Abstract
Emerging evidence indicates that cell surface receptors, such as the entire epidermal growth factor receptor (EGFR) family, have been shown to localize in the nucleus. A retrograde route from the Golgi to the endoplasmic reticulum (ER) is postulated to be involved in the EGFR trafficking to the nucleus; however, the molecular mechanism in this proposed model remains unexplored. Here, we demonstrate that membrane-embedded vesicular trafficking is involved in the nuclear transport of EGFR. Confocal immunofluorescence reveals that in response to EGF, a portion of EGFR redistributes to the Golgi and the ER, where its NH2-terminus resides within the lumen of Golgi/ER and COOH-terminus is exposed to the cytoplasm. Blockage of the Golgi-to-ER retrograde trafficking by brefeldin A or dominant mutants of the small GTPase ADP-ribosylation factor, which both resulted in the disassembly of the coat protein complex I (COPI) coat to the Golgi, inhibit EGFR transport to the ER and the nucleus. We further find that EGF-dependent nuclear transport of EGFR is regulated by retrograde trafficking from the Golgi to the ER involving an association of EGFR with γ-COP, one of the subunits of the COPI coatomer. Our findings experimentally provide a comprehensive pathway that nuclear transport of EGFR is regulated by COPI-mediated vesicular trafficking from the Golgi to the ER, and may serve as a general mechanism in regulating the nuclear transport of other cell surface receptors.
Keywords: nuclear epidermal growth factor receptor, nuclear transport, retrograde trafficking, coat protein complex I, Golgi, endoplasmic reticulum
Introduction
Endocytosis is characterized by membrane and vesicular trafficking along the secretory pathway, which transports budding vesicles from a donor membrane and then fuses them with an acceptor organelle. Luminal and membrane cargo proteins are carried by budding vesicles and sorted into destinations regulated by distinct assemblies of coat proteins [1]. Endosomal trafficking after endocytosis to the biosynthetic/secretory compartments, such as the endoplasmic reticulum (ER) and the Golgi apparatus, known as retrograde transport, is important for diverse cellular functions [2]. Several mammalian cargo proteins and exogenous viruses/toxins are routed from the early endosomes to the Golgi apparatus and the ER, respectively, by retrograde transport [2,3,4].
In eukaryotes, vesicular transport from the Golgi to the ER via a retrograde route is mediated by coat protein complex I (COPI) vesicles, which consist of the small GTPase ADP-ribosylation factor (ARF) and coatomer composed of seven subunits (α, β, β’, γ, δ, ε, and ζ) [5]. COPI coat assembly is initiated by the membrane recruitment and activation of ARF1, with GDP-GTP exchange mediated by ARF-guanine exchange factors [6]. Membrane-bound ARF1 recruits a preassembled coatomer complex, and cargo proteins are then recruited through multiple recognition sites on separate subunits [7]. Ultimately, disassembly from the membrane occurs when ARF-GTPase-activating protein hydrolyzes the GTP on ARF1 [7,8].
Multiple cell surface receptor tyrosine kinases (RTKs), such as insulin-like growth factor 1 receptor (IGF-1R), cMet, fibroblast growth factor receptor (FGFR), vascular endothelial growth factor receptor (VEGFR), and the four members of the epidermal growth factor receptor (EGFR) family, have been reported to localize in the nucleus [9,10,11,12,13,14]. Among these, nuclear EGFR/ErbB-1 has been shown to be involved in transcriptional regulation, cell proliferation, DNA repair, DNA replication, and chemo- and radio-resistance [15,16,17,18,19,20,21]. It has been reported that endocytosis is required for the nuclear translocation of EGFR [16,22,23]. However, after endocytosis, it is unclear which pathway routes EGFR proteins to the nucleus. In this study, we found that EGF-dependent nuclear transport of EGFR is regulated by a retrograde trafficking from the Golgi to the ER involving an association of EGFR with γ-COP.
