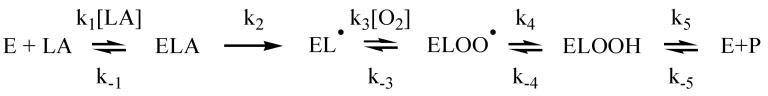Abstract
Human reticulocyte 15-lipoxygenase-1 (15-hLO-1) and human platelet 12-lipoxygenase (12-hLO) have been implicated in a number of diseases, with differences in their relative activity potentially playing a central role. In the current paper, we characterize the catalytic mechanism of these two enzymes with arachidonic acid (AA) as the substrate. Using variable-temperature kinetic isotope effects (KIE) and solvent isotope effects (SIE), we demonstrate that both kcat/Km and kcat for 15-hLO-1 and 12-hLO involve multiple rate-limiting steps that include a solvent dependent step and hydrogen atom abstraction. Nevertheless, an unexpectedly low kcat/Km KIE was determined for 15-hLO-1 (KIE = 8), which increases to well above semi-classical predictions (KIE = 18) upon the addition of the allosteric effector molecule, 12-hydroxyeicosatetraenoic acid (12-HETE), indicating a tunneling mechanism. Furthermore, the addition of 12-HETE lowers the observed kcat/Km SIE from 2.2 to 1.4, indicating that the rate-limiting contribution from solvent rearrangement in the reaction mechanism of 15-hLO-1 has decreased, with a concomitant increase in the C-H abstraction contribution. Finally, the allosteric binding of 12-HETE to 15-hLO-1 decreases the Km(O2) for AA, but increases the Km(O2) for LA, such that the Km(O2) values become similar for both substrates (∼20 μM). Considering that the oxygen concentration in cancerous tissue can be below 5 μM, this result may have cellular implications with respect to the substrate specificity of 15-hLO-1.
Keywords: lipoxygenase, arachidonic acid, kinetic isotope effect, solvent isotope effect
In the human cell, the hydroperoxidation of polyunsaturated fatty acids using molecular oxygen is accomplished by the human lipoxygenase (hLO) isozyme family (Scheme 1) (1). 5-hLO, 12-hLO and 15-hLO are the three main lipoxygenases in the cell and are named according to their positional specificity on arachidonic acid (AA), producing their respective hydroperoxyeicosatetraenoic acid (HPETE) products. The LO products are responsible for inflammatory response, but they are also implicated in a variety of other human diseases. 5-hLO is involved in asthma (2) and cancer (3, 4), 12-hLO is involved in psoriasis (5) and cancer (4, 6, 7) and 15-hLO is involved in atherosclerosis (8) and cancer (4, 9).
Scheme 1.
Recently, the substrate specificity of the 15-hLO isozymes has been suggested to play a role in prostate cancer since their respective products have different cellular responses (10-12). Reticulocyte 15-hLO-1 and epithelial 15-hLO-2 react with both LA and AA, although 15-hLO-1 reacts preferentially towards LA, while 15-hLO-2 reacts preferentially towards AA. However, this substrate specificity is highly dependent on the reaction conditions, such as the detergent used (13-15). In addition, the substrate specificity of both 15-hLO-1 and 15-hLO-2 can be affected by product binding to the allosteric site, suggesting an auto-regulatory mechanism (13). In contrast, 5-hLO and platelet 12-hLO are more selective and only react with AA. For comparison, soybean lipoxygenase-1 (sLO-1), a plant homologue and model enzyme for 15-hLO-1, also reacts preferentially with AA over LA (13, 16), even though AA is not a native substrate in soybeans.
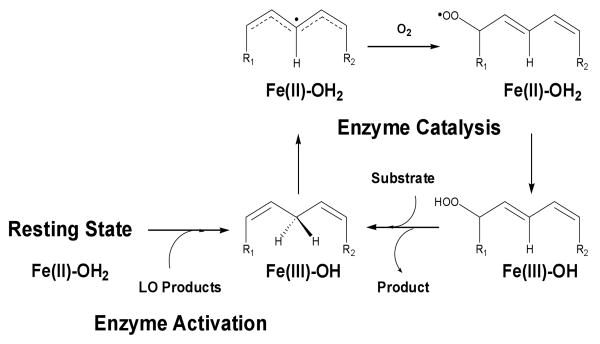
Regarding the mechanism by which these lipoxygenases oxygenate fatty acids, LOs are purified in the inactive Fe(II)-OH2 form and are activated to the Fe(III)-OH form by the hydroperoxide product, resulting in an observable kinetic lag phase (Scheme 1). A hydrogen atom is abstracted from the 1, 4-diene, forming a Fe(II)-OH2/ pentadienyl radical intermediate. Oxygen attacks the pentadienyl radical and the Fe(II)-OH2 reduces the hydroperoxide radical to form the product, leaving the active Fe(III)-OH species (1). The kinetics of this reaction with sLO-1 has been extensively studied and with LA as the substrate, the low temperature kcat/Km[LA] has three rate-determining steps (RDS's) consisting of diffusion, rearrangement and hydrogen atom abstraction, while kcat[LA] is solely limited by hydrogen abstraction (17). However, at high temperature both kcat/Km[LA] and kcat[LA] are solely limited by hydrogen abstraction. 15-hLO-1 has a similar kinetic behavior, with kcat/Km[LA] having multiple RDS's at low temperature (rearrangement and abstraction), but becoming solely limited by abstraction at high temperature. The kcat[LA] is solely limited by abstraction at all temperatures (18). These results prompted the question of whether this similarity in kinetics between sLO-1 and 15-hLO-1 was maintained with AA as the substrate.
Our laboratories subsequently synthesized AA, di-deuterated on C-13 (13,13-d2-AA), and determined for sLO-1 that the Dkcat with AA was similar to that with LA (19). A large Dkcat[AA] was observed with small activation energies, indicating a similar tunneling mechanism for the hydrogen atom abstraction of both substrates. Moreover, the extent of diffusion control on the kcat/Km[AA] was at a maximum at 20 °C, decreasing in prominence as temperature increased or decreased, comparable to that of sLO-1 with LA as substrate (17). In contrast, the Dkcat/Km[AA] for sLO-1 was shown to be small (∼8) and temperature independent, with no solvent isotope effect (SIE) at any temperature. These sLO-1 results with AA are distinct from the LA kinetics and can be best explained by an increase in affinity for AA, which results in an increase in commitment, and a subsequent decrease in Dkcat/Km[AA] (19).
For 15-hLO-1, it was determined using product branching experiments with d4-AA, that the Dkcat[AA] was small in comparison to the Dkcat[AA] for sLO-1, with a value of 11.6 ± 2.0 for C13 and 8.5 ± 4.0 for C10 hydrogen atom abstraction (20). These values were in the range of semi-classical kinetic isotope effects and raised the possibility that the hydrogen atom abstraction for 15-hLO-1 with AA was not proceeding through a tunneling mechanism, opposite from that of LA. This potential difference between the catalytic mechanism of AA and LA for 15-hLO-1 is intriguing because it was recently observed that substrate specificity of 15-hLO-1 with AA and LA could be changed through product regulation of an allosteric site (13). If the mechanisms of catalysis for AA and LA are fundamentally different, then it is conceivable that this could help explain the change in substrate specificity of 15-hLO-1 and provide a method for investigating the effect of the allosteric site. In order to investigate this possibility further, we determined the primary kinetic isotope effect and the solvent isotope effect for both 15-hLO-1 and 12-hLO, with AA as substrate.
