Abstract
The juxtacapsular nucleus of the anterior division of the BNST (jcBNST) receives robust glutamatergic projections from the basolateral nucleus of the amygdala (BLA), the postpiriform transition area, and the insular cortex as well as dopamine (DA) inputs from the midbrain. In turn the jcBNST sends GABAergic projections to the medial division of the central nucleus of the amygdala (CEAm) as well as other brain regions. We recently described a form of long-term potentiation of the intrinsic excitability (LTP-IE) of neurons of the juxtacapsular nucleus of BNST (jcBNST) in response to high-frequency stimulation (HFS) of the stria terminalis that was impaired during protracted withdrawal from alcohol, cocaine, and heroin and in rats chronically treated with corticotropin releasing factor (CRF) intracerebroventricularly. Here we show that DAergic neurotransmission is required for the induction of LTP-IE of jcBNTS neurons through dopamine (DA) D1 receptors. Thus, activation of the central CRF stress system and altered DAergic neurotransmission during protracted withdrawal from alcohol and drugs of abuse may contribute to the disruption of LTP-IE in the jcBNST. Impairment of this form of intrinsic neuronal plasticity in the jcBNST could result in inadequate neuronal integration and reduced inhibition of the CEA, contributing to the negative affective state that characterizes protracted abstinence in post-dependent individuals. These results provide a novel neurobiological target for vulnerability to alcohol and drug dependence.
Keywords: stress, reward, action potential threshold, neuronal integration, temporal precision
Introduction
Neuroadaptive changes in the extended amygdala circuits are believed to be key in the motivation for excessive alcohol and drug intake (Koob et al., 1998). In particular, many motivational aspects of addiction have been localized to the central nucleus of the amygdala and the lateral subdivision of the bed nucleus of the stria terminalis (BNST) (Koob, 2003). The BNST is a collection of about a dozen nuclei that are well positioned to integrate somatomotor, autonomic and affective responses. Multiple reinforcing drugs including nicotine, morphine, ethanol, and cocaine were found to increase dialysate DA in the BNST (Carboni et al., 2000). Additionally, D1-class (D1/D5) DA receptors in the dorsolateral BNST have been implicated in the reinforcing properties of self administered cocaine (Epping-Jordan et al., 1998) and alcohol (Eiler et al., 2003). The BNST has also been shown to be critical in mediating stress-induced reinstatement of cocaine self-administration in rodent models (Shaham et al., 2000). The BNST is also believed to play a central role in the regulation of stress response and in the long-term actions of drugs of abuse (Epping-Jordan et al., 1998; Aston-Jones et al., 1999; Delfs et al., 2000; Georges and Aston-Jones, 2001; Koob, 2003). Extracellular corticotropin-releasing factor (CRF) levels are increased in the BNST during ethanol withdrawal, and such an increase is reduced by subsequent ethanol intake (Olive et al., 2002).
The juxtacapsular nucleus of the BNST (jcBNST) is a small nucleus in the dorsal anterolateral BNST that is bounded laterally by the internal capsule and the caudoputamen complex, ventrally by the anterior commissure, and medially by the oval nucleus of the BNST (McDonald et al., 1999; Shammah-Lagnado and Santiago, 1999; Dong et al., 2001; Larriva-Sahd, 2004). The jcBNST contains gamma-aminobutyric acid (GABA)ergic neurons, lacks glutamatergic neurons (Larriva-Sahd, 2006; Francesconi et al., 2009) and has complex interactions, which are likely to play important roles in the regulation of stress and reward. In particular, the jcBNST has direct projections to the medial part of the central nucleus of the amygdala (CEAm) and can indirectly also influence the CEA through its projections to the basolateral amygdala (BLA and other cell groups that in turn send projections to the CEA (Dong et al., 2000). The CEAm is the major output nucleus of the amygdala (Pitkanen et al., 1997). Thus, changes in the integration properties of jcBNST neurons may contribute to the overall amygdala output and to the persistent emotional dysregulation associated with protracted withdrawal.
Plastic changes of neuronal circuits are believed to underlie learning and memory as well as adaptive and maladaptive changes induced by the individual life experiences (Kandel, 2001; LaBar and Cabeza, 2006; Sigurdsson et al., 2007; Bonci and Borgland, 2009). A growing body of evidence supports that, in addition to synaptic forms of plasticity, plastic changes of the intrinsic membrane properties of neurons are possible and that these forms of plasticity have significant implications for the integrative properties of neurons. Plastic changes of synaptic efficacy and of the membrane properties of neurons can either coexist or occur independently of each other. Recent work from our group has described an activity-dependent modification of the threshold for action potential generation of jcBNST neurons, i.e. a long-term potentiation of their intrinsic excitability (LTP-IE), that was impaired during protracted withdrawal in rats with histories of alcohol dependence, escalated cocaine or heroin self-administration and in rats chronically treated with CRF by intracerebroventricular (ICV) injections (Francesconi et al., 2009). Inhibition of CRF1 receptors restored capacity for LTP-IE in the jcBNST of animals with a history of alcohol dependence (Francesconi et al., 2009). In the present report we also provide evidence that DA D1 receptors contribute to the induction of this LTP-IE. The requirement for the activation of DA D1-class receptors for the induction of LTP-IE in the jcBNST suggests that altered DAergic neurotransmission may contribute to the disruption of this form of intrinsic neuronal plasticity in jcBNST neurons during protracted withdrawal from dependent drug use. The elucidation of the mechanisms behind the changes in the intrinsic neuronal plasticity that are induced by alcohol and drugs of abuse in the jcBNST is likely to have heuristic value for the understanding of the neurobiological bases of drugs of abuse.
The jcBNST at the interface between the stress and the reward systems
The extended amygdala is a neuroanatomical macrostructure stretching from the temporal lobe to the rostral forebrain that comprises several basal forebrain regions sharing similarities in morphology, neurochemistry and connectivity (Alheid and Heimer, 1988; Alheid et al., 1995). The brain regions that compose the extended amygdala include the central and medial nuclei of the amygdala, the BNST, and the cellular corridors that bridge the gap between these structures, dorsally along the path of stria terminalis, and ventrally, in the sublenticular region (Alheid and Heimer, 1988; Alheid et al., 1995). This anatomical system is composed of two major divisions: the medial and the central subdivision that are related to the medial and central amygdaloid nuclei, respectively (Alheid and Heimer, 1988; Alheid et al., 1995).