Materials and Methods
Experimental reagents
The following antibodies and chemicals were purchased for our study: anti-EGFR antibodies (Santa Cruz; NeoMarker); anti-calregulin, anti-calnexin, and anti-γ-COP antibodies (Santa Cruz); anti-tubulin antibodies, mouse IgG, brefeldin A, and recombinant human EGF (Sigma); anti-lamin B antibodies (Calbiochem); and Texas red-labeled EGF (Molecular Probes). siRNA oligonucleotides targeting γ-COP and nonspecific siRNA control were purchased from Drarmacon. The GalNAc-T2-GFP plasmid was a gift from Dr. B. Storrie (University of Arkansas for Medical Sciences). Expression vectors of ARF1/wt, ARF1/T31N, and ARF1/Q71L were provided by Dr. J.S. Bonifacino (National Institutes of Health).
Cell culture and EGF treatment
All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F12 supplemented with 10% fetal bovine serum and antibiotics. In this series of experiments, cells were treated with 50 ng/ml EGF under serum-starved conditions for 24 hr.
Double-labeled assay and triple-labeled assay
Cells maintained in a serum-starved medium for 24 hr were treated with or without EGF for 30 min. For a double-labeled assay, after fixation, the cells were permeabilized with 75 μM digitonin at 4°C for 6 min and incubated with the first primary antibodies. Subsequently, the cells were refixed, permeabilized with 0.5% Triton X-100 for 15 min, and incubated with the second primary antibodies. The third primary antibodies were added for a triple-labeled assay. Immunostained cells were analyzed using confocal microscopy.
Confocal microscopy
Cultured cells were washed three times with PBS, fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100 for 15 min, and incubated with 5% bovine serum albumin for 1 h. Cells were then incubated with the primary antibodies overnight at 4°C. Cells were washed with PBS and then further incubated with the appropriate secondary antibody diluted at 1:500 and tagged with fluorescein isothiocyanate, Texas red, or Alexa 647 (Molecular Probes) for 1 h at room temperature. Nuclei were stained with DAPI contained in the mounting reagent (Invitrogen). Confocal fluorescence images were captured using a Zeiss LSM 710 laser microscope. In all cases, optical sections through the middle planes of the nuclei as determined using nuclear counterstaining were obtained.
Cellular fractionation and ER purification
Non-nuclear and nuclear fractions were prepared as described previously [24]. Purification of the ER was performed using the OptiPrep density gradient medium with a slight modification (Sigma). In brief, cultured cells were harvested and resuspended in a homogenization buffer (10 mM Tris-HCl, pH 7.5, 250 mM sucrose, protease inhibitor cocktail). Cells were homogenized using 20 strokes with a Dounce homogenizer in the same buffer and then centrifuged at 12,000 × g for 20 min at 4°C. The resulting supernatant was further centrifuged at 100,000 × g for 45 min at 4°C. After centrifugation, the supernatant was collected as a non-nuclear/non-microsomal fraction, and the microsomal pellet was resuspended in the homogenizing buffer. The resulting mixture of 6.67 vol. of the microsomal suspension with 3.33 vol. of the OptiPrep density gradient medium was transferred to tubes (1 ml/tube) and centrifuged overnight at 200,000 × g. The ER fractions were then collected.