Material and Methods
Materials
All commercial fatty acids (Sigma-Aldrich Chemical Company) and LO products were re-purified using a Higgins HAIsil Semi-Preparative (5μm, 250 × 10 mm) C-18 column. Solution A was 99.9% MeOH and 0.1% acetic acid; solution B was 99.9% H2O and 0.1% acetic acid. An isocratic elution of 85% A:15% B was used to purify all fatty acids, which were stored at −80 °C for a maximum of 6 months. Perdeuterated LA (d31-LA) (98% deuterated, Cambridge Isotope Labs) was purified as previously described (21). The (10,10,13,13)-d4-AA was synthesized as previously described (20, 22, 23). All other chemicals were reagent grade or better and were used without further purification.
Overexpression and Purification of 15-Human Lipoxygenase-1 and 12-Human Lipoxygenase
Human reticulocyte 15-lipoxygenase-1 (15-hLO-1) and human platelet 12-lipoxygenase (12-hLO) are N-terminally, His6-tagged proteins, which were expressed and purified as previously published (24, 25). All enzymes were purified to greater than 90% purity, as evaluated by SDS-PAGE analysis. Iron content of 12-hLO and 15-hLO-1 were determined with a Finnigan inductively coupled plasma mass spectrometer (ICP-MS), using cobalt-EDTA as an internal standard. Iron concentrations were compared to standardized iron solutions and used to normalize enzyme concentrations.
Steady-State Kinetic Measurements
Lipoxygenase rates were determined by following the formation of the conjugated diene product at 234 nm (ε = 25,000 M-1 cm-1) with either a Perkin-Elmer Lambda 40 UV/Vis or a Cary 100 Bio spectrophotometer. All reactions were 2 mL in volume and constantly stirred using a magnetic stir bar at room temperature (22 °C) unless otherwise described. Assays were carried out in 25 mM Hepes buffer (pH 7.5) with substrate concentrations ranging from 1 μM – 20 μM, and were initiated by the addition of enzyme, as described below. The 12-hLO displays erratic behavior at low substrate concentrations (< 1 μM), resulting in large errors in the Km values. To circumvent this inherent problem, we determined that adding the 12-hLO first, and then quickly initiating the reaction with the addition of the appropriate amount of substrate, yielded significantly more reproducible results. Substrate concentrations were quantitatively determined by allowing the enzymatic reaction to go to completion. Kinetic data were obtained by recording initial enzymatic rates at each substrate concentration and were then fitted to the Michaelis-Menten equation using the KaleidaGraph (Synergy) program to determine kcat and kcat/Km values.
Determination of Kinetic Isotope Effect for 15-hLO-1 with AA as Substrate
The non-competitive kinetic isotope effect on the kcat (Dkcat[AA]) and kcat/Km (Dkcat/Km[AA]) values was determined by comparing the steady-state kinetic results of protiated arachidonic acid with that of d4-arachidonic acid as previously described (17, 18). Kinetic measurements were performed using a Cary 100 Bio spectrophotometer by following product formation at 234 nm, at temperatures ranging from 15 – 40 °C in 25 mM Hepes buffer at pH 7.5. Reactions were initiated using ∼16 nM and ∼40 nM (normalized to iron content) of 15-hLO-1 for protiated and deuterated arachidonic acid, respectively, with substrate concentrations ranging from 1– 15 μM. Reactions were performed in the presence of purified 13-HPODE (∼6 μM) (or 15-HPETE ∼6 μM) to activate 15-hLO-1 and remove the kinetic lag phase. Kinetic parameters were determined as described in the steady-state kinetic section.
Determination of Kinetic Isotope Effect for 12-hLO with AA as Substrate
The non-competitive kinetic isotope effect on the kcat (Dkcat[AA]) and kcat/Km (Dkcat/Km[AA]) values was determined as described above for 15-hLO-1. The steady-state KIE experiments were performed using a PE Lambda 40 spectrophotometer, using buffer conditions described above (25 mM Hepes, pH 7.5) at temperatures ranging from 15 – 40 °C. Reactions were initiated using ∼5 nM and ∼60 nM (normalized to iron content) of 12-hLO for protiated and deuterated arachidonic acid, respectively, with substrate concentrations ranging from 0.1 – 10 μM. All kinetic parameters were determined as described in the steady-state kinetic section.
Determination of Solvent Isotope Effect for 12-hLO and 15-hLO-1 with AA as Substrate
The solvent isotope effect was determined by comparing the steady-state kinetic results of assays performed in H2O and D2O under temperatures ranging from 15 – 40 °C as previously described (17, 18). Reactions were performed in 25 mM Hepes buffer at pH = 7.5 (pH meter reading was 7.1 for buffered D2O), and initiated using ∼5 nM and ∼7 nM enzyme concentration (normalized to iron content), for 12-hLO and 15-hLO-1 respectively. All kinetic parameters were determined as described in the steady-state kinetic section. The variable-temperature SIE experiments for 15-hLO-1 included the addition of 13-HPODE (6 μM) to remove the kinetic lag phase. In addition, the SIE was performed with and without 12-HETE (5μM) at 15 °C, to determine if allosteric product binding affected the solvent dependency of the reaction.
Determining the Effects of 12-HETE on the Competitive Kinetic Isotope Effect and the Solvent Isotope Effect of 15-hLO-1 with AA as Substrate
The Dkcat/Km[AA] ratio was determined similarly to the previously published competitive substrate specificity method (13), which utilizes a Finnigan LTQ liquid chromatography - tandem mass spectrometer (LC-MS/MS) to quantify the product turnover. The enzymatic reactions were initiated by the addition of 1 μM substrate, of a known molar ratio (1:1) of d4-AA:AA, with and without pre-incubation of 12-HETE (5 μM) (or 13-HODE, 5 μM) with 15-hLO-1 (∼4 nM). Enzymatic assays were performed using buffer conditions described above (25 mM Hepes, pH 7.5, 22 °C). A Phenomenex Synergi Hydro-RP (4 μm, 150 × 2.0 mm) column was used to detect the reduced LO products with an elution protocol consisting of 0.2 ml/min, isocratic mobile phase of 59.9% ACN:40% H2O:0.1% THF. The corresponding reduced product ion peak ratio was determined using negative ion MS/MS (collision energy = 35 eV), with the following masses; 15-HETE, parent m/z = 319, fragments m/z = 175 and 219, 12-HETE, parent m/z = 319, fragments m/z = 179 and 257, 13-HODE, parent m/z = 295, fragments m/z = 183 and 251, and perdeuterated 13-HODE, parent m/z = 325, fragments m/z = 213 and 281 (26). All extracted reaction mixtures were reduced with trimethylphosphite for LC-MS/MS analysis.
The effect of 12-HETE on the SIE of 15-hLO-1 was performed as described above; however, 12-HETE (5 μM) was added to each substrate concentration for steady-state kinetic analysis. Reactions were performed in 25 mM Hepes buffer (15 °C) at pH = 7.5 (pH meter reading was 7.1 for D2O), and initiated using ∼40 nM enzyme (normalized to iron content) of 15-hLO-1.
Reaction Rates at Varying O2 Concentrations
Reaction rates of 15-hLO-1 with AA and LA were determined by measuring the extent of oxygen consumption on a Clark oxygen monitor as previously described (27). Reactions were carried out as a function of oxygen concentrations in 1 ml solutions, which were stirred constantly and equilibrated under air at 25 °C (258 μM O2). The reaction was initiated by addition of ∼30 nM 15-hLO-1 (normalized to iron content), via a gastight Hamilton syringe to the reaction chamber. The experiments were repeated at variable concentrations of oxygen, established by passing mixtures of N2 and O2 over stirred solutions in the reaction chamber for 10 min. The established oxygen concentration was calibrated against the value of O2 dissolved in an air-saturated solution at 25 °C (258 μM O2). The rate of oxygen consumption was recorded at oxygen concentrations ranging from 5 - 500 μM, in 25 mM Hepes pH 7.5 (25 °C) and saturating conditions of substrate (25 μM). Further investigations were performed in the presence of the 12-HETE (5 μM), to determine if allosteric binding affected the Km(O2), with either substrate.