The medial subdivision of the extended amygdala is composed of the medial BNST, medial nucleus of the amygdala, medial sublenticular extended amygdala, and the medial supracapsular bed nucleus of the stria terminalis (Alheid and Heimer, 1988; Alheid et al., 1995). Afferents to the medial division include the accessory olfactory bulb, anterior olfactory nucleus, agranular insular cortex, and infralimbic cortex, ventral subiculum, and basomedial amygdala. Efferents from the medial division include the ventral striatum, olfactory amygdaloid nuclei and medial hypothalamic nuclei (Alheid and Heimer, 1988; Alheid et al., 1995; Canteras et al., 1995). Neural circuits involving the medial amygdala, which is the main output of the medial subdivision of the extended amygdala, are involved in sexual and maternal behavior and aggression, among other behaviors (Numan, 2007; Caldwell et al., 2008). The central division of the extended amygdala comprises the central nucleus of the amygdala, the lateral subdivision of bed nucleus of the stria terminalis, the lateral sublenticular extended amygdala and the lateral supracapsular bed nucleus of the stria terminalis (Alheid and Heimer, 1988; Alheid et al., 1995). These structures display an overall cytoarchitectural similarity to the central nucleus of the amygdala, are profusely interconnected among each other, and project to the lateral rather than the medial hypothalamus and are interconnected with the ventral tegmental area (VTA) (Alheid et al., 1995) The central subdivision of the extended amygdala receives afferents from insular and prefrontal cortices, the posterior basolateral amygdala, medial part of the ventral pallidum, subparafascicular thalamus, parabrachial area, while it projects to the lateral hypothalamus, VTA and substantia nigra compacta, tegmental pedunculopontine nucleus, and to the locus ceruleus and the nucleus of the solitary tract (Alheid and Heimer, 1988; Alheid et al., 1995; McDonald et al., 1999; Valentino and Van Bockstaele, 2008). The lateral subdivision of the extended amygdala is believed to be a substrate for the integration of the brain arousal and stress systems with hedonic processing systems (Koob et al., 1998; Koob, 2003, 2009).
The BNST has been shown to have an anterior subdivision - further divided into lateral and a medial – and a posterior subdivision that differ in their functional connectivities (Phelix et al., 1992, 1994; Dong et al., 2001; Kozicz, 2001). The lateral anterior subdivision of the BNST is considered to be a part of the central subdivision of the extended amygdala. The lateral BNST has high amounts of DA and noradrenergic (NA) terminals, CRF terminals, CRF cell bodies, NPY terminals, and galanin cell bodies, abundant GABA-ergic neurons, and receives afferents from the prefrontal cortex, insular cortex, central and basolateral nuclei of the amygdala (Allen et al., 1984; Gray and Magnuson, 1992; Phelix et al., 1992, 1994; Dong et al., 2001; Kozicz, 2001).
The jcBNST is unique in the lateral BNST as, unlike the other lateral BNST subregions, it does not receive inputs from the central nucleus of the amygdala (Dong et al., 2000; Larriva-Sahd, 2004), but receives dense gutamatergic projections from the posterior part of the BLA, the amygdalopiriform transition area (APir), and the gustatory and visceral sensory areas in the dysgranular insular region, as well as light projections form the infralimbic cortex (McDonald et al., 1999; Shammah-Lagnado and Santiago, 1999; Dong et al., 2001; Larriva-Sahd, 2004). In the BNST, dopamine transporter (DAT)- immunoreactive fibers are found in the highest concentrations in the jcBNST and in the dorsolateral BNST in general (Freedman and Cassell, 1994). Conversely, noradrenergic (NA) inputs from the A1 and A2 cell groups of the caudal medulla are mostly directed to the ventral BNST (Phelix et al., 1992; Freedman and Cassell, 1994; Dumont and Williams, 2004; Egli et al., 2005). Dopamine (DA) inputs to the lateral BNST are primarily form the A10dc DAergic neurons in the periaqueductal grey (PAG) and from the VTA (A10), and to a lesser extent from the A10dr group in the dorsal raphe and the retrorubral nucleus (A8) (Hasue and Shammah-Lagnado, 2002). The origin of the DAergic innervation of the lateral BNST is similar to that of the CeA and unlike the one of the shell of the nucleus accumbens in that projections to the latter are predominantly from the VTA (Hasue and Shammah-Lagnado, 2002).
The jcBNST projects strongly to the medial central amygdalar nucleus (CEAm) and the subcommissural zone and caudal anterolateral areas of the BNST, which are involved in visceromotor responses (Dong et al., 2000; Larriva- Sahd, 2006). It also sends dense projections to the sublenticular extended amygdala and the mesencephalic reticular nucleus and retrorubral area (Dong et al., 2000). The jcBNST also provides inputs to the ventromedial striatum and anterior basolateral amygdalar nucleus, which are believed to modulate somatomotor outflow (Dong et al., 2000). Lastly, the jcBNST provides light inputs to the prelimbic, infralimbic subdivisions of the prefrontal cortex, to the posterior basolateral, posterior basomedial, and lateral amygdalar nuclei, to the paraventricular and medial mediodorsal thalamic nuclei; to the subthalamic and parasubthalamic nuclei of the hypothalamus; to the ventrolateral periaqueductal gray, and to the brainstem (Dong et al., 2000). These projections have been suggested to indicate a role for the jcBNST in integrating autonomic responses with somatomotor activity in adaptive behaviors (Dong et al., 2000), as also discussed below.
Plasticity of the intrinsic membrane properties of neurons
Neuronal activity can induce persistent modifications in the way a neuron reacts to subsequent inputs, both by changing synaptic efficacy and/or intrinsic membrane properties. Synaptic plasticity has received greater attention, and it is generally accepted that changes in the strength of synaptic connections underlie memory storage and some of the maladaptive changes in reward processing induced by drugs of abuse (Thomas et al., 2000; Ungless et al., 2001; Melis et al., 2002; Saal et al., 2003; Malenka and Bear, 2004; Dumont et al., 2005). In the BNST, Dumont et al. found that excitatory synaptic transmission was enhanced in the ventral BNST of rats that performed an operant task to obtain cocaine or a palatable food but not in rats that received cocaine or food passively, supporting that synaptic plasticity in this area is involved in reward-seeking behaviors (Dumont et al., 2005). Additionally, chronic morphine treatment selectively increased AMPA-dependent excitatory postsynaptic currents evoked in ventral BNST neurons projecting to the VTA by stimulation of dorsolateral input likely conveying neocortical afferences (Dumont et al., 2008). Winder and associates showed that acutely bath applied ethanol reduced the potentiation of synaptic responses in the dorsal anterolateral BNST (Weitlauf et al., 2004).
However, the existence of non-synaptic forms of plasticity has been suggested from the very first description of synaptic LTP (i.e., LTP of EPSP) by Bliss and Lomo (1973). In fact, they observed that the increased probability of firing that followed the induction of LTP was not fully explained by the enhancement of EPSP magnitude (Bliss and Lomo, 1973). This phenomenon was later described as EPSP-to-Spike potentiation (E-S potentiation) (Andersen et al., 1980). Subsequent studies observed that plastic increases in excitability can occur even in the absence of EPSP potentiation (e.g., (Jester et al., 1995).
Other examples of plasticity of the action potential thresholds have been described that can be induced by either high-frequency stimulation (HFS) (Chavez-Noriega et al., 1990; Armano et al., 2000; Aizenman and Linden, 2000) or post-synaptic depolarization (Cudmore and Turrigiano, 2004; Aizenman and Linden, 2000).