Results and Discussion
Distribution and orientation of EGFR in the Golgi and ER membrane for EGF response in vivo
Previous studies were postulated that the nuclear transport of EGFR is via a retrograde route from the Golgi to the ER [25,26]. If the nuclear transport of EGFR requires a membrane-bound environment involving the intracellular organelles such as the Golgi/ER, we would expect the COOH- and NH2-terminal domains of EGFR to reside on opposite sides of the membrane of these organelles and the nucleus. To address this issue, we sought to determine the orientation of EGFR in human breast carcinoma MDA-MB-468 cells using a double-labeled assay adapted from established procedures, sequentially permeabilized by digitonin and Triton X-100 [27]. In brief, we fixed cells treated with EGF or left untreated and permeabilized them with digitonin (which left the intracellular organelles and nucleus structurally intact) [28] and then labeled them with the mouse anti-NH2-terminal antibody EGFR(N) (Fig. S1A-1). Subsequently, we refixed the cells, permeabilized them with Triton X-100 to expose the luminal compartment, and incubated them with the rabbit anti-COOH-terminal antibody EGFR(C) to define the localization and expression level of expression of EGFR (Fig. S1A-2). After digitonin permeabilization, we detected EGFR(N) predominantly on the cell surface membrane without EGF treatment (Fig. S1A-1 and S1A-3, upper panels). Interestingly, we could not detect EGFR(N) in the cytoplasmic area after EGF treatment (Fig. S1A-1 and S1A-3, lower panels), suggesting that the NH2-terminus of EGFR is masked inside the intracellular organelles upon EGF treatment. Once intracellular organelle membranes in the same cell were permeabilized by Triton X-100, EGFR(C) was able to stain EGFR inside the cells in response to EGF (Fig. S1A-2 and S1A-4). Furthermore, we detected yellow merged signals representing co-localization of EGFR(N) (red) and EGFR(C) (green) only on cell surfaces, not inside the cells (Fig. S1A-5 to S1A-7), supporting the notion that upon EGF treatment, the NH2-terminus of EGFR is masked inside intracellular organelles such as the Golgi/ER lumen and nuclear membrane. We performed a similar experiment using the reverse order of antibody staining. Specifically, we first stained the cells with EGFR(C) after digitonin treatment (Fig. S1B-1) and then stained them with EGFR(N) after Triton X-100 treatment (Fig. S1B-2). The results showed that EGFR(C) could be detected in the cytoplasmic area, including the area right next to the nuclear membrane, after digitonin treatment (Fig. S1B-1 and S1B-3). The co-localization (yellow) of EGFR(C) (green) and EGFR(N) (red) in the cytoplasm increased significantly after EGF treatment (Fig. S1B-5 to S1B-7), suggesting that the COOH-terminus of EGFR is exposed to the cytoplasm in response to EGF. These results suggested that full-length EGFR anchors to the membranes of intracellular organelles such as the Golgi/ER lumen with the COOH-terminus exposed to the cytoplasm and the NH2-terminus inside intracellular organelles upon EGF treatment.
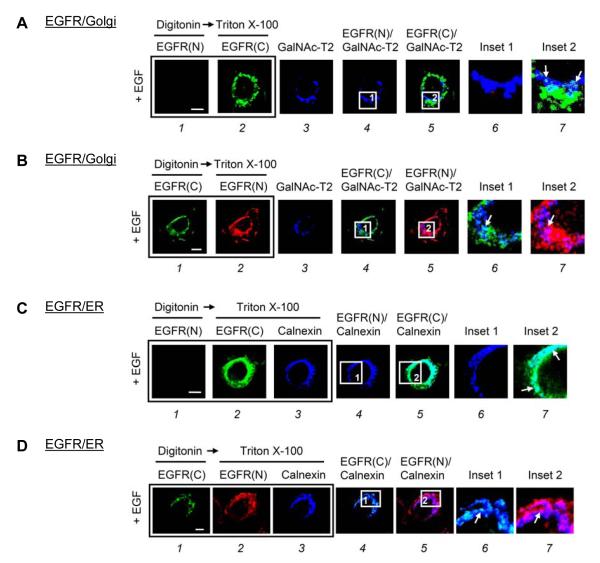
