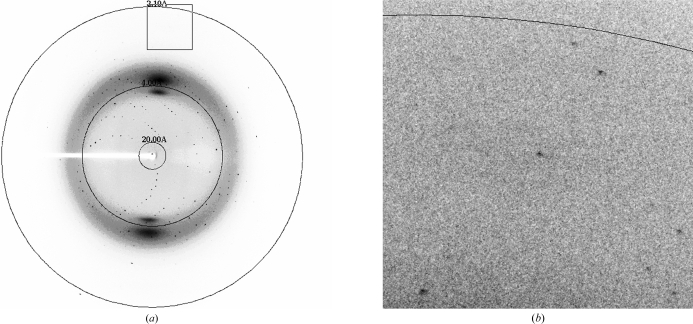
rATX crystals grew in a multitude of PEG-based conditions. However, the best crystals grew from PEG/Ion Screen (Hampton Research) condition No. 12 (20% PEG 3350, 0.2 M ammonium iodide) supplemented with 0.3 M sodium thiocyanate. The most reproducible single crystals grew from drops set up with seed stock. The crystals reached maximum dimensions of 0.15 × 0.05 × 0.05 mm in two weeks (Fig. 1 ▶). A complete X-ray data set was collected to 2.05 Å resolution from a single native rATX crystal, with some frames showing spots to 1.9 Å resolution (Fig. 2 ▶). Specific volume calculations based on the molecular weight of rATX and the unit-cell parameters indicated the presence of a single molecule in the asymmetric unit, with 47% solvent content and a Matthews coefficient of 2.3 Å3 Da−1 (Matthews, 1968 ▶). Structure determination was initiated using the Xac nucleotide pyrophosphatase/phosphodiesterase structure (PDB code 2gsn; Zalatan et al., 2006 ▶) as the initial model; this enzyme shares 30% sequence identity with the NPP domain of rATX. The program Phaser (McCoy et al., 2007 ▶) yielded a rotation-function Z score of 14.4 using reflections to 3.0 Å resolution. The high resolution and the presence of a single molecule in the asymmetric unit make this an appropriate crystal form for structure determination and refinement.
Figure 1.
Native rATX crystals obtained using 20% PEG 3350, 0.2 M ammonuim iodide solution supplemented with 0.3 M sodium thiocyanate. The approximate dimensions of the crystals are 0.15 × 0.05 × 0.05 mm.
Figure 2.
(a) X-ray diffraction pattern of an rATX crystal (oscillation range 1.0°); (b) an enlargement (with a different brightness and contrast) showing the highest resolution area (the resolution ring is at 2.1 Å).
Acknowledgments
The authors wish to thank the staff of the 23-ID-D (GM/CA-CAT), 21-ID-D (LS-CAT) and 24-ID-E (NE-CAT) beamlines at the APS for support during synchrotron data collection. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-06CH11357.
References
- Cui, P., McCalmont, W., Tomsig, J., Lynch, K. & Macdonald, T. (2007). Bioorg. Med. Chem.16, 2212–2225. [DOI] [PMC free article] [PubMed]
- Ferry, G., Moulharat, N., Pradère, J., Desos, P., Try, A., Genton, A., Giganti, A., Beucher-Gaudin, M., Lonchampt, M., Bertrand, M., Saulnier-Blache, J., Tucker, G., Cordi, A. & Boutin, J. (2008). J. Pharmacol. Exp. Ther.327, 809–819. [DOI] [PubMed]
- Hausmann, J., Christodoulou, E., Kasiem, M., De Marco, V., van Meeteren, L. A., Moolenaar, W. H., Axford, A., Owen, R. L., Evans, G. & Perrakis, A. (2010). Acta Cryst. F66, 1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, M., Ma, L., Aoki, J., Chun, J. & Ueda, H. (2008). Mol. Pain, 4, 4–6. [DOI] [PMC free article] [PubMed]
- Jansen, S., Callewaert, N., Dewerte, I., Andries, M., Ceulemans, H. & Bollen, M. (2007). J. Biol. Chem.282, 11084–11091. [DOI] [PubMed]
- Kanda, H., Newton, R., Klein, R., Morita, Y., Gunn, M. & Rosen, S. (2008). Nature Immunol.4, 415–423. [DOI] [PMC free article] [PubMed]
- Kehlen, A., Lauterbach, R., Santos, A. N., Thiele, K., Kabisch, U., Weber, E., Riemann, D. & Langner, J. (2001). Clin. Exp. Immunol.123, 147–154. [DOI] [PMC free article] [PubMed]
- Khurana, S., Tomar, A., George, S., Wang, Y., Siddiqui, M., Guo, H., Tigyi, G. & Mathew, S. (2008). Exp. Cell Res.3, 530–542. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst.40, 658–674. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Parrill, A., Echols, U., Nguyen, T., Pham, T., Hoeglund, A. & Baker, D. (2007). Bioorg. Med. Chem.16, 1784–1795. [DOI] [PubMed]
- Prestwich, G. D., Gajewiak, J., Zhang, H., Xu, X., Yang, G. & Serban, M. (2008). Biochim. Biophys. Acta, 9, 588–594. [DOI] [PMC free article] [PubMed]
- Saunders, L., Ouellette, A., Bandle, R., Chang, W., Zhou, H., Misra, R., De La Cruz, E. & Braddock, D. (2008). Mol. Cancer Ther.7, 3352–3362. [DOI] [PMC free article] [PubMed]
- Umezu-Goto, M., Kishi, Y., Taira, A., Hama, K., Dohmae, N., Takio, K., Yamori, T., Mills, G., Inoue, K., Aoki, J. & Arai, H. (2002). J. Cell Biol.158, 227–233. [DOI] [PMC free article] [PubMed]
- Van Meeteren, L., Brinkmann, V., Saulnier-Blache, J., Lynh, K. & Moolenaar, W. (2008). Cancer Lett.266, 203–208. [DOI] [PMC free article] [PubMed]
- Yuelling, L. M. & Fuss, B. (2008). Biochim. Biophys. Acta, 9, 525–530. [DOI] [PMC free article] [PubMed]
- Zalatan, J. G., Fenn, T. D., Brunger, A. T. & Herschlag, D. (2006). Biochemistry, 45, 9788–9803. [DOI] [PubMed]