The intrinsic membrane properties of dendrites, somas and action potential trigger zones are key in the coupling of synaptic inputs into activity output and can all be plastically modified. For instance, plastic changes in dendritic K+ currents such as the transient A-type outward current (IA) and the hyperpolarization activated current (Ih) can affect the amplitude EPSP and their spatial and temporal summation as well as the back-propagation of action potentials into dendrites (Wang et al., 2003; Frick et al., 2004; Chen et al., 2006; Kim et al., 2007). Channels that underlie the non-inactivating M-type K+ current (IM) have also been shown to undergo plastic changes altered firing patterns (Brown and Randall, 2009). Currents mediating spike after-potentials - such as small conductance (SK) Ca2+-activated K+ channels have also been shown to undergo plastic changes that result in altered firing patterns (Sourdet et al., 2003; Lin et al., 2008). Mounting evidence support that the intrinsic membrane properties of neurons are also targets of drugs of abuse. For instance the IA has been shown to be regulated by cannabinoids (Tang et al., 2005); the Ih has been shown to be altered in the VTA after chronic alcohol treatment (Hopf et al., 2007); SK and large conductance (BK) Ca2+-activated K+ channels have been shown to contribute to altered neuronal excitability following chronic ethanol consumption (Mulholland et al., 2009). Plastic modifications of the threshold for action potential generation can result in long-lasting changes in the likelihood of firing (Ganguly et al., 2000; Xu et al., 2005). As it will be discussed in the next section, LTP-IE in the jcBNST is characterized by a shift of the threshold for action potential generation and a reduction of the jitter, i.e. the temporal dispersion of the spike latencies, mediated by changes in the slow inactivating transient D-type K+ current (ID) (Shen et al., 2004).
Together these examples indicate that, in addition to synaptic forms of plasticity, multiple plastic changes of the membrane properties of neurons are possible and these forms of plasticity can have significant implications for the integration properties of neurons and neuroadaptations induced by alcohol and drug of abuse.
Long term potentiation of the intrinsic excitability (LTP-IE) of jcBNST neurons after stimulation of the stria terminalis
As discussed above, the jcBNST is a subdivision of the lateral BNST that receives a robust glutamatergic input through the stria terminalis (Dong et al., 2001; Larriva-Sahd, 2004). Therefore, a glutamatergic field potential can be readily recorded in acute brain slices of the jcBNST in vitro following stimulation of the stria terminalis (Francesconi et al., 2009), in agreement with earlier field recordings from the lateral BNST in brain slices (Sawada et al., 1980; Weitlauf et al., 2004) and in vivo with stimulation of the BLA (Adamec, 1989). In the jcBNST, such a field potential was characterized by two fast negative components (Francesconi et al., 2009). Application of the α-hydroxy-5-methyl-4-isoazolepropionic acid (AMPA) receptor inhibitor CNQX abolished the second, but not the first, negative component of the field potential. This suggests that the first negative component of the field potential represents the presynaptic volley, while the second component originates post-synaptically, as also noted by others in field recordings form the dorsal anterolateral BNST (Weitlauf et al., 2004). We interpret the second negative component of the evoked field potentials as primarily the manifestation of the population spike - i.e. the summation of synaptically-driven action potentials of jcBNST neurons – like in other neuronal populations characterized by an irregular neuronal and dendritic organization (Misgeld et al., 1979; Zhou and Poon, 2000; Walcott and Langdon, 2002). In fact, in the jcBNST, synaptic currents enter post-synaptic neurons via dendrites arrayed radially or on opposite sides of neurons (Larriva-Sahd, 2004; Francesconi et al., 2009). Such an arrangement of inputs does not favor the separation of charges into a layered dipole and the generation of a large net flow of macroscopic currents resulting in a discernable field EPSP by extracellular recordings.
Consistent with this interpretation, we observed that delivery of a specific pattern of high-frequency stimulation (HFS) of the stria terminalis in BNST slices in vitro induced a protracted enhancement of the field potential amplitude that was accompanied by only a transient potentiation of EPSPs in jcBNST neurons but was associated with a protracted decrease of the firing threshold and increased firing probability and temporal fidelity of firing (Francesconi et al., 2009). This was reminiscent of examples of activity-dependent plasticity of neuronal excitability (E-S potentiation) occurring without concomitant EPSP potentiation that can be elicited in the hippocampal CA1 region (e.g., (Jester et al., 1995)). However, the LTP-IE in the jcBNST differed from some of the early examples of E-S potentiation as it was not affected by changes in the inhibitory inputs (Abraham et al., 1987). Three functional types of jcBNST neurons can be recognized on the basis of their rectification properties and rebound depolarization in response to hyperpolarizing current injections (Egli and Winder, 2003; Hammack et al., 2007). HFS induced a significant shift toward hyperpolarization of the threshold for action potential generation in all the three functional types of neurons (Francesconi et al., 2009).
LTP-IE in jcBNST neurons was found to be due to the inhibition of postsynaptic D-type K+ current (ID), a slow inactivating transient current sensitive to 4- aminopyridine (4-AP) and α-dendrotoxin (α-DTX) (Shen et al., 2004). In fact, a brief application of micromolar 4-AP induced a similar protracted shift of the threshold for action potential generation toward hyperpolarization and increased temporal fidelity of firing as HFS of the stria terminalis even in the presence of inhibitors of synaptic transmission (Francesconi et al., 2009). Additionally, previous application of 4-AP occluded the effects of HFS (Francesconi et al, 2009 and unpublished). Together these results indicate that down-regulation of post-synaptic ID is required for the expression LTP-IE in the jcBNST. Long-lasting changes in the ID have also been observed in the prefrontal cortex during protracted abstinence from cocaine administration (Dong et al., 2005). The ID has also been implicated in the plastic regulation of the threshold of BLA neurons (Kroner et al., 2005). Other mechanisms for plastic changes of the action potential threshold e.g. regulation of sodium channel properties, have been found in other cell types (Ganguly et al., 2000; Xu et al., 2005).
Modification of intrinsic membrane properties in addition to altering the relationship between synaptic inputs and the probability of firing may also alter the temporal structure of the neuronal discharge. In fact the ID was first identified by Storm in hippocampal CA1 pyramidal neurons by its ability to delay firing action potentials (Storm, 1988). In the jcBNST, in addition to greater probability of firing, LTP-IE in the jcBNST was also associated with reduced variability of the latency to the first spike or “jitter” - a measure of the dispersion of firing. Similarly to our observation in jcBNST neurons (Francesconi et al., 2009), inhibition of Kv1.2 channels – the main mediators of the ID - has been found to decrease both the threshold and the latency for action potential in striatal medium spiny neurons (MSN) (Nisenbaum et al., 1994).