We next performed a triple-labeled assay to further investigate the distribution and orientation of EGFR in the Golgi and the ER. To label the Golgi, we used the Golgi stack enzyme N-acetylgalactosaminyltransferase-2 (GalNAc-T2) fused with the green fluorescent protein (GFP) [29]. After digitonin treatment, EGFR(N) (Fig. 1A-1), which should be located inside the lumen of organelles, did not co-localize with GalNAc-T2 (Fig. 1A-3, blue) for 15 min after EGF treatment (Fig. 1A-4 and 1A-6, still blue). However, once the Golgi membrane of the same cell was permeabilized by Triton X-100 and stained with EGFR(C), we detected the white merged signals (Fig. 1A-5 and 1A-7) representing co-localization of EGFR(C) (Fig. 1A-2, green) and GalNAc-T2 (Fig. 1A-3, blue), suggesting that the NH2-terminus of EGFR is masked inside the Golgi upon EGF treatment. In a similar experiment performed using the reverse order of antibody staining, EGFR(C) (Fig. 1B-1, green), which should be exposed to the cytoplasm, and GalNAc-T2 (Fig. 1B-3, blue) were co-localized as indicated by the white merged signals after digitonin treatment (Fig. 1B-4 and 1B-6), suggesting that the COOH-terminus of EGFR is exposed outside the Golgi upon EGF treatment. In addition, we identified the distribution and orientation of EGFR in the ER using a triple-labeled assay with an antibody against the COOH-terminal cytoplasmic portion of calnexin, an ER membrane-anchored protein. After digitonin treatment, EGFR(N) (Fig. 1C-1) did not co-localize with the cytoplasmic portion of calnexin (Fig. 1C-3, blue) for 30 min after EGF treatment (Fig. 1C-4 and 1C-6, still blue). However, EGFR(C) (Fig. 1D-1, green) could readily co-localize with calnexin (Fig. 1D-3, blue) as indicated by the merged white signals (Fig. 1D-4 and 1D-6). Once the ER membrane was permeabilized by Triton X-100, both EGFR(C) and EGFR(N) could co-localize with calnexin (Fig. 1C-5, 1C-7, 1D-5, and 1D-7). These results suggested that in response to EGF, the NH2-terminus of EGFR is masked inside the ER lumen, whereas the COOH-terminus of EGFR is exposed to the cytoplasm. Furthermore, Texas red-labeled EGF, which binds to the NH2-terminal ligand-binding domain of EGFR, co-localized with the ER lumen protein calregulin (Fig. S1C).
Fig. 1. The NH2-terminus of EGFR resided within the Golgi and ER lumen after EGF treatment in MDA-MB-468 cells.
(A and B) MDA-MB-468 cells transfected with GalNAc-T2-GFP as a Golgi marker were treated with EGF for 15 min. (C and D) MDA-MB-468 cells maintained in serum-starved medium for 24 h were treated with EGF for 30 min. Cells were then immunostained with the indicated antibodies in a triple-labeled assay and analyzed using confocal microscopy. Briefly, after fixation, the cells were permeabilized with digitonin and incubated with the first primary antibodies: (A-1 and C-1) mouse anti-NH2-terminal EGFR(N), and (B-1 and D-1) rabbit anti-COOH-terminal EGFR(C) antibodies. Subsequently, the cells were refixed and permeabilized with Triton X-100 to the second and third primary antibodies: (A-2 and C-2) EGFR(C), (B-2 and D-2) EGFR(N), and (C-3 and D-3) goat anti-calnexin antibodies. The boxed areas are shown in detail in the insets. Bar, 5 μm.
Together, these results indicated that upon EGF treatment, the NH2-terminus of EGFR resides in the Golgi and ER lumen, whereas the COOH-terminus of EGFR is exposed to the cytoplasm. It’s worthwhile to mention that we have identified the tripartite nuclear localization signals (NLSs) of EGFR in the juxtamembrane region within the intracellular COOH-terminus of EGFR [30], and importin β is known to interact through NLSs of proteins including EGFR [22] and ErbB-2 [31]. Here, we found, upon EGF treatment, the COOH-terminus of EGFR was indeed exposed to the cytoplasm, allowing the NLSs to interact with importin β.
