Abstract
Structural redesign of selected non-steroidal estrogen receptor binding compounds has previously been successful in the discovery of new inhibitors of tubulin assembly. Accordingly, tetra-substituted alkene analogues (21-30) were designed based in part on combinations of the structural and electronic components of tamoxifen and combretastatin A-4 (CA4). The McMurry coupling reaction was used as the key synthetic step in the preparation of these tri- and tetra-arylethylene analogues. The structural assignment of E, Z isomers was determined on the basis of 2D-NOESY experiments. The ability of these compounds to inhibit tubulin polymerization and cell growth in selected human cancer cell lines was evaluated. Although the compounds were found to be less potent than CA4, these analogues significantly advance the known structure activity relationship associated with the colchicine binding site on β-tubulin.
1. Introduction
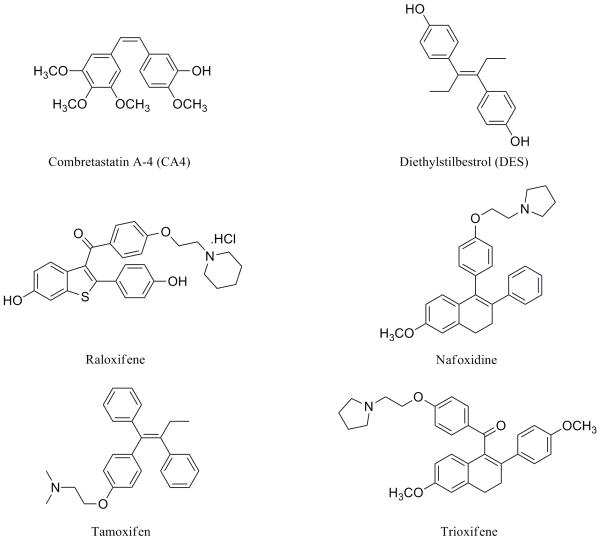
Structural diversity is an important theme describing the growing number of compounds that bind to the colchicine site on tubulin and inhibit tubulin assembly.1 The diarylethylene moiety in both combretastatin A-4 (CA4)2 and diethylstilbestrol (DES)3 (Fig. 1) inspired us to modify the molecular templates found in certain non-steroidal antiestrogenic compounds to explore the interaction of the resulting new compounds with the tubulin-microtubule protein system. This molecular design strategy proved highly successful for the synthesis of new benzo[b]thiophene,4 indole,5 and dihydronaphthalene6 analogues similar to raloxifene,7 nafoxidine,8 and trioxifene.9 Tamoxifen10 is a triarylethylene compound that has been widely used in the treatment of breast cancer, as well as hepatocellular, ovarian, colorectal, and pancreatic carcinomas.11 In contrast to CA4, 2-methoxyestradiol (2ME), and DES,12 tamoxifen does not have a significant effect on tubulin polymerization (IC50 > 40 μM; Table 2). Tamoxifen and its metabolites are thought to act primarily through inhibition of the estrogen receptor, but other mechanisms have been documented13 and include induction of apoptosis,14 interference with the insulin-like growth factor I receptor,15 and suppression of telomerase activity by inhibition of protein kinase C.16 The pronounced biological activity of tamoxifen has inspired the synthesis of numerous structural congeners.17
Figure 1.
Combretastatin A-4 and selected nonsteroidal antiestrogen compounds.
Table 2.
Cytotoxicity studies against human cancer cell lines DU-145, SK-OV-3, and NCI-H460, and assay for inhibition of tubulin polymerization
| Compound | Inhibition of Tubulin Polymerization IC50 (μM) |
GI50 (μM) SRB assaya |
||
|---|---|---|---|---|
| DU-145 | SK-OV-3 | NCI-H460 | ||
| Tamoxifen | >40 | 6.07b | 6.40b | 4.48b |
| 21 | >40 | 28.0 | 24.1 | 23.4 |
| 22 | >40 | 24.3 | 8.44 | 6.54 |
| 23 | >40 | 20.9 | 27.4 | 34.1 |
| 24 | >40 | 18.8 | 17.1 | 13.3 |
| 25 | >40 | 21.9 | 13.8 | 37.2 |
| 26 | >40 | 19.9 | 18.9 | 33.0 |
| 27 | >40 | 4.25 | 2.72 | 5.37 |
| 28 | >40 | 16.9 | 4.35 | 10.0 |
| 29 | >40 | 2.58 | 0.576 | 3.41 |
| 30 | >40 | 13.5 | 3.79 | 5.77 |
These data are an average of a minimum three separate experiments.
ref 26
Combretastatin A-4, a natural product found in the bush willow tree Combretum caffrum, is a potent inhibitor of tubulin assembly (IC50 = 1.2 μM)18 and is also strongly cytotoxic against selected human cancer cell lines (for example, GI50 = 2 nM against DU-145 prostate cancer cells).19 A water soluble phosphate prodrug (CA4P, fosbretabulin, ZYBERSTAT™) is currently in human clinical trials as a vascular disrupting agent.20
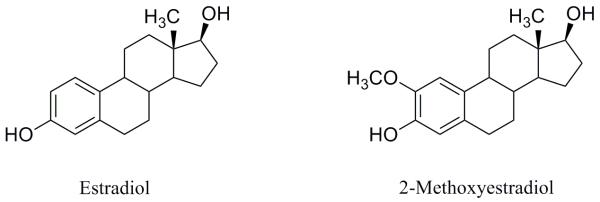
It is instructive to note that a number of derivatives of estradiol are strong inhibitors of tubulin polymerization.21 Interestingly, one of these derivatives, 2ME, is a natural metabolite of 17-β-estradiol in mammals (Fig. 2).22
Figure 2.
Estradiol and 2-methoxyestradiol.
The McMurry coupling reaction is an important methodology for the synthesis of highly-functionalized alkenes. This reaction, which has been used for the synthesis of tamoxifen and related compounds,23 was employed to synthesize a series of tri- and tetra-arylethylene compounds 21-30 that mimic the structural core of tamoxifen while incorporating features of CA4 and colchicine. These compounds that each contains trimethoxyphenyl and p-methoxy-m-hydroxyphenyl rings were evaluated for their ability to inhibit tubulin polymerization and for their cytotoxicity against selected human cancer cell lines.
2. Results and discussion
2.1. Chemistry
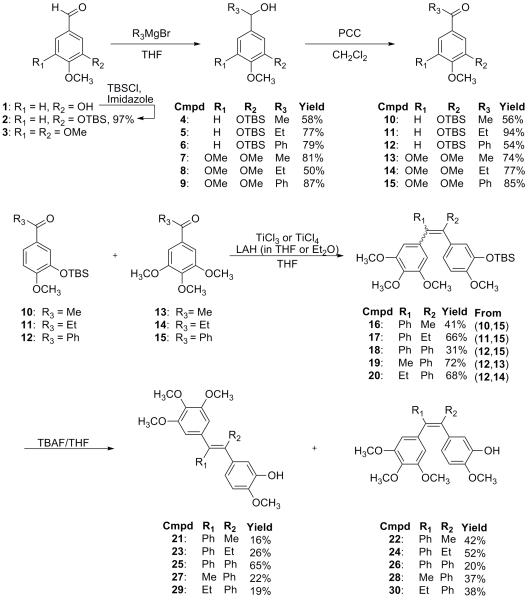
The requisite ketones necessary for the McMurry coupling reaction were prepared as outlined in Scheme 1. In brief, the appropriate aldehyde, upon treatment with the indicated organometallic reagent, formed the anticipated secondary alcohols 4-9 that were oxidized upon treatment with pyridinium chlorochromate (PCC) to their corresponding ketones 10-15. The low valent titanium (LVT) induced reductive deoxygenation of carbonyls to olefins (McMurry coupling) takes place in two successive steps: (i) reductive dimerization of the starting ketones to form a carbon-carbon bond and (ii) deoxygenation of the 1,2-diolate intermediate to give an alkene.24 Careful addition of LiAlH4 to the solution of TiCl3 or TiCl4 in THF followed by heating at reflux generated the LVT. The requisite ketones together with proton sponge as a solution in THF were heated at reflux to obtain 16-20. The mixture of TBS protected E, Z isomers 16-20 proved difficult to separate by column chromatography. However upon deprotection, the resulting phenolic E, Z isomers 21-30 were readily separable. The stereochemical assignments of the E, Z isomers were determined primarily on the basis of 2D-NOESY experiments. For example, the stereochemistry of compound 21 was determined based on its 2D-NOESY spectrum (supplementary data), obtained at 500 MHz. The methyl protons at 1.99 ppm demonstrate NOE cross peaks with protons at 6.50 ppm and 6.56 ppm of the 3′-hydroxy-4′-methoxyphenyl ring B as well as with the protons at 6.43 ppm on the 3,4,5-trimethoxyphenyl ring A. In addition, there is an absence of an NOE cross peak between the methyl protons and the protons at 6.91 ppm of the unsubstituted phenyl ring. Collectively, these NOE data establish the stereochemical assignment of compound 21 to be in the E configuration. Similarly, the stereochemistry of compound 22 was determined to be in the Z configuration based on its 2D-NOESY spectrum (supplementary data), obtained at 360 MHz. The methyl protons at 1.97 ppm demonstrate NOE cross peaks with protons at 6.58 ppm and 6.59 ppm of the 3′-hydroxy-4′-methoxyphenyl ring B as well as with the protons at 7.22 ppm on the phenyl ring. In addition, there is an absence of an NOE cross peak between the methyl protons and the protons at 6.11 ppm of the 3,4,5-trimethoxyphenyl ring A. A similar strategy using 2D-NOESY data was employed for the stereochemical assignment of compounds 23-30 (Table 1). Single crystal X-ray diffraction of compounds 23 and 27 (each recrystallized from 20% EtOAc in hexanes) confirms the stereochemical assignment for these compounds (supplementary data). 25
Scheme 1.