In other examples, Pouille and Scanziani (2001) have shown that feed-forward inhibition regulates jitter of the first spike by regulating the duration of EPSP in hippocampal pyramidal cells: greater feed-forward inhibition resulted in briefer EPSPs and reduced jitter (Pouille and Scanziani, 2001). Similarly, the temporal precision of neocortical GABAergic fast-spiking interneurons is regulated by inhibitory autapses (i.e., self-synapses) that reduce the jitter during a train of multiple action potential (Bacci and Huguenard, 2006). Thus, inhibitory neurotransmisison – both synaptic or autaptic – can be the source of increased temporal precision. The increased temporal precision that characterizes LTP-IE in the jcBNST appears to differ from these examples as, in the jcBNST, the reduction of jitter was seen also if action potentials were elicited by neuronal depolarization after blocking GABAergic and glutamatergic synaptic transmission (Francesconi et al., 2009) and therefore is intrinsic in nature. In jcBNST neurons, the reduced jitter may relate to the increase in the slope of the prepotential, i.e., the slow depolarization that precedes the fast action potential upstroke, which was seen to be steeper after either HFS or application of 4-AP (Francesconi et al., 2009). Thus, the dynamic threshold mechanism itself might enable the increased temporal fidelity that characterizes LTP-IE in the jcBNST as noted by Azouz and Gray in cortical neurons where rapid membrane depolarizations are also associated with lower thresholds and shorter latencies (Azouz and Gray, 2000). A plastic change of action potential threshold associated with a reduced latency and increased in the slope of the prepotential was also observed in a form of LTP-IE in layer V visual cortex (Cudmore and Turrigiano, 2004).
A plasticity of the temporal precision of firing was shown in layer V pyramidal neurons of the sensorimotor cortex, but, unlike jcBNST LTP-IE that is characterized by reduced jitter of the first spike, it involved subsequent action potentials (Sourdet et al., 2003). In those neurons, Sourdet et al. demonstrated a downregulation of SK channels and reduced IAHP that accelerated the rate of membrane depolarization preceding each action potential and decreased their jitter (Sourdet et al., 2003). Together, the studies by Sourdet et al. and Francesconi et al. show that plastic changes in the temporal precision of firing can result from changes in specific currents.
The reduced threshold for action potential generation and enhanced temporal fidelity of the spike induced by neuronal activity in jcBNST is likely to play a key role for neuronal coding as increased temporal fidelity of neuronal firing translates into more efficient use of the capacity of neural connections (Singer, 1999). In fact, an increasing synchrony is expected to strengthen the impact of the synchronously firing neurons onto common target cells if these target cells integrate their inputs over small time scales (Fries, 2005).
Multiple drugs of abuse induced a common disruption of potentiation of the field potential in the jcBNST after delivery of HFS to the stria terminalis during protracted withdrawal. We used a validated animal model of escalated dependent alcohol intake induced by exposure to alcohol vapors (Roberts et al., 2000; O'Dell et al., 2004). LTP-IE in the jcBNST, in alcohol dependent rats, was significantly impaired at the late time point tested during protracted withdrawal (4-6 weeks) (Francesconi et al., 2009). A partial impairment of LTP-IE was observed at an earlier time point during protracted withdrawal (1-2 weeks). However, during acute withdrawal (3-6 h) LTP-IE in dependent rats was not significantly different from controls. Conversely, LTP-IE in the jcBNST of non-dependent rats was partially impaired at the earlier time point during protracted withdrawal (1-2 weeks) but recovered by the late time point of 4-6 weeks after withdrawal. Thus alcohol dependence induces a protracted impairment of the capacity for plasticity in the jcBNST, while a transient impairment is seen after a history of non-dependent alcohol intake.
We also investigated the induction of LTP-IE in rats with or without extended access to cocaine or heroin self-administration sufficient to produce dependence (Koob et al., 2004; Ahmed et al., 2005; Chen et al., 2006a). Rats self-administering cocaine for 1h per day (ShA) show stable cocaine intake over time (Koob et al., 2004). Conversely, extended access to cocaine self-administration for 6h per day (LgA) induce an increase in drug intake, previously correlated with a persistent decrease in brain reward function during withdrawal (Koob et al., 2004). A strong impairment of LTP-IE was observed in the jcBNST of LgA rats 1-2 weeks after cessation of cocaine self-administration, while ShA rats showed only a partial impairment of LTP-IE at the same time point (Francesconi et al., 2009). Similar results were observed in rats with a history of heroin self-administration under LgA (23h per day) or ShA (1h per day) conditions (Francesconi et al., 2009). Thus, multiple drugs of abuse induced a common disruption of LTP-IE in the jcBNST. Such impairment was graded and was more pronounced and in rats that self-administered amounts of the drugs sufficient to maintain dependence.
D1-class DA receptors activation is required for LTP-IE of the jcBNST
Interaction of glutamate and DA neurotransmission is key to the effects of drugs of abuse. Dysregulations of these neurotransmitter systems brought about by excessive drug intake that has been implicated in drug dependence and addiction (Koob et al., 1998; Kalivas and Volkow, 2005). Hippocampal synaptic plasticity requires both NMDA and D1 DA activation (Huang and Kandel, 1995; Kerr and Wickens, 2001; Li et al., 2003; Navakkode et al., 2007). In the nucleus accumbens activation of DA D1 receptors increases AMPA receptor membrane insertion (Gao et al., 2006; Sun et al., 2008). Mounting evidence supports that NMDA receptors can directly interact with D1 DA receptors. Such interactions may involve effects on signal transduction (Cepeda et al., 1993), receptor trafficking (Dunah and Standaert, 2001; Scott et al., 2002; Gao et al., 2006), and direct protein-protein interactions (Lee et al., 2002; Fiorentini et al., 2003). Additionally, DA D1 receptors interact with mGluR5 to induce LTD in the prefrontal cortex (Otani et al., 1999) and LTP in the nucleus accumbens (Schotanus and Chergui, 2008). Therefore, as LTP-IE in the jcBNST required NMDA and mGluR5 receptors (Francesconi et al., 2009), we investigated the role of DAergic neurotransmission in its induction.
To this end, the D1-receptor antagonist SCH23390 was applied to the perfusion bath during delivery of HFS to the stria terminalis. We observed that SCH23390 prevented the induction of LTP-IE (Fig. 1). This effect was D1 specific as application of the D2 receptor blocker sulpiride did not affect the induction of LTP-IE (Fig. 1B). While several previous studies showed forms of synaptic plasticity that required the interaction of D1, NMDA and group I mGluR receptors (Otani et al., 1999; Kerr and Wickens, 2001; Huang and Kandel, 2006; Navakkode et al., 2007; Schotanus and Chergui, 2008), the present results show that also a form of plasticity of the intrinsic excitability of neurons can also require the both DA and glutamate receptors for its induction. We then explored if depletion of endogenous catecholamines impaired the induction of LTP-IE in the jcBNST. To this aim, we treated a set of rats with reserpine or vehicle before sacrifice. LTP-IE induction in the jcBNST slices from reserpine-treated rats displayed a decrease of the magnitude of LTP when compared to vehicle-treated rats (Fig. 1C). Bath application of the D1 agonist SKF81297 restored capacity for LTP-IE in catecholamine-depleted slices (Fig. 1D), suggesting that endogenous DA is required for LTP-IE induction. This finding is reminiscent of a previous report showing that DA D-1 receptor activation restores a synaptic form of LTP in DA-depleted neostriatal slices (Kerr and Wickens, 2001). The requirement for D1-receptor activation for the induction of LTP-IE in the jcBNST is also in agreement with reports in other cell types that DA D1 receptors modulate the ID current (Nisenbaum et al., 1998; Dong and White, 2003; Kroner et al., 2005), which, as discussed in the previous section, are key in mediating LTP-IE in the jcBNST.