Involvement of Golgi-to-ER retrograde trafficking in the EGFR nuclear transport
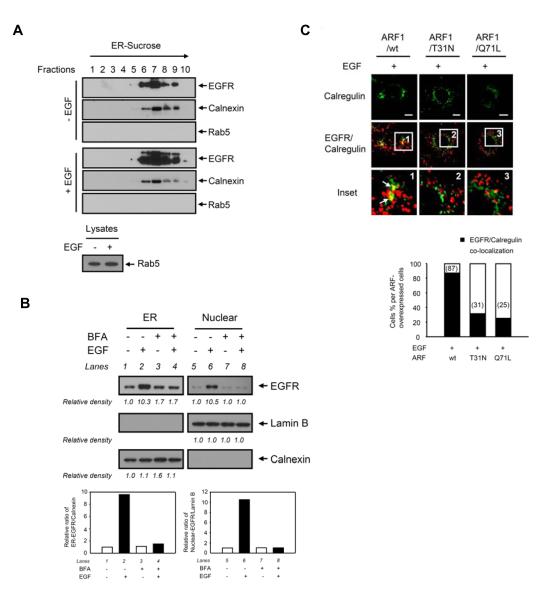
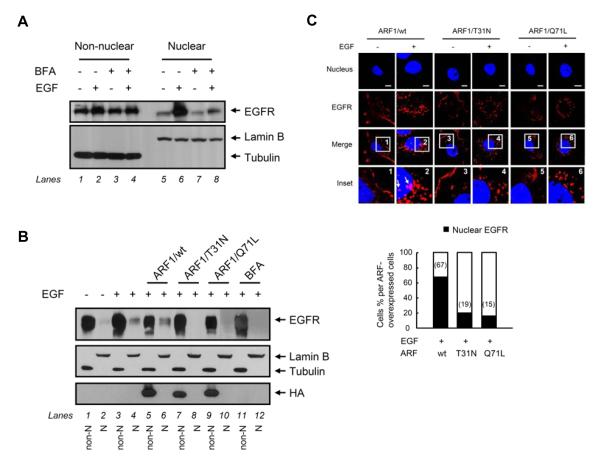
To study whether the Golgi-to-ER retrograde trafficking regulates EGFR transport to the ER membrane, we confirmed the localization of EGFR in the ER. In addition to the immunofluorescence studies (Fig. 1C and 1D), immunoblotting analysis of the ER fractions purified using an OptiPrep gradient technique identified EGFR in the ER membranes in which calnexin but not the early endosomal protein Rab5 was detected (Fig. 2A). We then asked whether the COPI-mediated retrograde trafficking from the Golgi to the ER regulates EGFR transport to the ER membrane because the COPI-coated retrograde vesicles, which consist of the small GTPase ARF and coatomer composed of seven subunits, is known to transport cargo proteins from the Golgi to the ER [5]. To this end, we pretreated cells with brefeldin A (BFA), a chemical that inhibits ARF1-guanine exchange factors and prevents the activation of ARF1, which resulted in the disassembly of the COPI coat to the Golgi [32]. We found that immunoblotting analysis of ER portions purified using an OptiPrep gradient technique showed that pretreatment with BFA decreased EGF-dependent EGFR translocation to the ER (Fig. 2B, lane 4 versus lane 2). We then used two dominant mutants of ARF1, ARF1/T31N and ARF1/Q71L, that inhibit COPI-coated vesicular trafficking from the Golgi to the ER [33] to further investigate the role of COPI-coated retrograde vesicles in the EGFR transport to the ER membrane. As shown in Fig. 2C, when the cells transfected with these two ARF1 mutants, the yellow merged image representing co-localization of EGFR and the ER marker calregulin was abolished (insets 2 and 3 versus inset 1, arrows), suggesting that EGFR could not be transported to the ER while ARF1 was inactivated. These results indicated that vesicular trafficking mediated by ARF1 activation plays a critical role in the EGF-induced EGFR transport to the ER. We further study whether the Golgi-to-ER retrograde trafficking regulates EGFR transport to the nucleus. We found that BFA pretreatment inhibited EGF-dependent EGFR nuclear transport (Fig. 2B, lane 8 versus lane 6; Fig. 3A, lane 8 versus lane 6), indicating the coat assembly is involved in the nuclear transport of EGFR. In addition, EGF-dependent nuclear transport of EGFR was inhibited in cells transfected with the two dominant mutants, ARF1/T31N (Fig. 3B, lane 8 versus lane 4) and ARF1/Q71L (Fig. 3B, lane 10 versus lane 4). The confocal immunofluorescence studies also supported the biochemical results (Fig. 3C). Together, these results suggested that COPI-coated vesicular trafficking mediated by ARF1 activation is involved in the nuclear transport of EGFR via the retrograde trafficking from the Golgi to the ER.