Lag Phase Investigations of 15-hLO-1
Observations that 15-hLO-1 has an extended lag phase (activation period) with AA compared to LA prompted us to investigate if this effect is due to differences in their affinity towards the ferrous enzyme, as previously seen with sLO-1 (19). Reactions were carried out as described for the steady-state experiments (25 mM Hepes. pH 7.5, 22 °C), with the assays being initiated by 15-hLO-1, with 12 nM and 18 nM (normalized to iron content) for LA and AA, respectively, at substrate-limiting conditions (5 μM). The 15-hLO-1 enzyme was pre-incubated with AA (25 μM), followed by addition of LA, and vice versa, to determine the effect on the lag phase, as previously described (19).
Results and Discussion
Mechanistic Investigations of Human 15-Lipoxygenase-1 with AA as Substrate
Non-Competitive Kinetic Isotope Effect
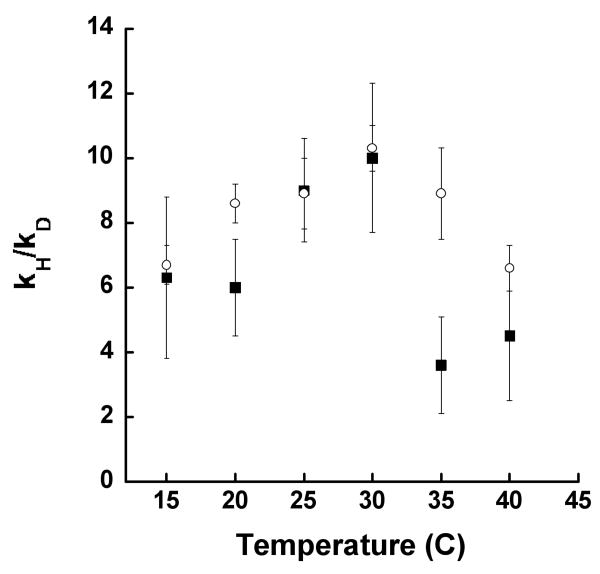
Previous variable-temperature KIE studies with LO isozymes were limited to deuterated linoleic acid (17, 18, 21), since appropriately deuterated AA is not commercially available and is difficult to synthesize. In the current study, (10,10,13,13)-d4-arachidonic acid (d4-AA) was synthesized as previously described (20, 22, 23), and utilized to investigate the primary KIE of 15-hLO-1 and 12-hLO. Deuterium labeling at both C10 and C13 was required to prevent changes in regioselectivity (20). Investigations of 15-hLO-1 with LA have previously demonstrated a temperature dependent Dkcat/Km[LA] and kcat/Km[LA] SIE, and a temperature independent Dkcat[LA] (∼40) and kcat[LA] SIE (18). These studies suggest 15-hLO-1 displays hydrogen atom tunneling and has multiple RDSs at low temperature for kcat/Km[LA], whereas the kcat[LA] is solely rate-limited by hydrogen atom abstraction. In the current work, the non-competitive, variable-temperature KIE of 15-hLO-1 with AA was significantly different, demonstrating temperature dependency for both Dkcat/Km[AA] and Dkcat[AA] and with markedly lower magnitudes (Figure 1). The Dkcat/Km[AA] was determined to be 6.3 ± 2.5, 6.0 ± 1.5, 9.0 ± 1.6, 10.0 ± 2.3, 3.6 ± 1.5, 4.5 ± 2.0, for 15 °C, 20 °C, 25 °C, 30 °C, 35 °C and 40 °C, respectively. The Dkcat[AA] data was determined to be 6.7 ± 0.6, 8.6 ± 0.6, 8.9 ± 1.1, 10.3 ± 0.7, 8.9 ± 1.4, 6.6 ± 0.9, for 15 °C, 20 °C, 25 °C, 30 °C, 35 °C and 40 °C, respectively. The change in Dkcat/Km[AA] and Dkcat[AA] with respect to temperature is suggestive of multiple rate-limiting steps, as seen for 15-hLO-1 with LA; however, unlike with LA, the Dkcat/Km[AA] and Dkcat[AA] values rise and then decrease at temperatures above 30 °C. In addition, the magnitude of both Dkcat/Km[AA] and Dkcat[AA] for 15-hLO-1 are considerably smaller than the previously reported Dkcat/Km[LA] and Dkcat[LA] values for 15-hLO-1 (18). The current values are within the range predicted by semi-classical mechanics (28, 29) and suggest that possibly hydrogen atom tunneling does not occur. This hypothesis is difficult to confirm with ΔEact and AH/AD investigations, as was reported for sLO-1 and 15-hLO-1 with LA (18, 30), due to multiple RDS's for the kcat[AA] of 15-hLO-1 at low temperature (vide infra). Moreover, the observed inactivation of 15-hLO-1 with AA at high temperatures makes the energy of activation (Eact) and the Arrhenius prefactor (A) for d4-AA unattainable.
Figure 1.
Temperature dependence of the apparent primary kH/kD for 15-hLO-1: Dkcat (open circles) and Dkcat/Km (closed squares). Enzymatic assays were performed in 25 mM Hepes buffer (pH 7.5) with 13-HPODE (6 μM) addition.
The non-competitive variable-temperature KIE data for 15-hLO-1 with AA was performed in the presence of 13-HPODE (6 μM) to remove the kinetic lag phase, which is more pronounced with AA than LA. However, this raises the concern that binding of 13-HPODE to the allosteric site could affect the KIE, as was seen with the addition of 12-HPETE/HETE to 15-hLO-1 and LA (13). The non-competitive KIE study was therefore performed in the presence of 15-HPETE (6 μM) (25 mM Hepes, pH 7.5, 25 °C), which demonstrated that addition of 15-HPETE afforded the same KIE results as with 13-HPODE added (Dkcat/Km[AA] = 11 ± 2 and Dkcat[AA] = 7.3 ± 0.6).
Competitive Kinetic Isotope Effect
To confirm the unexpectedly low Dkcat/Km[AA] value of 15-hLO-1, the competitive Dkcat/Km[AA] was determined with a mixture of d4-AA and AA at 22 °C, using the LC-MS/MS method previously described for substrate specificity studies (13). Using d4-AA, the Dkcat/Km[AA] values were determined to be 8 ± 1 and 10 ± 2 for abstraction from C13 and C10, respectively (Figure S1, Supplemental Material). These values are in good agreement with the averaged, non-competitive data at both C13 and C10 and with the previously published Dkcat[AA] values from product branching experiments (20). Considering that no product was added to the competitive experiments and yet the Dkcat/Km[AA] value was the same as the non-competitive results, with both 13-HPODE and 15-HPETE added, this indicates that there was no allosteric effect on the Dkcat/Km[AA] of 15-hLO-1 with these products. As mentioned above, the low KIE values suggest that the hydrogen atom abstraction does not proceed through a tunneling mechanism; however, the intrinsic KIE values could also be masked due to either a large kinetic commitment with AA as substrate or due to a decreased contribution of the abstraction step to the rate-limiting step (19, 21). To this end, 12-HETE (5 μM) was added to the competitive reaction mixture of d4-AA/AA with 15-hLO-1 and the observed Dkcat/Km[AA] increased from 8 ± 1 to 18 ± 3, indicating that tunneling is involved in the hydrogen atom abstraction process. A similar result was previously observed with the addition of 12-HPETE or 12-HETE to 15-hLO-1, with Dkcat/Km[LA] increasing from 24 to 44 (13). 13-HODE was also added to the competitive peroxidation of d4-AA/AA by 15-hLO-1 and was shown to have little effect on Dkcat/Km[AA] (10 ± 2), confirming the non-competitive KIE result (vide supra).