Fig. 1.
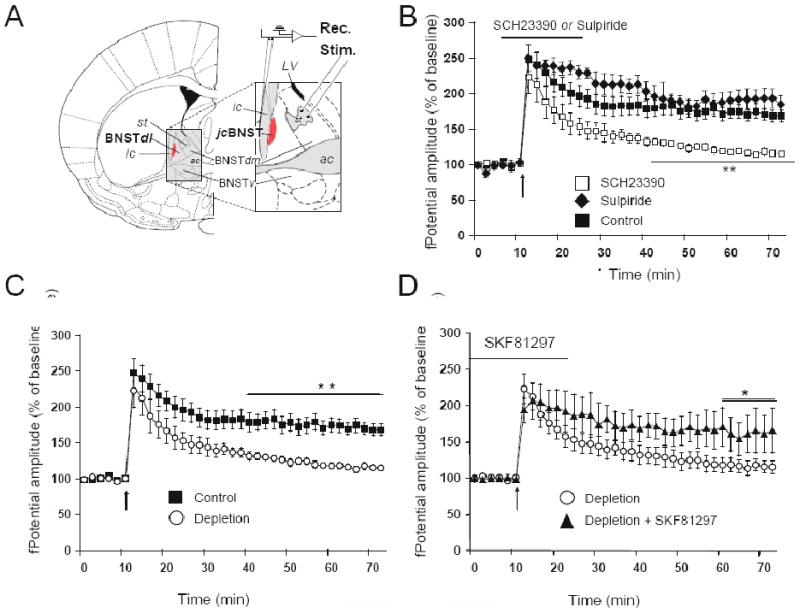
A) Extracellular recordings from the jcBNST in coronal brain slices of Wistar rats. Field potential recordings were carried out from the jcBNST in acute brain slices as previously described (Francesconi et al., 2009) and as shown in Fig. 1A. st: stria terminalis; jcBNST: juxtacapsular bed nucleus of the stria terminalis (shown in yellow); BNSTdl: dorsolateral bed nucleus of the stria terminalis; BNSTdm: dorsomedial bed nucleus of the stria terminalis; BNSTv: ventral bed nucleus of the stria terminalis; ac: anterior commissure; LV: lateral ventricle; ic: internal capsule. Modified from Paxinos and Watson (Paxinos and Watson, 1998). B) The D1-class receptors antagonist SCH23390 impairs LTP-IE in the jcBNST when applied during the induction paradigm. Field potential amplitude of slices that received a 30 min application (horizontal bar) of SCH23390 (2μM; open squares; n= 7) during delivery of HFS to the stria terminalis impairs LTP-IE induction. Field potential amplitude of control slices that received the same tetanization paradigm are shown (n = 7, filled squares). Application of the D2 receptor blocker sulpiride (50μM; n= 5) did not block the induction of LTP-IE (**SCH23390 different from Control and Sulpiride, p<0.01). C) Catecholamine depletion decreases LTP-IE in the jcBNST. Rats were treated with reserpine (5 mg/kg i.p.) 6 h before sacrifice to deplete endogenous catecholamines or vehicle. Comparison of LTP-IE in control (n= 7, filled square) and depleted (n= 4, open circle) jcBNST slices showed a decrease of the magnitude of LTP-IE after catecholamine depletion (**=p<0.01). D) The D1-class agonist SKF81297 substantially restores LTP-IE in catecholamine depleted slices. The D1 agonist SKF81297 (10 μM), applied 15 min before HFS for 30 min, facilitated LTP-IE induction in depleted slices (n=4, filled triangle). LTP-IE in depleted slices is shown for comparison (n=4, open circle) (*=p<0.05-0.01).
The cooperativity of NMDA, mGluR5 and D1 receptor in the induction of LTP-IE is consistent with the anatomical convergence of glutamatergic and DAergic projections in the jcBNST and mounting evidence suggesting extensive functional interactions between DA D1 and glutamatergic NMDA receptors (Scott and Aperia, 2009). In fact, some D1 effects have been shown to be dependent on coincident activation of NMDA receptors (Wu et al., 1993; Adriani et al., 1998; Smith-Roe and Kelley, 2000; David et al., 2004). Additionally, D1-receptor activation potentiates NMDA receptor-induced responses in neostriatal slices (Levine et al., 1996) and D1-receptors and the NMDA NR1 subunit have reciprocal effects on their trafficking between subcellular compartments (Dunah and Standaert, 2001; Scott et al., 2002; Gao et al., 2006).
Since the scientific literature suggests that protracted withdrawal is accompanied by changes in DAergic neurotransmission (Chen et al., 2008; Samuvel et al., 2008; Volkow et al., 2003), it is possible that altered DAergic function may contribute to the impaired LTP-IE in animals with histories of dependence on alcohol, cocaine or heroin (Francesconi et al., 2009). Chronic activation of the CRF system may contribute to a reduced DAergic tone in the jcBNST during protracted withdrawal. Izzo et al. showed that chronic CRF treatment impairs DAergic system function (Izzo et al., 2005) and the central CRF system, which is known to be sensitized during protracted drug withdrawal (Koob and Kreek, 2007), plays a role in the impairment of LTP-IE in the jcBNST (Francesconi et al., 2009). In fact, chronic ICV administration of CRF mimics the impairment of LTP-IE that is seen during protracted withdrawal and LTP-IE can be restored in alcohol post-dependent rats by repeated administration of a CRF1 receptor antagonist (Francesconi et al., 2009). Increased CRF activation could interact with glutamatergic receptors that are required for the induction of LTP-IE, as suggested by studies in other brain regions (Ungless et al., 2003), although the CRF2 receptor was implicated. Additionally, presynaptic CRF receptors may affect neurotransmitter release, as shown in other brain regions (Nie et al., 2004; Wang et al., 2005; Gallagher et al., 2008) as well as in the BNST (Kash et al., 2008).
Together, these observations indicate that activation of both glutamate NMDA and mGluR5 and DA D1 receptor is required for the induction of LTP-IE in the jcBNST and suggest that activation of the central CRF system and altered DAergic neurotransmission contribute to the disruption of this form of intrinsic neuronal plasticity in jcBNST neurons during protracted withdrawal from dependent drug use.