McMurry coupling to synthesize tri- and tetra-arylethylene analogues.
Table 1.
NOE correlations for compounds 21-30 in DMSO-d6.
| Cmpd | Proton shift (δH ppm) | NOE Correlation (δH ppm) | ||
|---|---|---|---|---|
| Ring Aa |
Ring Bb | Phenyl ring(s) |
||
| 21 c | 1.99 (s, 3H, CH3) | 6.43 (s, 2H) |
6.50 (dd, 1H, J = 8.1 Hz, J = 2.0 Hz) 6.56 (d, 1H, J = 2.0 Hz) |
|
| 22 d | 1.97 (s, 3H, CH3) | 6.58 (dd, 1H, J = 8.0 Hz, J = 2.0 Hz) 6.59 (d, 1H, J = 2.0 Hz) |
7.22 (d, 2H, J = 7.2 Hz) |
|
| 23 d | 0.88 (t, 3H, J = 7.2 Hz, CH2CH3) and 2.33 (q, 2H, J = 7.2 Hz, CH2CH3) |
6.45 (s, 2H) |
6.50 (dd, 1H, J = 8.3 Hz, 2.2 Hz) 6.56 (d, 1H, J = 2.2 Hz) |
|
| 24 d | 0.87 (t, 3H, J = 7.2 Hz, CH2CH3) and 2.30 (q, 2H, J = 7.2 Hz, CH2CH3) |
6.56 (dd, 1H, J = 7.9 Hz, 1.8 Hz) 6.57 (d, 1H, J = 1.8 Hz) |
7.23 (d, 2H, J = 6.8 Hz) |
|
| 25 d | 6.18 (s, 2H, ArH) | 7.02-7.16 (m, 10H) |
||
| 26 d | 6.25 (s, 2H, ArH) | 6.54 (dd, 1H, J = 8.3 Hz, 2.1 Hz) 6.62 (d, 1H, J = 2.0 Hz) |
7.00-7.12 (m, 10H) |
|
| 27 c | 2.11 (s, 3H, CH3) | 6.37 (s, 2H) |
6.59 (d, 1H, J = 2.0 Hz) 6.63 (dd, 1H, J = 8.2 Hz, 2.0 Hz) |
|
| 28 c | 2.03 (s, 3H, CH3) | 6.43 (s, 2H) |
7.19 (m, 2H) |
|
| 29 c | 0.92 (t, 3H, J = 7.4 Hz, CH2CH3) and 2.48 (q, 2H, J = 7.4 Hz, CH2CH3) |
6.34 (s, 2H) |
6.59 (d, 1H, J = 2.0 Hz), 6.63 (dd, 1H, J = 8.2 Hz, J = 2.0 Hz,) |
|
| 30 c | 0.89 (t, 3H, J = 7.4 Hz, CH2CH3) and 2.37 (q, 2H, J = 7.4 Hz, CH2CH3) |
6.40 (s, 2H) |
7.18 (d, 2H, J = 7.0 Hz) |
|
3,4,5-trimethoxyphenyl ring
3′-hydroxy-4′-methoxyphenyl ring
data determined at 500 MHz
data determined at 360 MHz
2.2. Biology
This series of tri- and tetra-substituted stilbene derivatives were evaluated by an in vitro cytotoxicity assay, which was carried out with a panel of three human cancer cell lines comprised of prostate cancer (DU-145), ovarian cancer (SK-OV-3), and lung carcinoma (NCI-H460), using doxorubicin as a reference compound. The screening procedure was based on the standard sulforhodamine B (SRB) assay method.6c,35 The GI50 values are shown in Table 2. A comparison of the triarylethylene analogues with R1 = phenyl (21-24) showed enhanced activity for the Z isomers (22 and 24) in SK-OV-3, and NCI-H460 human cancer cell lines. The reverse trend was observed for the triarylethylene analogues (27-30) in which R2 = phenyl. In this case, the E analogues (27 and 29) were more active in all three cancer cell lines. Collectively, compounds 27-30 were more active than compounds 21-24. There were no significant differences in cytotoxicity between the E and Z tetra-arylethylene analogues 25 and 26. Of this series of compounds, triarylethylene analogue 29 was the most cytotoxic across all three of the cell lines used in this study, and 29 was also more cytotoxic than tamoxifen against the three lines. It was especially active against SK-OV-3 cells (GI50 = 0.6 μM). Since the compounds in this study, like tamoxifen, did not significantly inhibit tubulin assembly (IC50 > 40 μM), the cytotoxicity demonstrated by analogue 29 is presumed to result from a different mechanism.
3. Conclusions
The McMurry coupling reaction was applied successfully to the synthesis of a series of new tri- and tetra-arylethylene analogues 21-30, which incorporate structural features of tamoxifen and CA4. In contrast to CA4, none of the compounds significantly inhibited tubulin assembly; however certain analogues (such as 27 and 29) demonstrated significant cytotoxicity against human cancer cell lines, suggesting an alternate mechanism of action.
4. Experimental27
Chemical reagents used in the synthetic procedures were obtained from various chemical suppliers (Sigma Aldrich, Acros Chemical Co., Alfa Aesar, Fisher Scientific, EMD Chemicals, and VWR). The following solvents were either used in their anhydrous form as obtained from the chemical suppliers or freshly distilled prior to use: methylene chloride (CH2Cl2) over calcium hydride, tetrahydrofuran (THF) over potassium metal and benzophenone, and hexanes over calcium hydride. Anhydrous Et2O or THF was used for organometallic reactions. Reactions were performed under an inert atmosphere using nitrogen gas unless specified. Thin layer chromatography (TLC) plates (pre-coated glass plates with silica gel 60 F254, 0.25 mm thickness, EMD chemicals, VWR) were used to monitor reactions. Silica gel (200-400 mesh, 60 Å), used for column chromatography, was obtained from either Silicycle Inc. or VWR. Purification of intermediates and products was carried out using manual flash column chromatography with silica gel or a Biotage® Isolera™ flash purification system using Biotage® KP-Sil SNAP columns. Intermediates and products synthesized were characterized on the basis of 1H NMR (Brüker DPX operating at 300 MHz or Brüker AMX operating at 360 MHz or Varian Inova operating at 500 MHz), and 13C NMR (Brüker DPX operating at 75 MHz or Brüker AMX operating at 90 MHz or Varian operating at 125 MHz). All the chemical shifts are expressed in ppm (δ), coupling constants (J) are presented in Hz, and peak patterns are reported as broad (br), singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). Elemental analysis was performed by Atlantic Microlab, Norcross, GA. High-resolution mass spectra (HRMS) were obtained using Electron Impact (EI) ionization on a VG Prospec Micromass spectrometer or Electrospray Ionization (ESI) technique on a Thermo Scientific LTQ Orbitrap Discovery Mass spectrometer in the Baylor University Mass Spectrometry Core Facility. Melting points were determined on a Thomas Hoover capillary melting point apparatus and are uncorrected. Purity of the compounds was further analyzed at 25 °C using an Agilent Series 1200 high performance liquid chromatography (HPLC) system with a diode-array detector with a wavelength range of 190-400 nm, a Zorbax XDB-C18 HPLC column (4.6 mm × 150 mm, 5 μm) and a Zorbax reliance cartridge guard-column; eluents, solvent A, water; solvent B, acetonitrile; gradient, 90% A/10% B →0% A/100% B over 0 to 10 min; flow rate 0.5 mL/min; injection volume 20 μL; monitored at 254 nm wavelength).