Solvent Isotope Effect
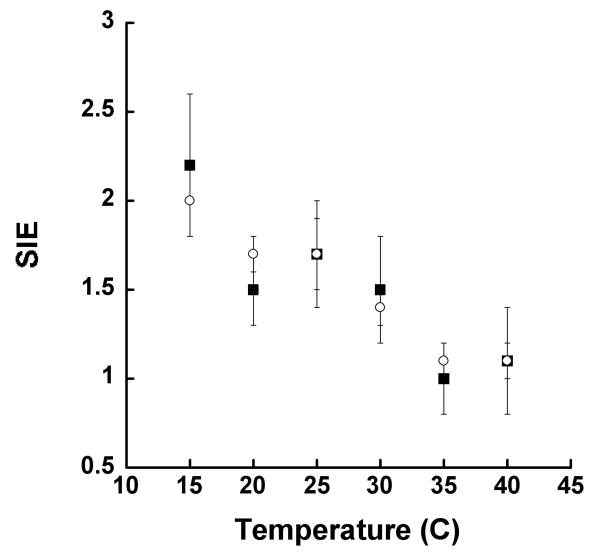
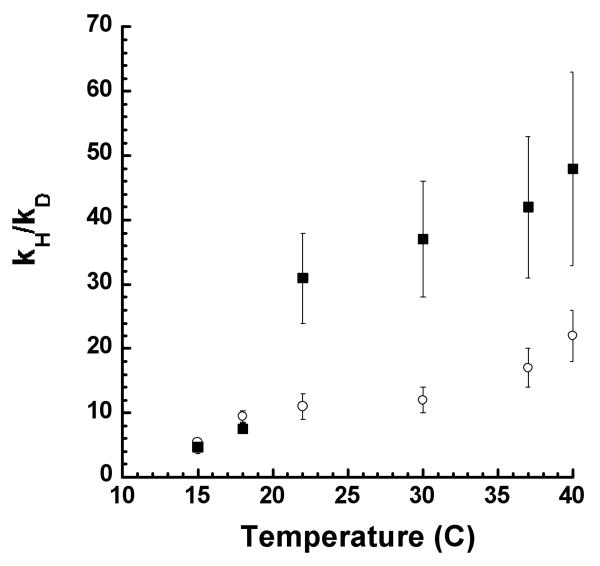
It was previously shown that the reaction of 15-hLO-1 with LA (no 13-HPODE added), displayed a temperature dependent kcat/Km[LA] SIE, yet no SIE for kcat[LA], indicating that kcat/Km[LA] is partially rate-limited by a solvent dependent step, while kcat[LA] is not (18). In contrast, the results for 15-hLO-1 with AA (13-HPODE added) demonstrate a temperature dependent SIE for both kcat/Km[AA] and kcat[AA] (Figure 2), indicating multiple rate-limiting steps at low temperature for both kcat/Km[AA] and kcat[AA]. This observation is consistent with the Dkcat/Km[AA] and Dkcat[AA] temperature dependence data. The observed SIE at lower temperatures also explains the decreased substrate KIE values at low temperature, as a solvent dependent step would partially mask the observed KIE of the hydrogen atom abstraction step, since abstraction is not fully rate-limiting. The SIE data, however, does not explain the decreasing KIE values at high temperature, since there is no SIE effect in this temperature range (vide infra).
Figure 2.
Temperature dependence of solvent isotope effect for 15-hLO-1: kcat (open circles) and kcat/Km (closed squares). Enzymatic assays were performed in 25 mM Hepes buffer (pH 7.5) with 13-HPODE (6 μM) addition.
As discussed above, the addition of 12-HETE increased the competitive Dkcat/Km[AA] to a magnitude well above semi-classical predictions, whereas 13-HPODE and 15-HPETE had little effect on the Dkcat/Km[AA]. The effect of 12-HETE on the reaction mechanism of 15-hLO-1 was investigated further by probing the SIE at low temperature (15 °C), where the SIE is greatest. It was determined that the addition of 12-HETE (5 μM) significantly lowered the SIE from 2.3 ± 0.2 to 1.4 ± 0.2 and 2.4 ± 0.3 to 1.4 ± 0.2 for kcat/Km[AA] and kcat[AA], respectively. This indicates that the solvent-dependent step for 15-hLO-1 has become less rate-limiting with the addition of 12-HETE, and corroborates the competitive Dkcat/Km[AA] results that show a larger substrate KIE under the same conditions. No effect on SIE was observed with the addition of 13-HPODE.
Reaction Rate at Varying O2 Concentration
Previously our laboratory performed O2 dependency experiments for the peroxidation of LA by 15-hLO-1 and AA by 12-hLO, and determined the Km(O2) values to be 4.2 ± 1.1 μM and 7.0 ± 1.4 μM, respectively ([Substrate] = 25 μM, at 25 °C) (18). In the current investigation, 15-hLO-1 was studied with AA and LA (25 °C) concurrently, for direct comparison, and Km(O2)[LA] was determined to be 9.8 ± 0.7 μM and Km(O2)[AA] to be 25 ± 6 μM, indicating a 2.5-fold difference in the Km(O2) between the two substrates (Table 1). The kcat values were determined to be 7.6 ± 0.1 s-1 and 5.6 ± 0.3 s-1 for LA and AA, respectively, and are in agreement with kcat values determined pectrophotometrically (13). The kcat/Km(O2) values were therefore determined to be 0.78 ± 0.07 s-1μM-1 and 0.23 ± 0.07 s-1μM-1 for LA and AA, respectively, indicating a greater than 3-fold preference for LA, in oxygen limiting conditions. The addition of 12-HETE (5 μM) demonstrated a decrease in the kcat/Km(O2) for LA (0.30 ± 0.07 s-1μM-1) and an increase in the kcat/Km(O2) for AA (0.32 ± 0.040 s-1μM-1), yielding similar kcat/Km(O2) values for the two substrates (Table 1). This is a remarkable result since the binding of 12-HETE to the allosteric site not only increases the fatty acid (kcat/Km)AA/(kcat/Km)LA ratio 4-fold (13), but it also increases the oxygen (kcat/Km)AA/(kcat/Km)LA ratio 3-fold. In both cases, 12-HETE increases the substrate specificity of 15-hLO-1 towards AA. The kcat values with the addition of 12-HETE were determined to be 6.6 ± 0.3 s-1 and 5.2 ± 0.1 s-1 for LA and AA, respectively, and are in agreement with the previously published spectroscopic data (13).
Table 1.
Comparison of the Steady-State Kinetic Parameters of 15-hLO-1 for Oxygen with AA and LA as Substratesa
| No Product Addition | 12-HETE Addition | ||
|---|---|---|---|
| AA | |||
| kcat | 5.6 ± 0.3 | 5.2 ± 0.1 | |
| Km(O2) | 25 ± 6 | 17 ± 2 | |
| kcat/Km(O2) | 0.23 ± 0.07 | 0.32 ± 0.04 | |
| LA | |||
| kcat | 7.6 ± 0.1 | 6.6 ± 0.3 | |
| Km(O2) | 9.8 ± 0.7 | 22 ± 4 | |
| kcat/Km(O2) | 0.78 ± 0.07 | 0.30 ± 0.07 |
Enzymatic assays were performed in 25 mM Hepes buffer (pH 7.5, 25 °C), with and without product addition, in the presences of 25 μM substrate. Oxygen consumption was detected using a Clark oxygen monitor.