Potential functional significance of plastic changes of jcBNST integration properties
A possible interpretation of the functional significance of plastic changes of jcBNST integration properties is depicted in Fig. 2. BLA and the APir efferents to the BNST course in the stria terminalis and, to a lesser extent, in the ansa peduncularis in the sublenticular region (Shammah-Lagnado and Santiago, 1999; Dong et al., 2001; and Shammah-Lagnado S.J., personal communication). The BLA is activated in humans and experimental animals by exposure to drug related environmental cues that elicit drug-seeking behavior (Ciccocioppo et al., 2001; Bonson et al., 2002; Hayes et al., 2003; Fuchs et al., 2005). The jcBNST also receive robust glutamatergic efference form the dysgranular insular cortex, and light projections from the infralimbic cortex (McDonald et al., 1999; Shammah-Lagnado and Santiago, 1999; Dong et al., 2001; Larriva-Sahd, 2004). Efferents form the insular cortex appear to reach the jcBNST by coursing medially along the temporal limb of the anterior commissure, with virtually no fibers in the stria terminalis (A. McDonald, personal communication). The insular cortex is involved in the perception of internal and visceral sensations that are associated with autonomic motor control and influence motivated behaviors such as drug-seeking both in humans (Gray and Critchley, 2007; Naqvi and Bechara, 2009) and rodents (Contreras et al., 2007; Hollander et al., 2008). The jcBNST also receives neocortical inputs as contingent of fiber collaterals from the internal capsule (Larriva-Sahd, 2004, and personal communication). The PAG DAergic group that innervates the lateral BNST (Hasue and Shammah-Lagnado, 2002), also projects to other neural areas implicated in reinforcing effects of drugs such as the CeA, BNST, sublenticular extended amygdala, and sublenticular extended amygdala (Ottersen, 1981; Grove, 1988; Hasue and Shammah-Lagnado, 2002). Findings form Fernandez-Espejo and associates indicate that these DA neurons are involved in the rewarding effects of heroin as measured by place preference (Flores et al., 2006).
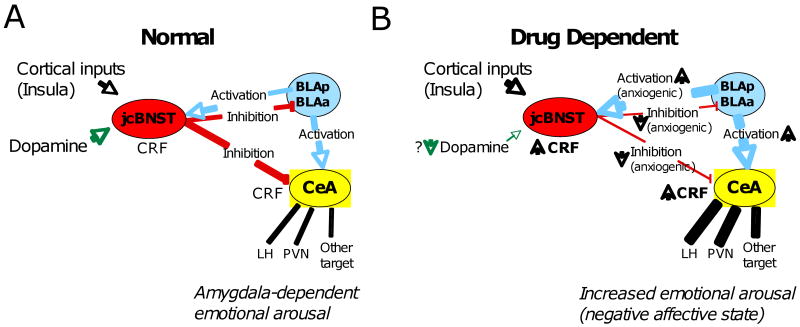
Fig. 2. Loss of jcBNST intrinsic neuronal plasticity could contribute to the negative affective state that characterizes protracted abstinence in postdependent individuals.
A) The jcBNST receives a glutamatergic input from the posterior BLA (BLAp) and the amygdalopiriform transition area (APir) through the stria terminalis, interoceptic sensory information from the insular cortex and dopaminergic afferences from the midbrain. The jcBNST provides GABAergic outputs to the anterior BLA (BLAa) and to the medial central nucleus of the amygdala (CEAm). The activity-dependent plasticity described here could allow the jcBNST to increase its ensemble activity at times of greater stimulation. Outputs of the CEA are key to emotional arousal by acting on targets that include the lateral hypothalamic area (LH) and the paraventricular nucleus (PVN) of the hypothalamus, among others. Arrowheads in the diagram indicate activation, while T bars indicate inhibition. B) Animals with histories of dependent drug use showed impaired plasticity of neuronal excitability and temporal fidelity possibly brought about by increased BLA activation, increase CRF during protracted withdrawal and altered dopaminergic (DA) neurotransmission (see text). Loss of the ability of the jcBNST to plastically increase its output may therefore result in a reduced GABAergic inhibition to the amygdala, which is likely to be anxiogenic. Thus, impaired plasticity of the jcBNST in animals with a history of dependent drug intake could contribute to the negative affective state that is experienced during abstinence in human addicts and that is key to their vulnerability to relapse. An alternative hypothesis is discussed in the text.
As the jcBNST sends a major projection back to the CEAm and anterior BLA, which is likely mostly or exclusively GABA ergic (Sun and Cassell, 1993; Francesconi et al., 2009), LTP-IE in the jcBNST can influence the activity of the CEA both directly and indirectly through its projections to BLA (Dong et al., 2000). Induction of LTP-IE in the jcBNST by stimulation of glutamatergic afference reaching the jcBNST through the stria terminalis (Francesconi et al., 2009) is expected to result in altered jcBNST activity also in response to other glutamatergic inputs to the jcBNST, as this form of plasticity is not likely to show input specificity. Hence, this LTP-IE will affect also the integration of the other glutamateric inputs to the jcBNST that reach the jcBNST independently of the stria terminalis such as the ones from the insular cortex. While it should be noted that convergence of the cortical and stria terminalis inputs on the same jcBNST neurons has not been formally demonstrated, two lines of evidence suggest this to be highly likely. Firstly, all jcBNST neurons tested in our work showed evoked EPSP after stimulation of the stria terminalis (unpublished observations) and, secondly, convergence of stria terminalis and cortical inputs was shown in the ventral BNST (Dumont et al., 2008). Thus the LTP-IE of the jcBNST has the potential to gate other inputs to the CEA in a manner somewhat reminiscent of hippocampal gating of prefrontal cortical throughput in nucleus accumbens neurons by regulating their likelihood of firing through the rhythmic modulation of their action potential threshold (O'Donnell et al., 1999).
Multiple studies suggest that the lateral BNST is involved in anxiety-like responses. In this regard, Walker and Davis showed that lateral BNST infusion of the AMPA-type glutamate receptor antagonist NBQX decreased the late (i.e., sustained) component of fear-potentiated auditory startle, that is akin to anxiety (Walker and Davis, 2008). Additionally, inhibition of GABA synthesis in the BNST elicits anxiety-like behavior (Sajdyk et al., 2008), while application of the anxiolytic benzodiazepine midazolam to the BLA and CEA produce anxiolytic effects (Pesold and Treit, 1995). The small size of the jcBNST makes it difficult to determine its exact contribution to lesion and in vivo pharmacological studies of the lateral BNST. However, consideration of the fact that the jcBNST receives a considerable amount of the BLA glutamatergic projection to the BNST (Dong et al., 2000) and, in turn, sends a robust GABAergic projection to the CEAm (Dong et al., 2000), it seem reasonable to hypothesize that the BLA-jcBNST-CEA pathway may play a significant role in the regulation of anxiogenic responses (Fig. 2A).
Similarly, to the jcBNST, recent studies suggest that another small amygdalar nuclear complex, the intercalated nuclei, are essential for regulating complex behaviors such as fear conditioning and extinction. In fact, as Alexander McDonald suggested in his 1983 paper, the morphology of many of the cells in the jcBNST closely resemble those of the intercalated nuclei, and its position laterally adjacent to the dorsolateral subdivision of the BNST is reminiscent of the relationship of the medial intercalated nuclei to the lateral subdivision of the central nucleus, which is arguably homologous to the dorsolateral subdivision of the BNST (McDonald, 1983 and personal communication).