4.1. Chemistry
4.1.1. 1-{3-[(tert-Butyldimethylsilyl)oxy]-4-methoxyphenyl}-1-ethanol (4):28
To a solution of 3-[(tert-butyldimethylsilyl)oxy]-4-methoxybenzaldehyde 2 (5.47 g, 20.5 mmol) in Et2O (anhydrous, 25 mL), cooled to 0 °C, MeMgBr (10.3 mL, 3.0 M soln. in Et2O) was added dropwise and stirred under N2. The reaction mixture was allowed to warm to room temperature and monitored for completion by TLC. After 8 h, the reaction mixture was quenched with water (25 mL) and extracted with EtOAc (2 × 100 mL). The organic layer was separated, washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alcohol 4 (3.39 g, 12.0 mmol, 58%) as a colorless liquid.
1H NMR (CDCl3, 500 MHz): δ 6.75 (dd, 1H, J = 8.0 Hz, J = 2.0 Hz, ArH), 6.72 (d, 1H, J = 2.0 Hz, ArH), 6.65 (d, 1H, J = 8.0 Hz, ArH), 4.63 (dq, 1H, J = 6.5 Hz, J = 3.0 Hz, CHOH), 3.63 (s, 3H, OCH3), 1.29 (d, 3H, J = 6.5 Hz, CHCH3), 0.84 (s, 9H, C(CH3)3), 0.00 (s, 6H, Si(CH3)2).
13C NMR (CDCl3, 125 MHz): δ 150.3, 145.0, 138.6, 118.5, 118.3, 112.0, 67.0, 55.6,
25.7, 25.0, 18.5, −4.6.
HRMS (ESI+) m/z: 305.1545, (Calculated for C15H26O3SiNa – 305.1549).
4.1.2. 1-{3-[(tert-Butyldimethylsilyl)oxy]-4-methoxyphenyl}-1-propanol (5)
To a solution of 3-[(tert-butyldimethylsilyl)oxy]-4-methoxybenzaldehyde 2 (5.46 g, 20.5 mmol) in THF (anhydrous, 25 mL) cooled to 0 °C, EtMgBr (10.5 mL, 2.8 M soln. in Et2O) was added dropwise and stirred under N2. The reaction mixture was allowed to warm to room temperature and monitored for completion by TLC. After 8 h, the reaction mixture was quenched with water (25 mL) and extracted with EtOAc (2 × 100 mL). The organic layer was separated, washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alcohol 5 (4.67 g, 15.8 mmol, 77%) as a colorless liquid.
1H NMR (CDCl3, 500 MHz): δ 6.78 (dd, 1H, J = 8.0 Hz, J = 3.0 Hz, ArH), 6.75 (d, 1H, J = 3.0 Hz, ArH), 6.72 (d, 1H, J = 8.0 Hz, ArH), 4.38 (t, 1H, J = 6.5 Hz, CHOH), 3.70 (s, 3H, OCH3), 1.59-1.69 (m, 2H, J = 6.5 Hz, CH2CH3), 0.90 (s, 9H, C(CH3)3), 0.78 (t, 3H, J = 6.5 Hz, CH2CH3), 0.06 (s, 6H, Si(CH3)2).
13C NMR (CDCl3, 125 MHz): δ 150.4, 145.0, 137.3, 119.2, 118.8, 111.9, 75.6, 55.5, 31.7
25.74, 18.5, 10.2, −4.6.
HRMS (ESI+) m/z: 319.1702, (Calculated for C16H28O3SiNa – 319.1705).
4.1.3. 1-{3-[(tert-Butyldimethylsilyl)oxy]-4-methoxyphenyl} benzyl alcohol (6)
To a solution of 3-[(tert-butyldimethylsilyl)oxy]-4-methoxybenzaldehyde 2 (5.35 g, 20.1 mmol ) in Et2O (anhydrous, 25 mL) cooled to 0 °C, PhMgBr (10.8 mL, 2.8 M soln. in Et2O) was added dropwise and stirred under N2. The reaction was left to warm to room temperature and monitored for completion by TLC. After 8 h, the reaction mixture was quenched with water (25 mL) and extracted with EtOAc (2 × 100 mL). The organic layer was separated, washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alcohol 6 (5.48 g, 15.9 mmol, 79%) as a colorless liquid.
1H NMR (CDCl3, 500 MHz): δ 7.21-7.27 (m, 4H, PhH), 7.14-7.17 (m, 1H, PhH), 6.80 (dd, 1H, J = 8.0 Hz, J = 2.0 Hz, ArH), 6.76 (d, 1H, J = 2.0 Hz, ArH), 6.70 (d, 1H, J = 8.0 Hz, ArH), 5.65 (d, 1H, J = 3.0 Hz, CHOH), 3.68 (s, 3H, OCH3), 2.16 (d, 1H, OH), 0.87 (s, 9H, C(CH3)3), 0.02 (s, 6H, Si(CH3)2).
13C NMR (CDCl3, 125 MHz): δ 150.4, 145.0, 144.0, 136.7, 128.4, 127.4, 126.4, 119.9,
119.6, 112.0, 75.7, 55.6, 25.7, 18.5, −4.7.
HRMS (ESI+) m/z: 367.1704, (Calculated for C20H28O3SiNa – 367.1705).
4.1.4. Phenyl-(3,4,5-trimethoxyphenyl)-methanol (9):29
To a solution of 3,4,5-trimethoxybenzaldehyde 3 (6.04 g, 30.8 mmol) in THF (anhydrous, 25 mL) cooled to 0 °C, PhMgBr (16.5 mL, 2.8 M soln. in Et2O) was added dropwise with stirring. The reaction mixture was allowed to warm to room temperature and monitored for completion by TLC. After 8 h, the reaction mixture was quenched with water (50 mL) and extracted with EtOAc (2 × 100 mL). The organic layer was separated, washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alcohol 9 (7.31 g, 26.6 mmol, 87%) as a white solid.
Melting point: 9 (110-112 °C).
1H NMR (CDCl3, 360 MHz): δ 7.27-7.40 (m, 5H, PhH), 6.62 (s, 2H, ArH), 5.78 (s, 1H, CHOH), 3.83 (s, 9H, OCH3), 2.44 (s, 1H, OH).
13C NMR (CDCl3, 90 MHz): δ 153.3, 143.6, 139.4, 137.4, 128.5, 127.7, 126.5, 103.7,
76.4, 60.8, 56.1.
HRMS (ESI+) m/z: 297.1099, (Calculated for C16H18O4Na – 297.1103).
4.1.5. A typical experimental procedure for the oxidation of alcohols 4-9 to ketones 10-15 using PCC
To a solution of the appropriate alcohol in CH2Cl2, at 0 °C, PCC was added in small portions under N2 with vigorous stirring. The reaction was monitored for completion by TLC. After the reaction was completed, water was added. The reaction mixture was extracted with CH2Cl2, washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. Additional details for these syntheses are found in the supplementary information.
4.1.5.1. 1-{3-[(tert-Butyldimethylsilyl)oxy]-4-methoxyphenyl}-propan-1-one (11)
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded ketone 11 (0.57 g, 1.9 mmol, 96%) as a white solid.
Melting point: 11 (50-52 °C).
1H NMR (CDCl3, 360 MHz): δ 7.59 (dd, 1H, J = 8.5 Hz, 2.2 Hz, ArH), 7.48 (d, 1H, J = 2.2 Hz, ArH), 6.86 (d, 1H, J = 8.5 Hz, ArH), 3.86 (s, 3H, OCH3), 2.92 (q, 2H, J = 7.2 Hz, CH2CH3), 1.20 (t, J = 7.2 Hz, CH3CH3), 1.00 (s, 9H, C(CH3)3), 0.16 (s, 6H, Si(CH3)2).
13C NMR (CDCl3, 90 MHz): δ 199.4, 155.1, 144.9, 130.3, 122.9, 120.4, 110.9, 55.5,
31.4, 25.7, 18.4, 8.4, −4.6.
HRMS (ESI+) m/z: 317.1546, (Calculated for C16H26O3SiNa – 317.1549).