Lag Phase Investigations
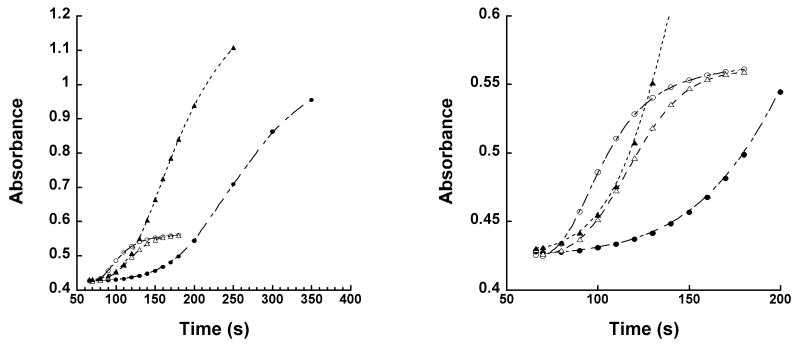
As mentioned, AA demonstrates higher affinity towards the ferrous sLO-1 enzyme than that of LA, manifested by a difference in their lag phase (19). This observation indicated that AA had a smaller KD than LA, increasing commitment with AA, thereby lowering the observed Dkcat/Km[AA]. As seen in Figure 3, 15-hLO-1 also displays a longer lag phase with AA than LA, at low substrate concentrations. Pre-incubation of 15-hLO-1 with AA (25 μM) followed by LA (5 μM) addition, drastically extends the lag phase of the enzyme, whereas little effect is seen on the lag phase under the reverse conditions (pre-incubation with LA (25 μM) followed by AA (5 μM) addition). These data suggest that the affinity of AA to the ferrous 15-hLO-1 is greater than 5-fold that of LA, since 5 μM AA out-competes the binding of pre-incubated 25 μM LA, such that no reduction in the lag phase is seen. The LO products, 13-HPODE and 12-HPETE, were shown to eliminate the lag phase for 15-hLO-1 with AA, but the reduced products, 13-HODE and 12-HETE, did not affect the lag phase. Finally, there is no auto-inactivation of 15-hLO-1 with either AA or LA at low substrate concentration. However, at high substrate concentrations, AA auto-inactivates 15-hLO-1 to a much greater extent, as seen by the lower overall product production. This difference may be relevant for cellular processes since the low substrate concentration is more similar to that in the cell.
Figure 3.
Lag phase comparison of 15-hLO-1 with AA (5 μM) (open triangles) and LA (5 μM) (open circles). Pre-incubation with AA (25 μM) followed by LA (5 μM) addition (closed circles), and pre-incubation of LA (25 μM) followed by AA (5 μM) addition (closed triangles). Enzymatic assays were performed in 25 mM Hepes buffer (pH 7.5, 22 °C). Insert: Magnified view of lag phase data.
Summary of 15-hLO-1
Kinetic investigations of lipoxygenase center around the primary KIE of hydrogen atom abstraction since it is the first irreversible step for fatty acid kinetics. With this in mind, the general method for LO kinetic studies is to determine if the observed KIE can be dependent on multiple rate-limiting steps, as seen by temperature, solvent and viscogen dependencies or by masking of the observed KIE by a large commitment (vide infra). Invariably, the observed KIE is affected by a combination of these processes and this specific study strives to differentiate between the relative influence of these processes in the oxidation of AA by 15-hLO-1. In the current investigation, our laboratories have applied this method to probing 15-hLO-1 with AA as its substrate and demonstrated that the kinetics of 15-hLO-1 with AA are remarkably different from the kinetics with LA as substrate (18). The Dkcat[AA] and Dkcat/Km[AA] values are small and slightly temperature dependent, with decreasing Dkcat/Km[AA] and Dkcat[AA] values at both low and high temperature. At low temperature, the decrease in Dkcat/Km[AA] and Dkcat[AA] is due to multiple RDS's (rearrangement and abstraction), as seen by an increasing SIE. However, at high temperature, no SIE was observed for either kcat/Km or kcat, indicating rearrangement is not responsible for the decrease in Dkcat/Km[AA] and Dkcat[AA]. This decrease could be due to inactivation of 15-hLO-1 at high temperature or because diffusion is a rate-determining step (RDS). Unfortunately, the diffusion step cannot be investigated because the available viscogens affect catalysis (18). These results are distinct from the 15-hLO-1/LA kinetic results, in that both kcat/Km and kcat for 15-hLO-1/AA are sensitive to rearrangement (SIE), and that the values of Dkcat/Km[AA] and Dkcat[AA] are lower than a tunneling mechanism would predict (28, 29). This latter issue was probed further by investigating the lag phase of 15-hLO-1. Previously, our laboratories postulated that the Dkcat/Km[AA] for sLO-1 was masked by a large commitment (k2/k-1, vide infra), since AA appeared to bind more tightly to the ferrous sLO-1 than LA, extending the lag phase (19). This experiment was repeated with 15-hLO-1 and it was observed that AA also extended the lag phase of LA catalysis. This result corroborates the hypothesis that AA binds more tightly to 15-hLO-1 than LA and thus has a larger commitment (k2/k-1). Commitment (k2/k-1) can be explained by examining the proposed mechanism of LO, minimally described by Scheme 2 (21, 31, 32).
Scheme 2.
| (1) |
According to Scheme 2, Dkcat/Km would be defined by eq 1, where substrate release (k-1) and hydrogen atom abstraction (k2) are the primary determinants for Dkcat/Km (this assumes k2 = kcat and the multiple steps observed at low temperature are included in k2). The Dkcat/Km increases to a maximum of k2H/k2D when commitment (k2/k-1) is small, and decreases to a value approaching 1, when commitment for 15-hLO-1 with AA is large, assuming that the intrinsic k2H/k2D remains unchanged. Therefore, a large commitment for 15-hLO-1 with AA would lower the observed KIE and potentially mask a large intrinsic KIE, if tunneling were present.
This hypothesis was investigated further by adding the allosteric effector molecule, 12-HETE, which increased the observed Dkcat/Km[AA] from 8 to 18. This latter value is well above the semi-classical prediction and suggests that the hydrogen atom abstraction of AA by 15-hLO-1 is occurring through a tunneling mechanism. Nevertheless, the reason for the increase in the KIE is most likely not due to a change in commitment since addition of 12-HETE increases the kcat/Km[AA] but does not affect kcat[AA] (13), indicating a decrease in Km[AA]. Considering that the large commitment for 15-hLO-1 with AA is due to a small k-1, it is unlikely that the observed decrease in Km[AA] is due to an increase in k-1 and a subsequent decrease in commitment. In addition, it was observed that while 13-HPODE and 12-HPETE reduced the lag phase for 15-hLO-1 with AA, 13-HODE and 12-HETE did not. This indicated that the release rate for AA (k-1), and hence commitment, was not changing upon allosteric binding.