Fig. 2. The Golgi-to-ER retrograde trafficking regulates EGF-dependent EGFR transport to the ER.
(A) EGFR was distributed in the ER in A431 cells. Cells maintained in a serum-starved medium for 24 hr were treated with or without EGF. The ER fraction was isolated using OptiPrep gradient purification and subjected to immunoblotting with the indicated antibodies. Calnexin and Rab5 were used as markers for the ER and early endosome, respectively. Rab5 antibody is validated in lower panel. (B) Pretreatment with BFA inhibited EGF-dependent EGFR translocation to the ER and to the nucleus in MDA-MB-468 cells. Cells maintained in a serum-starved medium for 24 hr were pretreated with BFA (5 μg/ml) for 30 min and then treated with or without EGF, followed by cellular fractionation and ER purification using OptiPrep gradient, and subjected to immunoblotting with the indicated antibodies. The bar diagram indicates the relative densities of the immunoblots as quantified using the ImageJ software program (1.38×; NIH). (C) The dominant ARF1mutants inhibited EGF-dependent EGFR translocation to the ER. Cells were transfected with ARF1/wt or two ARF1 mutants (ARF1/T31N and ARF1/Q71L) and then treated with EGF. Cells were immunostained with EGFR and calregulin and analyzed using confocal microscopy. Bar, 5 μm. The bar diagram indicates detection of nuclear localization of EGFR calculated for a total of 50 cells.
Fig. 3. The Golgi-to-ER retrograde trafficking regulates EGF-dependent EGFR transport to the nucleus.
(A) Pretreatment with BFA inhibited EGF-dependent EGFR translocation to the nucleus in MDA-MB-468 cells. Cells maintained in a serum-starved medium for 24 hr were pretreated with BFA (5 μg/ml) for 30 min and then treated with or without EGF, followed by cellular fractionation and subjected to immunoblotting with the indicated antibodies. (B and C) The dominant ARF1mutants inhibited EGF-dependent EGFR translocation to the nucleus. Cells were transfected with ARF1/wt or two ARF1 mutants (ARF1/T31N and ARF1/Q71L) and then treated with or without EGF. In (B), proteins from the non-nuclear fractions (non-N, odd-numbered lanes) and nuclear fractions (N, even-numbered lanes) by cellular fractionation were immunoblotted with the indicated antibodies. In (C), cells were immunostained with EGFR and analyzed using confocal microscopy. Bar, 5 μm. The bar diagram indicates detection of nuclear localization of EGFR calculated for a total of 50 cells.