4.1.5.2. {3-[(tert-Butyldimethylsilyl)oxy]-4-methoxyphenyl}-phenyl-methanone (12)
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded ketone 12 (1.79 g, 5.23 mmol, 55%) as a pale yellow liquid.
1H NMR (CDCl3, 360 MHz): δ 7.76 (dd, 2H, J = 9.8 Hz, 1.6 Hz, ArH), 7.38-7.49 (m, 5H, PhH), 6.89 (d, 1H, ArH), 3.88 (s, 3H, OCH3), 1.00 (s, 9H, C(CH3)3), 0.18 (s, 6H, Si(CH3)2).
13C NMR (CDCl3, 90 MHz): δ 195.4, 155.1, 144.7, 138.3, 131.8, 130.4, 129.7, 128.1,
125.5, 122.4, 110.7, 55.5, 25.6, 18.4, −4.6.
HRMS (ESI+) m/z: 365.1544, (Calculated for C20H26O3SiNa – 365.1549).
4.1.5.3. 1-(3,4,5-Trimethoxyphenyl)-1-ethanone (13):30
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded ketone 13 (7.56 g, 36.0 mmol, 74%) as a yellow solid.
Melting point: 13 (76-78 °C).
1H NMR (CDCl3, 500 MHz): δ 7.22 (s, 2H, ArH), 3.93 (s, 6H, OCH3), 3.92 (s, 3H, OCH3), 2.59 (s, 3H, CH3).
13C NMR (CDCl3, 125 MHz): δ 196.9, 153.0, 143.0, 132.5, 105.9, 61.0, 56.3, 26.4.
HRMS (ESI+) m/z: 233.0785, (Calculated for C11H14O4Na – 233.0790).
4.1.5.4. 1-(3,4,5-Trimethoxyphenyl)-1-propanone (14):31
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded ketone 14 (8.2 g, 37 mmol, 77%) as a yellow solid.
Melting point: 14 (49-50 °C).
1H NMR (CDCl3, 500 MHz): δ 7.22 (s, 2H, ArH), 3.93 (s, 6H, OCH3), 3.92 (s, 3H, OCH3), 2.98 (q, 2H, J = 7.5 Hz, CH2CH3), 1.23 (t, 3H, J = 7.5 Hz, CH2CH3).
13C NMR (CDCl3, 125 MHz): δ 199.6, 153.0, 142.4, 132.2, 105.5, 60.9, 56.3, 31.6, 8.4.
HRMS (ESI+) m/z: 247.0941, (Calculated for C12H16O4Na – 247.0946).
4.1.5.5. Phenyl-(3,4,5-trimethoxyphenyl)-methanone (15):32
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded ketone 15 (6.42 g, 23.6 mmol, 85%) as a yellow solid.
Melting point: 15 (74-76 °C).
1H NMR (CDCl3, 360 MHz): δ 7.82 (dd, J = 7.4 Hz, 2H, ArH), 7.58 (t, J = 7.4 Hz, 1H, PhH), 7.50 (t, J = 7.4 Hz, 2H, PhH), 7.08 (s, 2H, PhH), 3.95 (s, 3H, OCH3), 3.88 (s, 6H, OCH3).
13C NMR (CDCl3, 90 MHz): δ 195.7, 152.9, 142.2, 137.9, 132.6, 132.2, 129.8, 128.2,
107.9, 61.0, 56.3.
HRMS (ESI+) m/z: 295.0942, (Calculated for C16H16O4Na – 295.0946).
4.1.6. A typical experimental procedure for the McMurry coupling reaction using TiCl4 to form compounds 16-18
To a solution of titanium tetrachloride (1.7 g, 9.2 mmol, 1.0 mL) in anhydrous THF (50 mL) under N2 atmosphere, LiAlH4 (1.0 M soln. in ether) (0.17 g, 4.6 mL) was added dropwise. The solution was heated at reflux for 20 min, at which point a premixed solution of the ketone 15 (0.50 g, 1.8 mmol), and the appropriate ketone 10-12 (1.8 mmol), and 1,8-bis(dimethylamino)naphthalene (0.40 g, 1.8 mmol ) in THF (10 mL) was added dropwise to the reaction mixture. Reflux was continued for an additional 5 h. The reaction mixture was returned to room temperature, and a potassium carbonate solution (20% aqueous) was added dropwise until no further bubble formation was observed. The mixture was filtered, and the filtrate was extracted with Et2O (2 × 25 mL). The organic layer was separated, washed with water followed by brine, dried over anhydrous Na2SO4, and concentrated in vacuo.
4.1.6.1. (E/Z) 2-{[3′-(tert-Butyldimethylsilyl)oxy]-4′-methoxyphenyl}-1-phenyl-1-(3″,4″,5″-trimethoxyphenyl)-prop-1-ene (16)
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alkene 16 (0.40 g, 0.77 mmol, 42%) as a colorless, viscous oil, containing the E and Z isomers. The isomers could not be readily separated at this stage by chromatography and were carried on to the next step as a mixture.
4.1.6.2. (E/Z) 2-{[3′-(tert-Butyldimethylsilyl)oxy]-4′-methoxyphenyl}-1-phenyl-1-(3″,4″,5″-trimethoxyphenyl)-but-1-ene (17)
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alkene 17 (0.64 g, 1.2 mmol, 65%) as a colorless, viscous oil, containing the E and Z isomers. The isomers could not be readily separated at this stage by chromatography and were carried on to the next step as a mixture.
4.1.6.3. (E/Z) 2-{[3′-(tert-Butyldimethylsilyl)oxy]-4′-methoxyphenyl}-1,2-bis-phenyl-1-(3″,4″,5″-trimethoxyphenyl)-ethylene (18)
Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alkene 18 (0.27 g, 0.46 mmol, 25%) as a colorless, viscous oil, containing the E and Z isomers. The isomers could not be readily separated at this stage by chromatography and were carried on to the next step as a mixture.
4.1.7. (E/Z) 1-{[3′-(tert-Butyldimethylsilyl)oxy]-4′-methoxyphenyl}-1-phenyl-2-(3″,4″,5″-trimethoxyphenyl)-prop-1-ene (19)
To a solution of titanium trichloride (1.97 g, 12.8 mmol) in anhydrous THF (50 mL) under a N2 atmosphere, LiAlH4 (0.25 g, 2.5 M, 6.5 mmol, 2.6 mL) was added dropwise. The solution was heated at reflux for 20 min, at which point a premixed solution of ketone 13 (0.385 g, 1.83 mmol), ketone 12 (0.628 g, 1.83 mmol), and 1,8-bis(dimethylamino)naphthalene (0.398g, 1.83 mmol) in THF (10 mL) was added dropwise to the reaction mixture. Reflux was continued for an additional 5 h. The reaction mixture was returned to room temperature, at which point a potassium carbonate solution (20% aqueous) was added dropwise until no further bubble formation was observed. The solution was filtered, and the filtrate was extracted with Et2O (3 × 25 mL). The organic layer was washed with water followed by brine, dried over anhydrous MgSO4, and concentrated in vacuo. Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alkene 19 (0.687 g, 1.32 mmol 72%) as a colorless, viscous oil, containing the E and Z isomers. The isomers could not be readily separated at this stage by chromatography and were carried on to the next step as a mixture.
4.1.8. (E/Z) 1-{[3′-(tert-Butyldimethylsilyl)oxy]-4′-methoxyphenyl}-1-phenyl-2-(3″,4″,5″-trimethoxyphenyl)-but-1-ene (20)
To a solution of titanium trichloride (2.26 g, 14.7 mmol) in anhydrous THF (50 mL) under a N2 atmosphere, LiAlH4 (0.28 g, 2.5 M, 7.5 mmol, 3 mL) was added dropwise. The solution was heated at reflux for 20 min, at which point a premixed solution of ketone 14 (0.537 g, 2.39 mmol), ketone 12 (0.82 g, 2.4 mmol), and 1,8-bis(dimethylamino)naphthalene (0.398 g, 1.83 mmol) in THF (10 mL) was added dropwise to the reaction mixture. Reflux was continued for an additional 5 h. The reaction mixture was returned to room temperature, at which point a potassium carbonate solution (20% aqueous) was added dropwise until no further bubble formation was observed. The solution was filtered, and the filtrate was extracted with Et2O (3 × 25 mL). The organic layer was washed with water followed by brine, dried over anhydrous MgSO4, and concentrated in vacuo. Purification by flash chromatography (silica gel, 20:80, EtOAc:hexanes) afforded alkene 20 (0.868 g, 1.62 mmol 68%) as a colorless, viscous oil, containing the E and Z isomers. The isomers could not be readily separated at this stage by chromatography and were carried on to the next step as a mixture.