The ability of jcBNST neurons to increase their ensemble activity through the LTP-IE discussed here could result in a stronger inhibitory control on the output of the CEA at times of greater BLA activation (Fig. 2A). Reduced capacity for LTP-IE in animals with histories of drug dependence could result in inadequate feedback inhibition of the CEA, which may contribute to increased CEAm activity and increased emotional arousal (Fig. 2B). However, an alternative interpretation is that increased temporal precision of jcBNST firing could result in a greater temporally precision of CEAm neurons and greater synchronization of the CEAm neurons that fire action potentials. If this hypothesis is correct, impaired LTP-IE in animals during protracted withdrawal could be a homeostatic event aimed at reducing the precision - and therefore, the efficacy - of CEAm output during protracted withdrawal. Although the present model is admittedly simplified, it is suitable for further experimentation and analysis as it gathers factual anatomical and functional evidences. The elucidation of the mechanisms behind the changes in intrinsic neuronal plasticity induced by alcohol and drugs of abuse in the jcBNST is likely to have heuristic value for the understanding of the neurobiological bases of drugs of abuse.
Materials and Methods
Coronal rat brain slices were obtained from the rostral cerebrum of Wistar rats with a Leica (Wetzlar, Germany) VT1000E at the level shown in Fig. 1A. Brain slices were collected in oxygenated artificial CSF (ACSF) [in mM: 130 NaCl, 3.5 KCl, 24 NaHCO3, 1.25 NaH2PO4, 2.2 CaCl2, 10 glucose, and 2 MgSO4, pH 7.4 (oxygenated by bubbling a mixture of 95% O 2-5%CO 2)] and preincubated for at least 1 hr at room temperature. For recording, slices were transferred to a submerged recording chamber, perfused with oxygenated ACSF, and maintained at 31 ± 1°C. They were further incubated for at least 40 min before the recording session. The flow was maintained at 1.2 ml/min in a chamber volume of 1.5 ml. Field potential recordings were made from jcBNST as indicated in Fig. 1B. Microelectrodes pulled from 1.5 mm outer diameter glass tubing with a Flaming/Brown micropipette puller (Sutter Instruments, Novato, CA) and filled with ACSF (resistance of 3-5 MΩ) were used. A bipolar stimulating electrode was placed in the area of the stria terminalis (Fig. 1B). Field potential recordings were made with an Axoclamp 2B (Axon Instruments, Foster City, CA). Stimulation intensity was adjusted to obtain field potentials approximately one third of the maximum amplitude (pulse duration 80 μsec). An average of five stimulus-evoked responses was collected every 2 min. The acquisition and analysis were performed with the LABVIEW software package (National Instruments, Austin, TX). The boundary of the jcBNST is in good agreement with H. Dong et al. (2000) and was operationally defined by the area where a glutamatergic field potential was readily evoked by stimulation of the stria terminalis. For catecholamine depletion, reserpine (5 mg/kg i.p.) was administered acutely to the rats 6 h before sacrifice. Student's t test (paired or unpaired as appropriate) or analysis of variance (ANOVA) were used to analyze the behavioral and electrophysiological data using StatView (SAS Institute) or Microsoft Excel. ANOVA was followed by Fisher LSD post-hoc analysis. All results are expressed as mean ± SEM. Cut-off p values of <0.05 were considered to be statistically significant.
Table 1. List of abreviations.
| 4-AP | 4-aminopyridine |
| α-DTX | α-dendrotoxin |
| AMPA | α-hydroxy-5-methyl-4-isoazolepropionic acid |
| APir | amygdalopiriform transition area |
| jcBNST | juxtacapsular nucleus of the anterior division of the BNST |
| BLA | basolateral nucleus of the amygdala |
| BNST | bed nucleus of the stria terminalis |
| CEA | central nucleus of the amygdala |
| CEAm | central nucleus of the amygdala, medial division |
| CRF | corticotropin releasing factor |
| DA | dopamine |
| DAT | dopamine transporter |
| EPSP | excitatory postsynaptic potential |
| HFS | high-frequency stimulation |
| GABA | gamma-aminobutyrate |
| IA | A-type K+ current |
| IAHP | Afterhyperpolarization current |
| ID | D-type K+ current |
| Ih | hyperpolarization activated current |
| IM | M-type K+ current |
| ICV | intracerebroventricular |
| LTP-IE | long-term potentiation of the intrinsic excitability |
| NA | noradrenergic |
| NMDA | N-methyl-D-aspartic acid |
| NPY | neuropeptide Y |
| PAG | periaqueductal grey |
| VTA | ventral tegmental area |
Acknowledgments
Supported by grants AA016587 (WF), DA013821, AA013191 (PS), AA008459, DA004043, DA004398, AA006420 (GFK), and by the Pearson Center for Alcoholism and Addiction Research. We thank Dr. Luigi Pulvirenti of TSRI, Dr. Jorge Larriva-Sahd of UNAM, Querétaro, Mexico, Dr. Alexander McDonald of the University of South Carolina, and Dr. Sara Shammah-Lagnado of the University of São Paulo, Brazil for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham W, Gustafsson B, Wigstrom H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J Physiol Paris. 1987;394:367–380. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec R. The relationship between the amygdala and bed nucleus of the stria terminalis in the cat: an evoked potential and single cell study. Behav Neural Biol. 1989;52:295–320. doi: 10.1016/s0163-1047(89)90427-5. [DOI] [PubMed] [Google Scholar]
- Adriani W, Felici A, Sargolini F, Roullet P, Usiello A, Oliverio A, Mele A. Nmethyl- D-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp Brain Res. 1998;123:52–59. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- Aizenman C, Linden D. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neuronsV. Nat Neurosci. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- Alheid G, de Olmos J, CA B. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. 2nd. San Diego: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of the substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Allen YS, Roberts GW, Bloom SR, Crow TJ, Polak JM. Neuropeptide Y in the stria terminalis: evidence for an amygdalofugal projection. Brain Res. 1984;321:357–362. doi: 10.1016/0006-8993(84)90193-8. [DOI] [PubMed] [Google Scholar]
- Andersen P, Sundberg SH, Sveen O, Swann JW, Wigstrom H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol. 1980;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, D'Angelo E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci. 2000;20:5208–5216. doi: 10.1523/JNEUROSCI.20-14-05208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci U S A. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron. 2006;49:119–130. doi: 10.1016/j.neuron.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Borgland S. Role of orexin/hypocretin and CRF in the formation of drugdependent synaptic plasticity in the mesolimbic system. Neuropharmacology. 2009;56 1:107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brown JT, Randall AD. Activity-dependent depression of the spike after depolarization generates long-lasting intrinsic plasticity in hippocampal CA3 pyramidal neurons. J Physiol. 2009 doi: 10.1113/jphysiol.2008.167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Halliwell JV, Bliss TV. A decrease in firing threshold observed after induction of the EPSP-spike (E-S) component of long-term potentiation in rat hippocampal slices. Exp Brain Res. 1990;79:633–641. doi: 10.1007/BF00229331. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Cudmore R, Turrigiano G. Long-term potentiation of intrinsic excitability in LV visual cortical neurons. J Neurophysiol. 2004;92:341–348. doi: 10.1152/jn.01059.2003. [DOI] [PubMed] [Google Scholar]