Regulation of EGFR nuclear transport from the Golgi to the ER by COPI-mediated retrograde trafficking
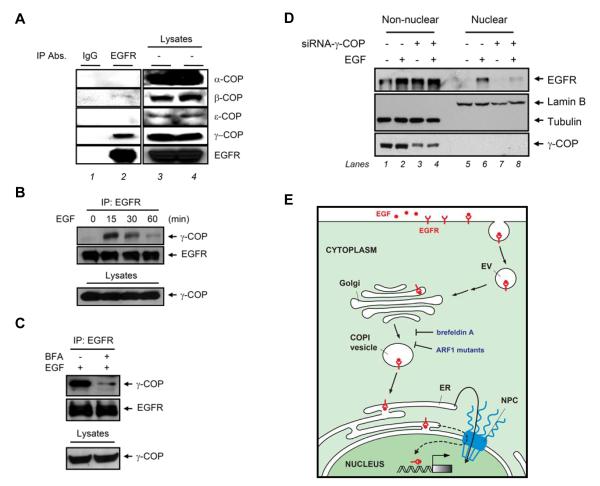
We observed one of the subunits of the COPI coatomer, γ-COP, strongly interacted with EGFR (Fig. 4A, lane 2). Another COPI subunit, β-COP, was weakly associated with EGFR, but other subunits did not interact with EGFR, including α-COP and ε-COP (Fig. 4A, lane 2). Furthermore, the interaction between γ-COP and EGFR in response to EGF was in a time-dependent manner, and the maximum association occurred in cells treated with EGF for 15 min (Fig. 4B), which was inhibited by BFA pretreatment (Fig. 4C). We further found that knocking down γ-COP using small interfering RNA (siRNA) approach inhibited EGF-dependent EGFR nuclear translocation (Fig. 4D, lane 8 versus lane 6). Non-nuclear EGFR was not affected while knocking down the expression of γ-COP (Fig. 4D, lane 4 versus lane 2). In summary, these results strongly suggested that nuclear transport of EGFR is regulated by vesicular trafficking from the Golgi to the ER through the association of EGFR with COPI vesicle (Fig. 4E).
Fig. 4. COPI-mediated retrograde trafficking from the Golgi to the ER regulates EGF-dependent EGFR nuclear transport.
(A) Analysis of EGF-dependent association of EGFR with COP subunits. Total lysates from MDA-MB-468 cells treated with EGF were immunoprecipitated with anti-EGFR antibodies and subjected to immunoblotting with the indicated antibodies. Immunoprecipitation performed with IgG was used as a negative control. Protein expression levels were duplicated. (B) EGFR associated with γ-COP in response to EGF. (C) Pretreatment with BFA inhibited the association of EGFR and γ-COP by EGF treatment for 15 min. (D) Knockdown of γ-COP expression by γ-COP siRNA downregulated EGF-dependent EGFR nuclear translocation in HeLa cells. (E) Diagram of retrograde trafficking from the Golgi to the ER transport by EGF treatment. The scale of the diagram does not reflect the relative sizes of different molecules or subcellular structures. The dashed lines linking the ER and the nucleus are based on postulate from a previous report [26]. EV, endocytic vesicle; COPI, coat protein complex I; ER, endoplasmic reticulum; NPC, nuclear pore complex.
Although it is well established that the endosome-to-Golgi retrograde trafficking occurs in several mammalian cargo proteins [2], the Golgi-to-ER retrograde transport is known to occur only in the exogenous proteins such as Shiga toxin, cholera toxin, the plant toxin ricin, and viruses [3]. After binding to their respective receptors at the cell surface, the toxin-receptor complexes enter a retrograde transport pathway and traffic via the Golgi to the ER [4]. Our finding that assembly of COPI initiated by ARF1 activation is involved in EGFR transport to the ER identified membrane-embedded EGFR as a cellular protein that uses retrograde trafficking to travel from the Golgi to the ER and then translocate into the nucleus. Thus, the retrograde trafficking thought to be used only by exogenous proteins actually is a normal mechanism that plays a role to transfer membrane embedded RTKs such as EGFR and ErbB-2 into the nucleus. Further investigation as to how ER membrane-embedded EGFR transports to the nucleus through the nuclear pore complexes, in addition to a potential route for EGFR nuclear translocation through the ER-associated degradation pathway [25], may clarify this area of EGFR trafficking, which has been overlooked for decades, and may contribute to understanding the functions of nuclear EGFR.