The SIE of 15-hLO-1 was subsequently probed with the addition of 12-HETE to determine if the percent contributions of the rate-limiting steps were changing and the kcat/Km[AA] SIE was observed to decrease from 2.3 to 1.4. This result indicates that 12-HETE binding decreases the contribution of the solvent dependency on the rate-limiting step and subsequently increases the contribution of hydrogen atom abstraction for AA. The increase in contribution of hydrogen atom abstraction to the rate-limiting step would therefore increase the observed KIE, as is seen experimentally for AA. It should be noted that previously our laboratory demonstrated that 12-HETE increased the observed KIE for 15-hLO-1 with LA (13) and we postulated that the increase was due to a decrease in commitment, as seen by the decrease in kcat[LA] (i.e. k2). Nevertheless, given the current data, it is possible that the solvent dependent step could be affected as well. It should be noted that the change in Dkcat/Km upon addition of 12-HETE could also be due to a change in the intrinsic k2H/k2D, but this can not be probed using the temperature dependency of kcat, as previously done for sLO-1 and 15-hLO-1 with LA (18, 30), since kcat for 15-hLO-1 with AA is not fully rate-limited by hydrogen atom abstraction, and 15-hLO-1 auto-inactivates at high temperatures with AA. Interestingly, 13-HPODE does not increase the Dkcat/Km[AA] nor does it decrease the SIE, even though it has an allosteric effect on substrate specificity, similar to 12-HETE. This result suggests that 12-HETE and 13-HPODE affect the microscopic rate constants differently upon binding to the allosteric site.
The competitive Dkcat/Km[AA] for hydrogen atom abstraction from C13 is comparable to that for abstraction from C10, which corroborates the Dkcat[AA] data previously determined (20). This result suggests that the hydrogen atom abstraction at these two structurally distinct positions is of a comparable mechanism. This is an unexpected result since the active site would have to spatially accommodate these two disparate positions for the same hydrogen atom abstraction process. Further investigations are in progress to probe this further.
Another difference between 15-hLO-1 kinetics with AA and LA is the difference in their kcat/Km(O2) values, and the differential effect of the allosteric effector molecule, 12-HETE. The kcat/Km(O2) for AA is over 3-fold less than that of LA, suggesting LA binds in a preferred conformation for dioxygen attack. This result is similar to that seen for the kcat/Km(O2) of sLO-1 with AA, which is over 9-fold less than that for LA (under saturating conditions of 13-HPODE) (33). The addition of 12-HETE raises the kcat/Km(O2) value of 15-hLO-1 with AA and decreases the kcat/Km(O2) value for LA, such that their kcat/Km(O2) values become equivalent for both substrates. These results indicate that upon allosteric binding, either the position of the substrate relative to the O2 channel has changed or that the O2 channel itself has changed. This result may be relevant for human disease models since the oxygen concentration varies from 20 μM in normal tissue (34), to as low as 6 μM in prostate cancer (35), and hence oxygen concentration could regulate LO substrate specificity in the cell.
Mechanistic Investigations of Human 12-Lipoxygenase with AA as Substrate
Non-Competitive Kinetic Isotope Effect
Variable-temperature KIE experiments demonstrated that the Dkcat/Km[AA] and Dkcat[AA] of 12-hLO were temperature dependent, with values ranging from 4.7 ± 0.9 to 48 ± 15 and 5.5 ± 0.6 to 22 ± 4 between 15 °C and 40 °C, respectively (Figure 4).
Figure 4.
Temperature dependence of apparent primary kH/kD for 15-hLO-1: Dkcat (open circles) and Dkcat/Km (closed squares). Enzymatic assays were performed in 25 mM Hepes buffer (pH 7.5).
The magnitude of both Dkcat/Km[AA] and Dkcat[AA], at high temperature, are indicative of hydrogen atom tunneling, as previously seen for 15-hLO-1 with LA (18). The value of Dkcat/Km[AA] at high temperature does not match with the value of Dkcat[AA], which could be due to the inactivation of 12-hLO, as seen by the increased error, and is similar to that seen for 15-hLO-1 with LA (18). The temperature dependence of both Dkcat/Km[AA] and Dkcat[AA] for 12-hLO is indicative of multiple RDS's in the reaction mechanism that contribute differently to the overall rate at the different temperatures.
Solvent Isotope Effect
Previously, our laboratory determined that 12-hLO displayed a temperature dependent SIE for kcat[AA], but a temperature independent kcat/Km[AA] SIE, albeit with a large degree of error (18). However, in the current study 12-hLO displayed a temperature dependent KIE for both Dkcat/Km[AA] and Dkcat[AA], suggesting a possible discrepancy between these two results. Considering that the original SIE data (18) and the current KIE data were performed under different conditions (25 mM Tris, pH 7.5 versus 25 mM Hepes, pH 7.5, respectively), the SIE experiments were re-examined using the KIE reaction conditions of this investigation. Under these new conditions, the error was reduced considerably and the solvent isotope effect for 12-hLO at pD 7.1 (pH 7.5) was determined to be approximately 1.4 at 15 °C, decreasing with increasing temperature to approximately 1.0, for both the kcat/Km[AA] and kcat[AA] (Figure 5). The new SIE data correlates well with the substrate KIE data, providing further support for multiple rate-limiting steps at low temperature for both kcat/Km[AA] and kcat[AA].
Figure 5.
Temperature dependence of solvent isotope effect 12-hLO: Dkcat (closed squares) and Dkcat/Km (open circles). Enzymatic assays were performed in 25 mM Hepes buffer (pH 7.5).
Temperature dependency of hydrogen atom abstraction
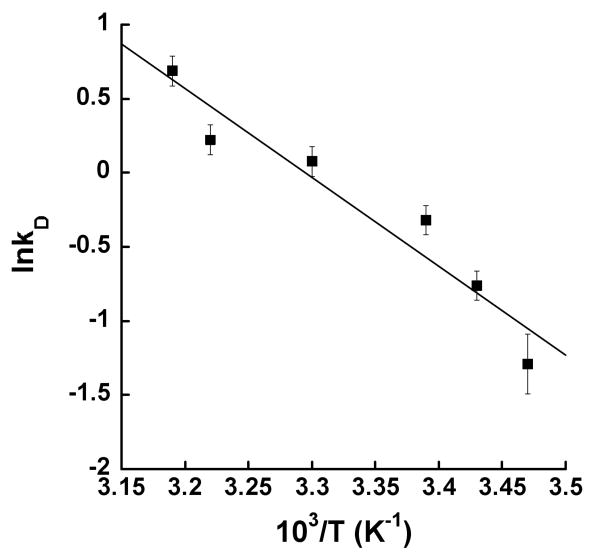
Considering that there are multiple RDS's for the kcat[AA] of 12-hLO at low temperature, it is not possible to determine the ΔEact and AH/AD, as previously determined for sLO-1 and 15-hLO-1 with LA (18, 30). Nevertheless, the energy of activation (Eact) and the Arrhenius prefactor (A) for the d4-AA can be estimated, since the peroxidation of deuterated substrate is most likely solely rate-limited by deuterium atom abstraction and there is no auto-inactivation at higher temperature, as seen for 15-hLO-1. Fitting the data with the empirical Arrhenius equation (k = A exp-Eact/RT), where R represents the gas constant, T is the absolute temperature, Eact is the energy of activation, and A is the Arrhenius prefactor (Figure 6), the Eact was determined to be 11.9 ± 1.0 kcal/mol and the AD to be 4 x 108 s-1 (Table 2). In comparing the 12-hLO/d4-AA data with the 15-hLO-1/d31-LA data, one observes that their Eact values are much smaller than the predicted value of 76 kcal/mol for the pentadienyl C-H homolytic bond cleavage (36), that their AD values are much smaller than the predicted Arrhenius prefactor (ATST ∼1013 s-1) from the semi-classical model (i.e. the bond-stretch model) (37), and that the magnitude of Dkcat/Km[AA] and Dkcat[AA] at high temperature are much greater than semi-classical predictions (7-10) (28, 29). These combined 12-hLO kinetic data are therefore consistent with a hydrogen tunneling mechanism (28).