4.1.9. A typical experimental procedure for the deprotection of TBS ether derivatives to form compounds 21-30
To a solution of the appropriate alkene 16-20 in CH2Cl2 at 0 °C under N2, tetrabutylammonium fluoride was added slowly. The reaction mixture was stirred for one hour (0 °C to rt). The reaction was quenched with water and extracted with CH2Cl2. The organic layer was separated, washed with brine, dried over MgSO4, and concentrated in vacuo.
4.1.9.1. 2-{3′-Hydroxy-4′-methoxyphenyl}-1-phenyl-1-(3″,4″,5″-trimethoxyphenyl)-prop-1-ene (E = 21, Z = 22)
Purification by flash chromatography (silica gel, 5:95, EtOAc:hexanes) afforded 21 (0.047 g, 0.12 mmol 16%, E-isomer) and 22 (0.130 g, 0.320 mmol, 42%, Z-isomer) as white solids.
Melting point: 21 (159-160 °C), 22 (151-152 °C).
21 E-isomer: 1H NMR (DMSO-d6, 360 MHz): δ 8.72, (s, 1H, OH), 7.1-6.98 (m, 3H, PhH), 6.91 (d, 2H, J = 7.4 Hz, PhH), 6.68 (d, 1H, J = 8.2 Hz, ArH), 6.56 (d, 1H, J = 2.0 Hz, ArH), 6.50 (dd, 1H, J = 8.1 Hz, J = 2.0 Hz, ArH), 6.43 (s, 2H, ArH), 3.71 (s, 6H, OCH3), 3.68 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 1.99 (s, 3H, CH3).
13C NMR (DMSO-d6, 90 MHz): δ 152.7, 146.0, 145.7, 142.7, 139.0, 137.8, 136.1, 136.0,
134.9, 130.0, 127.5, 125.8, 120.0, 116.4, 111.4, 106.7, 60.0, 55.9, 55.4, 23.4.
21 E-isomer: HRMS (EI+) m/z: 406.1787, (Calculated for C25H26O5 – 406.1780).
22 Z-isomer: 1H NMR (DMSO-d6, 360 MHz): δ 8.79 (s, 1H, OH), 7.38 (m, 2H, PhH), 7.28 (m, 1H, PhH), 7.22 (d, 2H, J = 7.2 Hz, PhH), 6.77 (d, 1H, J = 8.0 Hz, ArH), 6.59 (d, 1H, J = 2.0 Hz, ArH), 6.58 (dd, 1H, J = 8.0 Hz, J = 2.0 Hz, ArH), 6.11 (s, 2H, ArH), 3.84 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 3.43 (s, 6H, OCH3), 1.97 (s, 3H, CH3).
13C NMR (DMSO-d6, 90 MHz): δ 151.8, 146.2, 146.0, 142.8, 138.2, 137.7, 136.5, 135.6,
135.0, 129.6, 128.2, 126.7, 119.5, 116.1, 116.0, 111.8, 108.0, 59.9, 55.6, 55.5,
23.3.
Analysis Calculated for C25H26O5 22 Z: C, 73.87, H, 6.45. Found: C, 73.57, H, 6.50. 22 Z-isomer: HRMS (EI+) m/z: 406.1766, (Calculated for C25H26O5 – 406.1780).
4.1.9.2. 2-{3′ -Hydroxy-4′ -methoxyphenyl}-1-phenyl-1-(3″ ,4″ ,5″ -trimethoxyphenyl)-but-1-ene (E = 23, Z = 24)
Purification by flash chromatography (silica gel, 5:95, EtOAc:hexanes) afforded 23 (0.13 g, 0.31 mmol, 26%, E-isomer) and 24 (0.26 g, 0.62 mmol, 52 %, Z-isomer) as white solids.
Melting point: 23 (150-151 °C), 24 (126-127 °C).
23 E-isomer: 1H NMR (DMSO-d6, 360 MHz): δ 8.75 (s, 1H, OH), 6.99-7.10 (m, 3H,
PhH), 6.91-6.94 (m, 2H, PhH), 6.72 (d, 1H, J = 8.6 Hz, ArH), 6.56 (d, 1H, J = 2.2
Hz, ArH), 6.50 (dd, 1H, J = 8.3 Hz, 2.2 Hz, ArH), 6.45 (s, 2H, ArH), 3.74 (s, 6H,
OCH3), 3.70 (s, 3H, OCH3), 3.68 (s, 3H, OCH3), 2.33 (q, 2H, J = 7.2 Hz
CH2CH3), 0.88 (t, 3H, J = 7.2 Hz, CH2CH3).
13C NMR (DMSO-d6, 90 MHz): δ 152.7, 146.0, 145.7, 142.5, 141.2, 138.9, 137.4, 136.1,
133.9, 129.9, 127.5, 125.7, 120.4, 116.6, 111.4, 106.2, 60.0, 55.8, 55.3, 28.9, 13.4.
23 E-isomer: HRMS (EI+) m/z: 420.1940, (Calculated for C26H28O5 – 420.1937).
24 Z-isomer: 1H NMR (DMSO-d6, 360 MHz): δ 8.79 (s, 1H, OH), 7.42-7.37 (m, 2H, PhH), 7.32-7.28 (m, 1H, PhH), 7.23 (d, 2H, J = 6.8 Hz, PhH), 6.79 (d, 1H, J = 7.9 Hz, ArH), 6.57 (d, 1H, J = 1.8 Hz, ArH), 6.56 (dd, 1H, J = 7.9 Hz, 1.8 Hz, ArH), 6.11 (s, 2H, ArH), 3.72 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.44 (s, 6H, OCH3), 2.30 (q, 2H, J = 7.2 Hz, CH2CH3), 0.87 (t, 3H, J = 7.2 Hz, CH2CH3).
13C NMR (DMSO-d6, 90 MHz): δ 151.7, 146.1, 145.9, 142.7, 141.3, 138.0, 137.3, 135.4,
134.4, 128.9, 128.2, 126.6, 119.8, 116.4, 111.7, 107.9, 59.8, 55.45, 55.36, 28.6,
13.3.
Analysis Calculated for C26H28O5 24 Z: C, 74.26, H, 6.71. Found: C, 74.09, H, 6.79.
24 Z-isomer: HRMS (EI+) m/z: 420.1939, (Calculated for C26H28O5 – 420.1937).
4.1.9.3. 2-{3′ -Hydroxy-4′ -methoxyphenyl}-1,2-bis-phenyl-1-(3″ ,4″ ,5″ -trimethoxyphenyl)-ethylene (E = 25, Z = 26)
Purification by flash chromatography (silica gel, 5:95, EtOAc:hexanes) afforded 25 (0.14 g, 0.30 mmol, 65%, E-isomer) and 26 (0.04 g, 0.09 mmol, 20%, Z-isomer) as white solids.
Melting point: 25 (198-199 °C), 26 (184-186 °C).
25 E-isomer: 1H NMR (CDCl3, 500 MHz): δ 7.02-7.16 (m, 10H, PhH), 6.59 (d, 1H, J = 2.0 Hz, ArH), 6.57 (d, 1H, J = 8.4 Hz, ArH), 6.50 (dd, 1H, J = 8.4 Hz, 2.0 Hz, ArH), 6.18 (s, 2H, ArH), 5.37 (s, 1H, OH), 3.81 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.49 (s, 6H, OCH3).
13C NMR (DMSO-d6, 90 MHz): δ 151.9, 146.2, 145.6, 144.0, 143.0, 140.2, 139.2, 138.6,
135.9, 135.6, 130.6, 130.2, 127.7, 126.4, 126.2, 121.9, 117.9, 111.2, 108.5, 59.9,
55.4, 55.2.
Analysis Calculated for C30H28O5 25 E: C, 76.90, H, 6.02. Found: C, 76.55, H, 6.05.
25 E-isomer: HRMS (EI+) m/z 468.1928, (Calculated for C26H28O5 – 468.1937).
26 Z-isomer: 1H NMR (CDCl3, 500 MHz): δ 7.00-7.12 (m, 10H, PhH), 6.62 (d, 1H, J = 2.0 Hz, ArH), 6.61 (d, 1H, J = 8.2 Hz, ArH), 6.54 (dd, 1H, J = 8.3 Hz, 2.1 Hz, ArH), 6.25 (s, 2H, ArH), 5.40 (s, 1H, OH), 3.83 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 3.55 (s, 6H, OCH3).