- David HN, Sissaoui K, Abraini JH. Modulation of the locomotor responses induced by D1-like and D2-like dopamine receptor agonists and Damphetamine by NMDA and non-NMDA glutamate receptor agonists and antagonists in the core of the rat nucleus accumbens. Neuropharmacology. 2004;46:179–191. doi: 10.1016/j.neuropharm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong Y, White FJ. Dopamine D1-class receptors selectively modulate a slowly inactivating potassium current in rat medial prefrontal cortex pyramidal neurons. J Neurosci. 2003;23:2686–2695. doi: 10.1523/JNEUROSCI.23-07-02686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nasif F, Tsui J, Ju W, Cooper D, XT H, Malenka R, White F. Cocaine induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784:105–115. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S, Fernandez-Espejo E. Role for dopamine neurons of the rostral linear nucleus and periaqueductal gray in the rewarding and sensitizing properties of heroin. Neuropsychopharmacology. 2006;31:1475–1488. doi: 10.1038/sj.npp.1300946. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Thurbon D, Lekic D, Greenwell T, Specio S, Zorrilla EP, Richardson H, Chen S, O'Dell L, Morales M, Koob G, Sanna P. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosc. 2009 doi: 10.1523/JNEUROSCI.5129-08.2009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Kiss L, Poo M. Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat Neurosci. 2000;3:1018–1026. doi: 10.1038/79838. [DOI] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Grove EA. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988;277:315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine seeking behavior in the rat. Psychopharmacology (Berl) 2003;168:75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Age-related enhancement of a protein synthesis dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus. Learn Mem. 2006;13:298–306. doi: 10.1101/lm.166906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo E, Sanna P, Koob G. Impairment of dopaminergic system function after chronic treatment with corticotropinreleasing factor. Pharmacology Biochemistry and Behavior. 2005 doi: 10.1016/j.pbb.2005.04.017. in press. [DOI] [PubMed] [Google Scholar]
- Jester JM, Campbell LW, Sejnowski TJ. Associative EPSP--spike potentiation induced by pairing orthodromic and antidromic stimulation in rat hippocampal slices. J Physiol. 1995;484(Pt 3):689–705. doi: 10.1113/jphysiol.1995.sp020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1- dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Kozicz T. Axon terminals containing tyrosine hydroxylase- and dopamine-betahydroxylase immunoreactivity form synapses with galanin immunoreactive neurons in the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. 2001;914:23–33. doi: 10.1016/s0006-8993(01)02770-6. [DOI] [PubMed] [Google Scholar]
- Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. Juxtacapsular nucleus of the stria terminalis of the adult rat: extrinsic inputs, cell types, and neuronal modules: a combined Golgi and electron microscopic study. J Comp Neurol. 2004;475:220–237. doi: 10.1002/cne.20185. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. Histological and cytological study of the bed nuclei of the stria terminalis in adult rat. II. Oval nucleus: extrinsic inputs, cell types, neuropil, and neuronal modules. J Comp Neurol. 2006;497:772–807. doi: 10.1002/cne.21011. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, Drago J, Sibley DR, Westphal H. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R, Bear M. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the bed nucleus of the stria terminalis: a golgi study in the rat. Brain Res Bull. 1983;10:111–120. doi: 10.1016/0361-9230(83)90082-5. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U, Okada Y, Hassler R. Locally evoked potentials in slices of rat neostriatum: a tool for the investigation of intrinsic excitatory processes. Exp Brain Res. 1979;34:575–590. doi: 10.1007/BF00239150. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Hopf FW, Bukiya AN, Martin GE, Liu J, Dopico AM, Bonci A, Treistman SN, Chandler LJ. Sizing up Ethanol-Induced Plasticity: The Role of Small and Large Conductance Calcium-Activated Potassium Channels. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J Neurophysiol. 1994;71:1174–1189. doi: 10.1152/jn.1994.71.3.1174. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Mermelstein PG, Wilson CJ, Surmeier DJ. Selective blockade of a slowly inactivating potassium current in striatal neurons by (+/-) 6-chloro- APB hydrobromide ( SKF82958) Synapse. 1998;29:213–224. doi: 10.1002/(SICI)1098-2396(199807)29:3<213::AID-SYN3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of cell firing in the nucleus accumbens. Ann N Y Acad Sci. 1999;877:157–175. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999;19:9788–9802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–356. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd. San Diego: Academic Press; 1998. [Google Scholar]
- Pesold C, Treit D. The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res. 1995;671:213–221. doi: 10.1016/0006-8993(94)01318-c. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull. 1994;33:109–119. doi: 10.1016/0361-9230(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol. 2008;22:633–641. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. J Pharmacol Exp Ther. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- Sawada S, Takada S, Yamamoto C. Electrical activity recorded from thin sections of the bed nucleus of the stria terminalis, and the effects of neurotensin. Brain Res. 1980;188:578–581. doi: 10.1016/0006-8993(80)90058-x. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Dopamine D1 receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accumbens. Neuropharmacology. 2008;54:837–844. doi: 10.1016/j.neuropharm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Scott L, Aperia A. Interaction between N-methyl-d-aspartic acid receptors and D1 dopamine receptors: An important mechanism for brain plasticity. Neuroscience. 2009;158:62–66. doi: 10.1016/j.neuroscience.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Scott L, Kruse MS, Forssberg H, Brismar H, Greengard P, Aperia A. Selective upregulation of dopamine D1 receptors in dendritic spines by NMDA receptor activation. Proc Natl Acad Sci U S A. 2002;99:1661–1664. doi: 10.1073/pnas.032654599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Santiago AC. Projections of the amygdalopiriform transition area (APir). A PHA-L study in the rat. Ann N Y Acad Sci. 1999;877:655–660. doi: 10.1111/j.1749-6632.1999.tb09295.x. [DOI] [PubMed] [Google Scholar]
- Shen W, Hernandez-Lopez S, Tkatch T, Held JE, Surmeier DJ. Kv1.2-containing K+ channels regulate subthreshold excitability of striatal medium spiny neurons. J Neurophysiol. 2004;91:1337–1349. doi: 10.1152/jn.00414.2003. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci. 2003;23:10238–10248. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol. 2005;94:2983–2986. doi: 10.1152/jn.01187.2004. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci. 2000;20:5581–5586. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott EC, Langdon RB. Synaptically driven spikes and long-term potentiation in neocortical layer 2/3. Neuroscience. 2002;112:815–826. doi: 10.1016/s0306-4522(02)00131-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu NL, Wu CP, Duan S, Poo MM. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron. 2003;37:463–472. doi: 10.1016/s0896-6273(02)01189-3. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Brudzynski SM, Mogenson GJ. Functional interaction of dopamine and glutamate in the nucleus accumbens in the regulation of locomotion. Can J Physiol Pharmacol. 1993;71:407–413. doi: 10.1139/y93-061. [DOI] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Poon CS. Field potential analysis of synaptic transmission in spiking neurons in a sparse and irregular neuronal structure in vitro. J Neurosci Methods. 2000;94:193–203. doi: 10.1016/s0165-0270(99)00144-2. [DOI] [PubMed] [Google Scholar]