Figure 6.
Arrhenius plot of kinetic data for 12-hLO with d4-AA. Nonlinear fit is shown as a solid line. Enzymatic assays were performed in 25 mM Hepes, pH 7.5.
Table 2.
Energy of Activation and Arrhenius Prefactors Determination for Human Lipoxygenase Deuterium Abstractiona
| Substrate | Dkcatb | Eact (kcal/mol) |
AD (s-1) |
|
|---|---|---|---|---|
| 12-hLO | AA | 12.0 (2.0) | 11.9 (1.0) | 4 × 108 |
| 15-hLO-1 | LAc | 40.0 (8.0) | 8.7 (0.2) | 4 × 105 |
Data were collected between 15 – 40 °C in 25 mM Hepes pH 7.5 for 15-hLO-1, and 25mM Tris pH 7.5 for 12-hLO.
Dkcat = kcatH/kcatD at 30 °C.
From previously published data (18).
These results demonstrate that not only is 12-hLO distinct from 15-hLO-1 in both its fatty acid metabolism (only reacting with AA) and its primary sequence (80% similar/65% identical), but also in its reaction mechanism with AA. Our data demonstrates that both Dkcat/Km and Dkcat increase to large values at high temperature, whereas 15-hLO-1/AA KIE is at a maximum at 30 °C. However, the catalytic mechanism of 12-hLO is similar to that of 15-hLO-1 with AA in that its hydrogen atom abstraction proceeds through a tunneling mechanism, and both isozymes demonstrate a solvent dependent step for both kcat/Km and kcat, at low temperature. Interestingly, the magnitude of the SIE value for 12-hLO is lower than that for 15-hLO-1, suggesting a smaller participation in the rate-limiting step. Unfortunately, it could not be determined if diffusion is also a rate-limiting step, as seen for sLO-1 with LA (17), since viscogens inhibit catalysis of 12-hLO (18). It should be noted that although both 15-hLO-1 and 12-hLO display a temperature dependent KIE due to multiple rate-limiting steps, which include hydrogen atom abstraction and a solvent dependent step, gating cannot be completely discounted as a factor. The environmentally coupled tunneling model predicts that even if hydrogen abstraction is the sole RDS, changes in the gating energy can lead to a slightly temperature dependent KIE (28-30). Since the kcat of both enzymes are not fully rate-limited by hydrogen atom abstraction, this aspect of both 15-hLO-1 and 12-hLO catalysis could not be investigated.
Conclusion
In conclusion, this investigation illustrates four important properties of mammalian lipoxygenases. First, the general catalytic mechanism of 15-hLO-1/AA is similar to that of 12-hLO/AA with respect to both having multiple RDSs at low temperature (rearrangement and abstraction) and that the abstraction proceeds through a tunneling mechanism. However, the commitment (k2/k-1) of 15-hLO-1/AA is large enough, relative to its intrinsic KIE, to lower the observed Dkcat/Km[AA] significantly. Second, the kcat/Km[O2] with AA of 15-hLO-1 is over 3-fold higher compared to 12-hLO with AA, which could affect their relative activity in cells, where oxygen concentration is limited. Third, the binding of 12-HETE to the allosteric site of 15-hLO-1 lowers the solvent dependent step (SIE) such that hydrogen atom abstraction (KIE) becomes the predominant rate-limiting step, and increases the AA/LA ratio for kcat/Km[O2], such that there is no substrate preference between LA and AA, under limiting oxygen concentrations. These results, as well as our previous discovery that 12-HETE affects the AA/LA ratio for kcat/Km[Fatty Acid], suggest that under cellular conditions, the allosteric binding of 12-HETE to 15-hLO-1 would increase its substrate specificity towards AA over LA, which may have important implications in cancer progression. Finally, it appears that hydrogen atom tunneling is a common feature in the lipoxygenase mechanism, despite the variations between the microscopic rate constants of the various LO isozymes (sLO-1, 15-hLO-1 and 12-hLO), with the two substrates (AA and LA).
Supplementary Material
Abbreviations
- LO
lipoxygenase
- sLO-1
soybean lipoxygenase-1
- 15-hLO-1
human reticulocyte 15-lipoxygenase-1
- 12-hLO
human platelet 12-lipoxygenase
- AA
arachidonic acid
- 15-HPETE
15-(S)-hydroperoxyeicosatetraenoic acid
- 15-HETE
15-(S)-hydroxyeicosatetraenoic acid
- 12-HPETE
12-(S)-hydroperoxyeicosatetraenoic acid
- 12-HETE
12-(S)-hydroxyeicosatetraenoic acid
- d4-AA
(10,10,13,13)-d4-AA
- LA
linoleic acid
- d31-LA
fully deuterated LA
- 13-HPODE
13-(S)-hydroperoxyoctadecadienoic acid
- 13-HODE
13-(S)-hydroxyoctadecadienoic acid
- perdeuterated 13-HPODE
fully deuterated 13-(S)-HPODE
- perdeuterated 13-HODE
fully deuterated 13-(S)-HODE
- kcat
the rate constant for product release
- kcat/Km
the rate constant for substrate capture
- kcat/Km(O2)
the rate constant for oxygen binding, Dkcat/Km, primary kinetic isotope effect for kcat/Km
- Dkcat
primary kinetic isotope effect for kcat
Footnotes
This work was supported by the National Institutes of Health (GM44911, WAV and GM56062, TRH) and an American Heart Association pre-doctoral fellowship (0615604Z, CJ).