Supplementary Material
Acknowledgments
We thank Dr. B. Storrie (University of Arkansas for Medical Sciences) for providing the GalNAc-T2-GFP plasmid and Dr. J.S. Bonifacino (National Institutes of Health) for the ARF1/wt, ARF1/T31N, and ARF1/Q71L expression vectors. This study was partially funded by the National Institutes of Health grants RO1 109311 and PO1 099031; the National Breast Cancer Foundation, Inc.; the Sister Institutional fund from China Medical University Hospital and M.D. Anderson Cancer Center (to M.-C.H.); and National Science Council Taiwan Merit Postdoctoral Scholarship TMS-94-2B-001 (to Y.-N.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rodman J. Somsel, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113(Pt 2):183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- [2].Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- [3].Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sandvig K, van Deurs B. Membrane traffic exploited by protein toxins. Annu Rev Cell Dev Biol. 2002;18:1–24. doi: 10.1146/annurev.cellbio.18.011502.142107. [DOI] [PubMed] [Google Scholar]
- [5].Springer S, Spang A, Schekman R. A primer on vesicle budding. Cell. 1999;97:145–148. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- [6].Taylor TC, Kahn RA, Melancon P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell. 1992;70:69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- [7].Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- [8].Lippincott-Schwartz J, Liu W. Insights into COPI coat assembly and function in living cells. Trends Cell Biol. 2006;16:e1–4. doi: 10.1016/j.tcb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [9].Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun. 1999;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- [10].Carpenter G, Liao HJ. Trafficking of receptor tyrosine kinases to the nucleus. Experimental Cell Research. 2009;315:1556–1566. doi: 10.1016/j.yexcr.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sehat B, Tofigh A, Lin Y, Trocme E, Liljedahl U, Lagergren J, Larsson O. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Sci Signal. 3:ra10. doi: 10.1126/scisignal.2000628. [DOI] [PubMed] [Google Scholar]
- [12].Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2008;283:4344–4351. doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bryant DM, Stow JL. Nuclear translocation of cell-surface receptors: lessons from fibroblast growth factor. Traffic. 2005;6:947–954. doi: 10.1111/j.1600-0854.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- [14].Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- [16].Massie C, Mills IG. The developing role of receptors and adaptors. Nat Rev Cancer. 2006;6:403–409. doi: 10.1038/nrc1882. [DOI] [PubMed] [Google Scholar]
- [17].de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ, Levy DE, Depinho RA, Bonni A. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev. 2008;22:449–462. doi: 10.1101/gad.1606508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res. 2010;8:232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, McIntush EW, Li LY, Hawke DH, Kobayashi R, Hung MC. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol. 2006;8:1359–1368. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- [20].Wells A, Marti U. Signalling shortcuts: cell-surface receptors in the nucleus? Nat Rev Mol Cell Biol. 2002;3:697–702. doi: 10.1038/nrm905. [DOI] [PubMed] [Google Scholar]
- [21].Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13:6555–6560. doi: 10.1158/1078-0432.CCR-07-1610. [DOI] [PubMed] [Google Scholar]
- [22].Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J Cell Biochem. 2006;98:1570–1583. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- [23].Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- [24].Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- [25].Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell. 2007;18:1064–1072. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang YN, Yamaguchi H, Hsu JM, Hung MC. Nuclear trafficking of the epidermal growth factor receptor family membrane proteins. Oncogene. 2010 doi: 10.1038/onc.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adam SA, Marr RS, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Storrie B, White J, Rottger S, Stelzer EH, Suganuma T, Nilsson T. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem. 2007;282:10432–10440. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- [31].Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, Jackson CL. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell. 1999;3:275–285. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- [33].Ooi CE, Dell’Angelica EC, Bonifacino JS. ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.