13C NMR (DMSO-d6, 90 MHz): δ 151.9, 146.3, 145.8, 143.4, 142.8, 140.2, 139.2, 138.6,
136.2, 136.1, 130.7, 130.5, 127.61, 127.56, 126.4, 126.2, 121.4, 117.6, 111.6,
108.4, 59.9, 55.5.
Analysis Calculated for C30H28O5 26 Z: C, 76.90, H, 6.02. Found: C, 76.38, H, 6.07.
26 Z-isomer: HRMS (EI+) m/z 468.1934, (Calculated for C26H28O5 – 468.1937).
4.1.9.4. 1-{3′ -Hydroxy-4′ -methoxyphenyl}-1-phenyl-2-(3″ ,4″ ,5″ - trimethoxyphenyl)-prop-1-ene (E = 27, Z = 28)
Purification by flash chromatography (silica gel, 5:95, EtOAc:hexanes) afforded 27 (0.116 g, 0.29 mmol 22%, E-isomer) and 28 (0.200 g, 0.49 mmol, 37%, Z-isomer) as white solids.
Melting point: 27 (134-135 °C), 28 (167-168 °C).
27 E-isomer: 1H NMR (DMSO-d6, 500 MHz): δ 8.93 (s, 1H, OH), 7.09 (t, 2H, J = 7.0
Hz, PhH), 7.03 (t, 1H, J = 7.2 Hz, PhH), 6.90 (d, 1H, J = 8.2 Hz, ArH), 6.88 (d,
1H, J = 7.3 Hz, PhH), 6.63 (dd, 1H, J = 8.2 Hz, J = 2.0 Hz, ArH), 6.59 (d, 1H, J =
2.0 Hz, ArH), 6.37 (s, 2H, ArH), 3.77 (s, 3H, OCH3), 3.57 (s, 3H, OCH3), 3.51 (s,
6H, OCH3), 2.11 (s, 3H, CH3).
13C NMR (CDCl3, 125 MHz): δ 152.5, 145.3, 145.2, 143.5, 139.2, 139.0, 136.9, 136.4,
134.9, 130.4, 127.5, 125.8, 121.8, 116.3, 110.2, 106.9, 60.9, 55.92, 55.9, 22.8.
Analysis Calculated for C25H26O5 27 E: C, 73.87, H, 6.45. Found: C, 73.78, H, 6.36.
27 E-isomer: HRMS (EI+) m/z: 406.1769, (Calculated for C25H26O5 – 406.1780).
28 Z-isomer: 1H NMR (DMSO-d6, 500 MHz): δ 8.68 (s, 1H, OH), 7.36 (t, 2H, J = 7.4
Hz, PhH), 7.25 (t, 1H, J = 7.4 Hz, PhH), 7.19 (m, 2H, PhH), 6.65 (d, 1H, J = 8.4
Hz, ArH), 6.43 (s, 2H, ArH), 6.34 (d, 1H, J = 2.1 Hz, ArH), 6.29 (dd, 2H, J = 8.3
Hz, 2.2 Hz, ArH), 3.65 (s, 3H, OCH3), 3.60 (s, 3H, OCH3), 3.56 (s, 6H, OCH3),
2.03 (s, 3H, CH3).
13C NMR (CDCl3, 125 MHz): δ 152.5, 144.8, 144.7, 143.5, 139.4, 139.0, 136.8, 136.4,
134.7, 129.8, 128.1, 126.5, 122.5, 116.7, 109.8, 106.7, 60.9, 56.0, 55.8, 22.9.
Analysis Calculated for C25H26O5 28 Z: C, 73.87, H, 6.45. Found: C, 73.44, H, 6.44.
28 Z-isomer: HRMS (EI+) m/z: 406.1763, (Calculated for C25H26O5 – 406.1780).
4.1.9.5. 1-{3′ -Hydroxy-4′ -methoxyphenyl}-1-phenyl-2-(3″ ,4″ ,5″ - trimethoxyphenyl)-but-1-ene (E = 29, Z = 30)
Purification by flash chromatography (silica gel, 5:95, EtOAc:hexanes) afforded 29 (0.130 g, 0.309 mmol, 19%, E-isomer) and 30 (0.262 g, 0.623 mmol, 38%, Z-isomer) as white solids.
Melting point: 29 (140-142 °C), 30 (165-166 °C).
29 E-isomer: 1H NMR (DMSO-d6, 500 MHz): δ 8.95 (s, 1H, OH), 7.07 (t, 2H, J = 7.3 Hz, PhH), 7.01 (t, 1H, J = 7.3 Hz, PhH), 6.90 (d, 1H, J = 8.3 Hz, ArH), 6.86 (d, 2H, J = 7.1 Hz, ArH), 6.63 (dd, 1H, J = 8.2 Hz, 2.0 Hz, ArH), 6.59 (d, 1H, J = 2.0 Hz, ArH), 6.34 (s, 2H, ArH), 3.77 (s, 3H, OCH3), 3.58 (s, 3H, OCH3), 3.52 (s, 6H, OCH3), 2.48 (q, 2H, J = 7.4 Hz, CH2CH3), 0.92 (t, 3H, J = 7.4 Hz, CH2CH3).
13C NMR (CDCl3, 125MHz): δ 152.5, 145.3, 145.2, 143.5, 141.6, 138.4, 137.4, 136.9,
136.4, 130.3, 127.4, 125.7, 121.1, 115.8, 110.2, 107.2, 60.9, 56.0, 55.9, 28.5, 13.8.
Analysis Calculated for C26H28O5 29 E: C, 74.26, H, 6.71, O, 19.02. Found: C, 74.14, H, 6.53, O, 18.85.
29 E-isomer: HRMS (EI+) m/z 420.1933, (Calculated for C26H28O5 – 420.1937).
30 Z-isomer: 1H NMR (DMSO-d6, 500 MHz): δ 8.65 (s, 1H, OH), 7.36 (t, 2H, J = 7.5 Hz, PhH), 7.26 (m, 1H, J = 7.5 Hz, PhH), 7.18 (d, 2H, J = 7.0 Hz, ArH), 6.62 (d, 1H, J = 8.4 Hz, ArH), 6.40 (s, 2H, ArH), 6.32 (d, 1H, J = 2.1 Hz, ArH), 6.27 (dd, 1H, J = 8.3 Hz, 2.1 Hz, ArH), 3.63 (s, 3H, OCH3), 3.61 (s, 3H, OCH3), 3.57 (s, 6H, OCH3), 2.37 (q, 2H, J = 7.4 Hz, CH2CH3), 0.89 (t, 3H, J = 7.4 Hz, CH2CH3).
13C NMR (CDCl3, 125MHz): δ 152.6, 144.66, 144.65, 143.5, 141.3, 138.4, 137.6, 136.8,
136.4, 129.3, 128.1, 126.5, 122.4, 116.6, 109.7, 107.0, 60.9, 56.0, 55.8, 28.6, 13.7.
Analysis Calculated for C26H28O5 30 Z: C, 74.26, H, 6.71. Found: C, 74.34, H, 6.67.
30 Z-isomer: HRMS (EI+) m/z 420.1936, (Calculated for C26H28O5 – 420.1937).
4.2. Biology
4.2.1. Effects on tubulin polymerization
Bovine brain tubulin was purified as described previously.33 To evaluate the effect of the compounds on tubulin assembly in vitro, varying concentrations were preincubated with 10 μM tubulin (1.0 μg/mL) in glutamate buffer at 30 °C and then cooled to 0 °C. After the addition of GTP, the mixtures were transferred to 0 °C cuvettes in a recording spectrophotometer and warmed to 30 °C. The assembly of tubulin was observed turbidimetrically.34 The IC50 was defined as the compound concentration that inhibited the extent of assembly by 50% after a 20 min incubation.
4.2.2. Cell lines
All cell lines were maintained and grown on 60 cm2 dishes at 37 °C in a humidified atmosphere containing 5% CO2. The DU-145 prostate cancer and the SK-OV-3 ovarian cancer cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) cell culture medium (Biowhittaker®, Cat# 12-614F) containing final concentrations of the following ingredients: 10% fetal bovine serum (Gibco One Shot™, Cat# 16000-077), 2 mM L-glutamine (Glutamax®, Gibco, Catalog# 35050-061), 100 IU/mL penicillin, and 100 μg/mL streptomycin. The NCI-H460 lung cancer cell line was cultured in RPMI-1640 culture medium (ATCC®, Cat# 30-2001) containing 5% fetal calf serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin.