References
- 1.Solomon EI, Zhou J, Neese F, Pavel EG. New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem Biol. 1997;4:795–808. doi: 10.1016/s1074-5521(97)90113-7. [DOI] [PubMed] [Google Scholar]
- 2.Nakano H, Inoue T, Kawasaki N, Miyataka H, Matsumoto H, Taguchi T, Inagaki N, Nagai H, Satoh T. Synthesis and biological activities of novel antiallergic agents with 5-lipoxygenase inhibiting action. Bioorg Med Chem. 2000;8:373–80. doi: 10.1016/s0968-0896(99)00291-6. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:13182–7. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kellof GJ. Lipoxygenase Inhibitors as Potential Cancer Chemopreventives. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:467–483. [PubMed] [Google Scholar]
- 5.Hussain H, Shornick LP, Shannon VR, Wilson JD, Funk CD, Pentland AP, Holtzman MJ. Epidermis contains platelet-type 12-lipoxygenase that is overexpressed in germinal layer keratinocytes in psoriasis. Am J Physiol. 1994;266:C243–53. doi: 10.1152/ajpcell.1994.266.1.C243. [DOI] [PubMed] [Google Scholar]
- 6.Connolly JM, Rose DP. Enhanced angiogenesis and growth of 12-lipoxygenase gene-transfected MCF-7 human breast cancer cells in athymic nude mice. Cancer Lett. 1998;132:107–12. doi: 10.1016/s0304-3835(98)00171-2. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan R, Nadler J. Role of lipoxygenases in breast cancer. Front Biosci. 1998;3:E81–8. doi: 10.2741/a369. [DOI] [PubMed] [Google Scholar]
- 8.Harats D, Shaish A, George J, Mulkins M, Kurihara H, Levkovitz H, Sigal E. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2100–5. doi: 10.1161/01.atv.20.9.2100. [DOI] [PubMed] [Google Scholar]
- 9.Kamitani H, Geller M, Eling T. Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J Biol Chem. 1998;273:21569–77. doi: 10.1074/jbc.273.34.21569. [DOI] [PubMed] [Google Scholar]
- 10.Shappell SB, Manning S, Boeglin WE, Guan YF, Roberts RL, Davis L, Olson SJ, Jack GS, Coffey CS, Wheeler TM, Breyer MD, Brash AR. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3:287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler R, M SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Diff. 2000;11:49–61. [PubMed] [Google Scholar]
- 12.Hsi LC, Wilson L, Nixon J, Eling TE. 15-lipoxygenase-1 metabolites down-regulate peroxisome proliferator-activated receptor gamma via the MAPK signaling pathway. J Biol Chem. 2001;276:34545–52. doi: 10.1074/jbc.M100280200. [DOI] [PubMed] [Google Scholar]
- 13.Wecksler AT, Kenyon V, Deschamps JD, Holman TR. Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation. Biochemistry. 2008;47:7364–75. doi: 10.1021/bi800550n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–52. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilty I, Logan A, Vickers PJ. Differential characteristics of human 15-lipoxygenase isozymes and a novel splice variant of 15S-lipoxygenase. Eur J Biochem. 1999;266:83–93. doi: 10.1046/j.1432-1327.1999.00818.x. [DOI] [PubMed] [Google Scholar]
- 16.Ruddat VC, Mogul R, Chorny I, Chen C, Perrin N, Whitman S, Kenyon V, Jacobson MP, Bernasconi CF, Holman TR. Tryptophan 500 and arginine 707 define product and substrate active site binding in soybean lipoxygenase-1. Biochemistry. 2004;43:13063–71. doi: 10.1021/bi0489098. [DOI] [PubMed] [Google Scholar]
- 17.Glickman MH, Klinman JP. Nature of rate-limiting steps in the soybean lipoxygenase-1 reaction. Biochemistry. 1995;34:14077–92. doi: 10.1021/bi00043a013. [DOI] [PubMed] [Google Scholar]
- 18.Segraves EN, Holman TR. Kinetic investigations of the rate-limiting step in human 12- and 15-lipoxygenase. Biochemistry. 2003;42:5236–43. doi: 10.1021/bi0273462. [DOI] [PubMed] [Google Scholar]
- 19.Jacquot C, Peng S, van der Donk WA. Kinetic isotope effects in the oxidation of arachidonic acid by soybean lipoxygenase-1. Bioorg Med Chem Lett. 2008;18:5959–62. doi: 10.1016/j.bmcl.2008.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquot C, Wecksler AT, McGinley CM, Segraves EN, Holman TR, van der Donk WA. Isotope sensitive branching and kinetic isotope effects in the reaction of deuterated arachidonic acids with human 12- and 15-lipoxygenases. Biochemistry. 2008;47:7295–303. doi: 10.1021/bi800308q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis E, Johnson E, Holman T. Large Competitive Kinetic Isotope Effects in Human 15-Lipoxygenase Catalysis Measured by a Novel HPLC Method. J Am Chem Soc. 1999;121:1395–1396. [Google Scholar]
- 22.Peng S, Okeley NM, Tsai AL, Wu G, Kulmacz RJ, van der Donk WA. Synthesis of isotopically labeled arachidonic acids to probe the reaction mechanism of prostaglandin H synthase. J Am Chem Soc. 2002;124:10785–96. doi: 10.1021/ja026880u. [DOI] [PubMed] [Google Scholar]
- 23.Peng S, McGinley CM, van der Donk WA. Synthesis of site-specifically labeled arachidonic acids as mechanistic probes for prostaglandin H synthase. Org Lett. 2004;6:349–52. doi: 10.1021/ol0361711. [DOI] [PubMed] [Google Scholar]
- 24.Amagata T, Whitman S, Johnson TA, Stessman CC, Loo CP, Lobkovsky E, Clardy J, Crews P, Holman TR. Exploring sponge-derived terpenoids for their potency and selectivity against 12-human, 15-human, and 15-soybean lipoxygenases. J Nat Prod. 2003;66:230–5. doi: 10.1021/np020462l. [DOI] [PubMed] [Google Scholar]
- 25.Chen XS, Brash AR, Funk CD. Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system. Eur J Biochem. 1993;214:845–52. doi: 10.1111/j.1432-1033.1993.tb17988.x. [DOI] [PubMed] [Google Scholar]
- 26.Deems R, Buczynski MW, Bowers-Gentry R, Harkewicz R, Dennis EA. Detection and Quantitation of Eicosanoids via High Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. Methods Enzymol. 2007;432:59–82. doi: 10.1016/S0076-6879(07)32003-X. [DOI] [PubMed] [Google Scholar]
- 27.Knapp MJ, Klinman JP. Kinetic studies of oxygen reactivity in soybean lipoxygenase-1. Biochemistry. 2003;42:11466–75. doi: 10.1021/bi0300884. [DOI] [PubMed] [Google Scholar]
- 28.Knapp MJ, Klinman JP. Environmentally coupled hydrogen tunneling. Linking catalysis to dynamics. Eur J Biochem. 2002;269:3113–21. doi: 10.1046/j.1432-1033.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- 29.Klinman JP. The role of tunneling in enzyme catalysis of C-H activation. Biochim Biophys Acta. 2006;1757:981–7. doi: 10.1016/j.bbabio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Knapp MJ, Rickert K, Klinman JP. Temperature-dependent isotope effects in soybean lipoxygenase-1: correlating hydrogen tunneling with protein dynamics. J Am Chem Soc. 2002;124:3865–74. doi: 10.1021/ja012205t. [DOI] [PubMed] [Google Scholar]
- 31.Mogul R, Johansen E, Holman TR. Oleyl sulfate reveals allosteric inhibition of soybean lipoxygenase-1 and human 15-lipoxygenase. Biochemistry. 2000;39:4801–7. doi: 10.1021/bi992805t. [DOI] [PubMed] [Google Scholar]
- 32.Ruddat VC, Whitman S, Holman TR, Bernasconi CF. Stopped-flow kinetic investigations of the activation of soybean lipoxygenase-1 and the influence of inhibitors on the allosteric site. Biochemistry. 2003;42:4172–8. doi: 10.1021/bi020698o. [DOI] [PubMed] [Google Scholar]
- 33.Peng S, van der Donk WA. An unusual isotope effect on substrate inhibition in the oxidation of arachidonic acid by lipoxygenase. J Am Chem Soc. 2003;125:8988–9. doi: 10.1021/ja035977t. [DOI] [PubMed] [Google Scholar]
- 34.Hudetz AG. Mathematical model of oxygen transport in the cerebral cortex. Brain Res. 1999;817:75–83. doi: 10.1016/s0006-8993(98)01200-1. [DOI] [PubMed] [Google Scholar]
- 35.Parker C, Milosevic M, Toi A, Sweet J, Panzarella T, Bristow R, Catton C, Catton P, Crook J, Gospodarowicz M, McLean M, Warde P, Hill RP. Polarographic electrode study of tumor oxygenation in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:750–7. doi: 10.1016/S0360-3016(03)01621-3. [DOI] [PubMed] [Google Scholar]
- 36.McMillen DF, G DM. Hydrocarbon Bond Dissociation Energies. Ann Rev Phys Chem. 1982;33:493–532. [Google Scholar]
- 37.Kim Y, Kreevoy MM. The experimental manifestations of corner-cutting tunneling. Journal of the American Chemical Society. 1992;114:7116–7123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.