During the SRB assay, cells were plated in media containing the same serum, glutamine, and penicillin/streptomycin concentrations as described above and allowed to grow for 24 h before addition of compounds to be assayed. Compounds to be assayed were added as serial dilutions in media appropriate to the cell line, containing 5% fetal bovine serum, 2 mM L-glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin.
4.2.3. SRB assay (cell growth inhibition assay)
Inhibition of human cancer growth was assessed using the National Cancer Institute’s standard SRB assay, as previously described.35 Briefly, cells were distributed into 96-well plates (Costar®, Corning Inc., New York) in 100 μL of medium at a final concentration of 1 × 104 cells/well and incubated for 24 h, followed by treatment with study compounds and doxorubicin as a control at concentrations between 0.000005 and 50.0 μg/mL at 37 °C for 48 h. A growth inhibition of 50% in comparison to untreated controls (GI50 or the drug concentration causing a 50% reduction in net protein increase) was calculated by nonlinear regression analysis.
Supplementary Material
Acknowledgements
The authors are grateful to the Welch Foundation (grant no. AA-1278 to K.G.P.), and the Department of Chemistry and Biochemistry at Baylor University for their generous financial support of this project, and to the National Science Foundation for funding both the Varian 500 MHz NMR spectrometer (Award CHE-0420802), and the Bruker X8 APEX diffractometer (grant CHE-0321214). The authors express their appreciation to Dr. Kevin Klausmeyer and Mr. Cody Carson, Department of Chemistry and Biochemistry, Baylor University, for assisting with structural analysis of selected compounds. The authors would also like to thank Dr. James Karban and Dr. Alejandro Ramirez (Mass Spectrometry Core Facility, Baylor University) for mass spectra analyses and Dr. James Karban and Dr. Michelle Nemec (Director) of the Molecular Biosciences Center at Baylor University for use of these facilities. The authors are also grateful to Professor George R. Pettit (Arizona State University) for providing initial cell line data for some of the described compounds (data not included in this manuscript).
Footnotes
Supplementary data Details regarding structural characterization of final compounds (21-30) including 1H NMR, 13C NMR, 2D-NOESY, and HRMS spectra along with the thermal ellipsoid plots at 50% probability for compounds 23 and 27 have been made available. Supplementary data associated with this article can be found, in the online version, at (to be filled in once DOI is available).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1(a).Pinney KG, Jelinek C, Edvardsen K, Chaplin DJ, Pettit GR. In: Antitumor Agents from Natural Products. Kingston D, Newman D, Cragg G, editors. CRC Press; Boca Raton, FL: 2005. pp. 23–46. [Google Scholar]; (b) Pinney KG. In: Vascular-targeted Therapies in Oncology. Siemann DW, editor. John Wiley & Sons; London, UK: 2006. pp. 95–121. [Google Scholar]; (c) Pettit GR, Rhodes MR, Herald DL, Hamel E, Schmidt JM, Pettit RK. J. Med. Chem. Vol. 48. 2005. p. 4087. [DOI] [PubMed] [Google Scholar]
- 2(a).Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E. Mol. Pharmacol. 1988;34:200. [PubMed] [Google Scholar]; (b) Lin CM, Ho HH, Pettit GR, Hamel E. Biochemistry. 1989;28:6984. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 3.Kalach J-J, Joly-Pharaboz M-O, Chantepie J, Nicolas B, Descotes F, Mauduit C, Benahmed M, Andre J. J. Steroid Biochem. Mol. Biol. 2005;96:119. doi: 10.1016/j.jsbmb.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 4(a).Pinney KG, Bounds AD, Dingeman KM, Mocharla VP, Pettit GR, Bai R, Hamel E. Bioorg. Med. Chem. Lett. 1999;9:1081. doi: 10.1016/s0960-894x(99)00143-2. [DOI] [PubMed] [Google Scholar]; (b) Pinney KG, Pettit GR, Mocharla VP, del Pilar Mejia M, Shirali A. WO 9839323, 1998. PCT Int. Appl. 1998;129:245037. Chem. Abstr.; (c) Mullica DF, Pinney KG, Mocharla VP, Dingeman KM, Bounds AD, Sappenfield EL. J. Chem. Cryst. 1998;28:289. [Google Scholar]; (d) Mullica DF, Pinney KG, Dingeman KM, Bounds AD, Sappenfield EL. J. Chem. Cryst. 1996;26:801. [Google Scholar]
- 5(a).Hadimani MB, Kessler RJ, Kautz JA, Ghatak A, Shirali AR, O’Dell H, Garner CM, Pinney KG. Acta Cryst. 2002;C58:330. doi: 10.1107/s0108270102003669. [DOI] [PubMed] [Google Scholar]; (b) Pinney KG, Wang F, Hadimani M, del Pilar Mejia M. US 2007082872 U.S. Pat. Appl. Publ. 2007, U.S. Ser. No. 70,484.
- 6(a).Ghatak A, Dorsey JM, Garner CM, Pinney KG. Tetrahedron Lett. 2003;44:4145. [Google Scholar]; (b) Pinney KG, Mocharla VP, Chen Z, Garner CM, Ghatak A, Hadimani M, Kessler J, Dorsey JM, Edvardsen K, Chaplin DJ, Prezioso J, Ghatak UR. 20040043969 A1 U.S. Patent Appl. Publ. 2004; (c) Sriram M, Hall JJ, Grohmann NC, Strecker TE, Wootton T, Franken A, Trawick ML, Pinney KG. Bioorg. Med. Chem. 2008;16:8161. doi: 10.1016/j.bmc.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 7(a).Jones CD, Suarez T, Massey EH, Black LJ, Tinsley FC. J. Med. Chem. 1979;22:962. doi: 10.1021/jm00194a015. [DOI] [PubMed] [Google Scholar]; (b) Jones CD, Jevnikar MG, Pike AJ, Peters MK, Black LJ, Thompson AR, Falcone JF, Clemens JA, Bryant HU. J. Med. Chem. 1984;27:1057. doi: 10.1021/jm00374a021. [DOI] [PubMed] [Google Scholar]
- 8(a).Bloom HJC, Roe FJC, Mitchley BCV. Cancer. 1967;20:2118. doi: 10.1002/1097-0142(196712)20:12<2118::aid-cncr2820201209>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (b) Camerman N, Chan LYY, Camerman A. J. Med. Chem. 1980;23:941. doi: 10.1021/jm00182a023. [DOI] [PubMed] [Google Scholar]; (c) Sato M, Grese TA, Dodge JA, Bryant H, Turner CH. J. Med. Chem. 1999;42:1. doi: 10.1021/jm980344o. [DOI] [PubMed] [Google Scholar]; (d) Lednicer D, Babcock JC, Lyster SC, Duncan WG. Chem. Ind. (London) 1963:408. [Google Scholar]
- 9.Black LJ, Goode RL. Life Sci. 1980;26:1453. doi: 10.1016/0024-3205(80)90049-1. [DOI] [PubMed] [Google Scholar]
- 10.Harper MJK, Walpole AL. Nature (London) 1966;212:87. doi: 10.1038/212087a0. [DOI] [PubMed] [Google Scholar]
- 11(a).Hua W, Christianson T, Rougeot C, Rochefort H, Clinton GM. J. Steroid Biochem. Mol. Biol. 1995;55:279. doi: 10.1016/0960-0760(95)00187-5. [DOI] [PubMed] [Google Scholar]; (b) Lam L, Hu X, Aktary Z, Andrews DW, Pasdar M. Breast Cancer Res. Treat. 2008 Nov. 11 doi: 10.1007/s10549-008-0231-y. doi: 10.1007/s10549-008-0231-y. [DOI] [PubMed] [Google Scholar]; (c) Tan CK, Chow PK, Findlay M, Wong C, Machin D. J. Gastroenterol. Hepatol. 2000;15:725. doi: 10.1046/j.1440-1746.2000.02235.x. [DOI] [PubMed] [Google Scholar]
- 12.D’Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E. Proc. Natl. Acad. Sci. USA. 1994;91:3964. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13(a).Colletta AA, Benson JR, Baum M. Breast Cancer Res. Treat. 1994;31:5. doi: 10.1007/BF00689672. [DOI] [PubMed] [Google Scholar]; (b) Long X, Fan M, Bigsby RM, Nephew KP. Mol. Cancer Ther. 2008;7:2096. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kuo CC, Liu JF, Shiah HS, Ma LC, Chang JY. Int. J. Cancer. 2007;121:2293. doi: 10.1002/ijc.22927. [DOI] [PubMed] [Google Scholar]
- 14.Mandlekar S, Kong AN. Apoptosis. 2001;6:469. doi: 10.1023/a:1012437607881. [DOI] [PubMed] [Google Scholar]
- 15(a).Guvakova MA, Surmacz E. Cancer Res. 1997;57:2606. [PubMed] [Google Scholar]; (b) Lau KM, LaSpina M, Long J, Ho SM. Cancer Res. 2000;60:3175. [PubMed] [Google Scholar]
- 16(a).O’Brian CA, Liskamp RM, Solomon DH, Weinstein IB. Cancer Res. 1985;45:2462. [PubMed] [Google Scholar]; (b) Brandt S, Heller H, Schuster KD, Grote J. J. Cancer Res. Clin. Oncol. 2005;131:120. doi: 10.1007/s00432-004-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17(a).Schneider MR, Ball H, Schiller CD. J. Med. Chem. 1986;29:1355. doi: 10.1021/jm00158a006. [DOI] [PubMed] [Google Scholar]; (b) Kim SH, Katzenellenbogen JA. Bioorg. Med. Chem. 2000;8:785. doi: 10.1016/s0968-0896(00)00016-x. [DOI] [PubMed] [Google Scholar]
- 18.Siles R, Ackley JF, Hadimani MB, Hall JJ, Mugabe BE, Guddneppanavar R, Monk KA, Chapuis JC, Pettit GR, Chaplin DJ, Edvardsen K, Trawick ML, Garner CM, Pinney KG. J. Nat. Prod. 2008;71:313. doi: 10.1021/np070377j. [DOI] [PubMed] [Google Scholar]
- 19(a).Pettit GR, Toki B, Herald DL, Verdier-Pinard P, Boyd MR, Hamel E, Pettit RK. J. Med. Chem. 1998;41:1688. doi: 10.1021/jm970644q. [DOI] [PubMed] [Google Scholar]; (b) Pettit GR, Grealish MP, Herald DL, Boyd MR, Hamel E, Pettit RK. J. Med. Chem. 2000;43:2731. doi: 10.1021/jm000045a. [DOI] [PubMed] [Google Scholar]
- 20(a).Chaplin DJ, Pettit GR, Hill SA. Anticancer Res. 1999;19:189. [PubMed] [Google Scholar]; (b) Siemann DW, Chaplin DJ, Walicke PA. Expert. Opin. Investig. Drugs. 2009;18:189. doi: 10.1517/13543780802691068. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mooney CJ, Nagaiah G, Fu P, Wasman JK, Cooney MM, Savvides PS, Bokar JA, Dowlati A, Wang D, Agarwala SS, Flick SM, Hartman PH, Ortiz JD, Lavertu PN, Remick SC. Thyroid. 2009;19:233. doi: 10.1089/thy.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Akerley WL, Schabel M, Morrell G, Horvath E, Yu M, Johnsson B, Arbogast K. J. Clin. Oncol. (2007 ASCO Annual Meeting Proceedings) 2007;25:14060. [Google Scholar]; (e) Nathan PD, Judson I, Padhani A, Harris A, Carden CP, Smythe J, Collins D, Leach M, Walicke P, Rustin GJ. J. Clin. Oncol. (2008 ASCO Annual Meeting Proceedings) 2008;26:3550. [Google Scholar]; (f) [accessed July 31, 2009]; www.oxigene.com.
- 21.Cushman M, Katzenellenbogen JA, Lin CM, Hamel E. J. Med. Chem. 1995;38:2041. doi: 10.1021/jm00012a003. [DOI] [PubMed] [Google Scholar]
- 22.Hamel E, Lin CM, Flynn E, D’Amato RJ. Biochemistry. 1996;35:1304. doi: 10.1021/bi951559s. [DOI] [PubMed] [Google Scholar]
- 23.Coe PL, Scriven CE. J. Chem. Soc., Perkin Trans. I. 1986:475. [Google Scholar]
- 24(a).McMurry JE. Chem. Rev. 1989;89:1513. [Google Scholar]; (b) Ishaida A, Mukaiyama T. Chem. Lett. 1976:1127. [Google Scholar]; (c) Mukaiyama T, Sato J, Hanna J. Chem. Lett. 1973:1041. [Google Scholar]
- 25.Crystallographic data for structures 23 (deposition number CCDC-735898) and 27 (deposition number CCDC-734844) reported in this paper have been deposited with the Cambridge Crystallographic Data Centre. Copies of the data can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +41 (0) 1223 336033 or deposit@ccdc.cam.ac.uk).
- 26.These data are from the NCI Cancer Screen Data, Developmental Therapeutics Program, 08/2008. http://dtp.nci.nih.gov/dtpstandard/dwindex/index.jsp.
- 27(a).Tanpure RP, Pinney KG. Abstracts of Papers. 222nd National Meeting of the American Chemical Society; Chicago, IL. August 26-30, 2001; Washington, DC: American Chemical Society; 2001. MEDI-068. (b) Portions of this work have been reported in the following dissertation: Tanpure RP. Ph.D. Dissertation. Baylor University; Waco, TX: 2003. New synthetic methodology for the stereoselective preparation of functionalized conjugated dienes from alkynyl oxirane precursors: synthesis and biological evaluation of functionalized triarylalkenes as tubulin binding agents. (c) Portions of this work have been reported in the following thesis: Harkrider AR. M.S. Thesis. Baylor University; Waco, TX: 1998. Designed Inhibitors of Tubulin polymerization: Tamoxifen-Based Analogs.
- 28.Pinney KG, Wang F, Hadimani MB, del Pilar Mejia M. PCT Int. Appl. 2004:75. CODEN: PIXXD2 WO 2004099139 A1 20041118 CAN 141:424108 AN 2004:996124. [Google Scholar]
- 29(a).Moffett RB, Hanze AR, Seay PH. J. Med. Chem. 1964;7:178. doi: 10.1021/jm00332a013. [DOI] [PubMed] [Google Scholar]; (b) Tuerkmen H, Denizalti S, Oezdemir I, Cetinkaya E, Cetinkaya B. J. Organomet. Chem. 2008;693:425. [Google Scholar]; (c) Kilincarslan R, Yigit M, Ozdemir I, Cetinkaya E, Cetinkaya B. J. Heterocycl. Chem. 2007;44:69. [Google Scholar]
- 30(a).Saxena HO, Faridi U, Srivastava S, Kumar JK, Darokar MP, Luqman S, Chanotiya CS, Krishna V, Negi AS, Khanuja SPS. Bioorg. Med. Chem. Lett. 2008;18:3914. doi: 10.1016/j.bmcl.2008.06.039. [DOI] [PubMed] [Google Scholar]; (b) Zhang G, Zhang Y, Xi T, Peng S. Zhongguo Yaowu Huaxue Zazhi. 2004;14:84. [Google Scholar]; (c) Holt H, Jr., LeBlanc R, Dickson J, Brown T, Maddox JR, Lee M. Heterocycl. Commun. 2005;11:465. [Google Scholar]
- 31(a).Liu J, Bolstad DB, Smith AE, Priestley ND, Wright DL, Anderson AC. Chem. Biol. Drug Des. 2009;73:62. doi: 10.1111/j.1747-0285.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang EC, Wu CH, Chien SC, Chiang WC, Kuo YH. Tetrahedron Lett. 2007;48:7706. [Google Scholar]; (c) Joshi BP, Sharma A, Sinha AK. Can. J. Chem. 2005;83:1826. [Google Scholar]; (d) Sharma A, Joshi BP, Singh NP, Sinha AK. Tetrahedron. 2006;62:847. [Google Scholar]
- 32(a).Klemm LH, Wolbert HJ, Ho BT. J. Org. Chem. 1959;24:952. [Google Scholar]; (b) Zhang Y, Rovis TJACS. 2004. p. 15964. [DOI] [PubMed]; (c) Benington F, Morin RD, Clark LC., Jr. J. Org. Chem. 1960;25:1912. [Google Scholar]
- 33.Hamel E, Lin CM. Biochemistry. 1984;23:4173. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- 34(a).Verdier-Pinard P, Lai JY, Yoo HD, Yu J, Marquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E. Mol. Pharmacol. 1998;53:62. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]; (b) Hamel E. Cell Biochem. Biophys. 2003;38:1. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 35(a).Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolf A. J. Natl. Cancer Inst. 1991;83:757. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]; (b) Vichai V, Kirtikara K. Nat. Protocols. 2006;1:1112. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.