Abstract
Papillary thyroid carcinomas (PTC) are the most common type of thyroid malignancy. Most PTC carry one of the two mutations, RET/PTC rearrangement or BRAF mutation. Both mutations are able to activate the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling transduction pathway leading to cellular proliferation, differentiation, and apoptosis. PD0325901 is a specific MEK1/2 inhibitor and therefore is a promising drug to treat thyroid cancers with either RET/PTC or BRAF mutation. In this study we tested the effects of PD0325901 on PTC cells harboring either mutation in vitro by growth curves and Western blots and in vivo using a murine orthotopic xenograft model. We found that 50% growth inhibition (GI50) by PD0325901 was 11 nmol/L for the PTC cells with the RET/PTC1 rearrangement and 6.3 nmol/L for PTC cells with a BRAF mutation, with both concentrations readily achievable in serum. After 1 week of oral administration of PD0325901 (20–25 mg/kg/day) in mice, no tumor growth was detected in mice inoculated with PTC cells bearing a BRAF mutation. For PTC with the RET/PTC1 rearrangement, the average tumor volume of the orthotopic tumor was reduced by 58% as compared with controls. In conclusion, our data suggested that PTC cells carrying a BRAF mutation were more sensitive to PD0325901 than were PTC cells carrying the RET/PTC1 rearrangement. Our findings support the clinical evaluation of PD0325901 for patients with PTC and potentially other carcinomas with BRAF mutations.
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of malignancy in the thyroid (1). Most PTC carry one of two mutations, a BRAF mutation and RET/PTC rearrangement. The most common BRAF mutation is a T to A substitution at nucleotide 1799 in exon 15 that results in the conversion of a valine to glutamic acid at codon 600 (V600E) of the BRAF protein (2, 3). The negative charge introduced by glutamic acid mimics the effect of phosphorylation at an adjacent site when BRAF is activated and results in constitutive activation of BRAF. The incidence of BRAF mutations ranges from 29% to 83% depending on the cohort studied (4). RET/PTC rearrangements are unique to thyroid cancer, with 11 different RET/PTC rearrangements reported thus far (5–8). RET/PTC1, RET/PTC2, and RET/PTC3 rearrangements are the most studied in PTC. These rearrangements result from the generation of chimeric oncogenes in which the 3′ end of the kinase domain from the RET kinase fuses with the H4 gene as RET/PTC1 (6), regulatory subunit RIα of cyclic AMP-dependent protein kinase A as RET/PTC2 (5), or the RFG gene (or ELE1 gene) as RET/PTC3 (9). These chimeric oncogenes retain the kinase activity of RET and result in the constitutive activation of RET. The incidence of RET/PTC rearrangements in primary PTC is lower than that of BRAF mutation depending on the cohort studied (7, 10). Recent reviews of primary PTC have indicated that the frequency of RET/PTC rearrangements in PTC is approximately 20% (11, 12). Either BRAF mutation or RET/PTC rearrangement can activate the mitogen-activated protein kinase kinase (MEK1/2 or MAPKK) and downstream MAPK (ERK1/2) signaling transduction pathway, resulting in the activation of a variety of transcription factors that regulate cellular proliferation, differentiation, and apoptosis (2, 13, 14).
We and others have shown several inhibitors to inhibit the MEK/ERK signal transduction pathway in PTC (15–18). The multikinase inhibitor sorafenib (BAY 43-9006 or Nexavar, Bayer and Onyx Pharmaceuticals) was more sensitive in PTC cells carrying the RET/PTC1 rearrangement than in PTC cells carrying a BRAF mutation, with concentrations needed to inhibit 50% cell growth (GI50) of 0.14 μmol/L and 2.5 μmol/L, respectively. In vivo, sorafenib seemed to be more effective in PTC cells with RET/PTC1 rearrangement where 94% to 100% of tumor growth was inhibited than in PTC cells carrying a BRAF mutation where 53% to 54% tumor reduction was detected (18). PD98059, U0126, and CI-1040 (PD184352; Pfizer) are specific MEK1/2 inhibitors, and these inhibitors can inhibit the expression of phospho-ERK1/2 (p-ERK1/2) for various lengths of time in vitro (15–17). CI-1040 reduced tumor growth by 31.3% in mice inoculated with PTC cells carrying a BRAF mutation and by 47.5% in mice inoculated with PTC cells bearing the RET/PTC1 rearrangement (17). PD0325901 is a second-generation small-molecule inhibitor from Pfizer with specific activity against MEK1/2 (19–22). Compared with CI-1040, PD0325901 exhibits more potency and fewer side effects. PD0325901 has been tested in other cancers, including colon cancer, breast cancer, non–small cell lung cancer (NSCLC), and melanoma, and was well tolerated by patients in phase I–II trials (23, 24). In this study, we tested the effects of PD0325901 in PTC cell lines possessing either a BRAF mutation or RET/PTC1 rearrangement, both of which constitutively activate the BRAF-MEK1/2-ERK1/2 pathway. We found that PD0325901 was able to inhibit PTC cell growth both in vitro and in vivo.
Materials and Methods
Cell lines
A PTC cell line carrying the RET/PTC1 rearrangement (TPC-1) was kindly provided by Dr. Jerome Hershman (VA Greater Los Angeles Healthcare System, Los Angeles, CA; refs. 25–27). TPC-1 cells were maintained in RPMI1640 medium (Mediatech, Inc.) containing 10% fetal bovine serum (Hyclone), nonessential amino acid mixture (Cambrex BioScience), 1 mmol/L sodium pyruvate (Cambrex BioScience), and 2 mmol/L L-glutaminen in a 37°C incubator supplied with 95% air and 5% CO2. A PTC cell line with a BRAF mutation (K2, kindly provided by Dr. D. Wynford-Thomas from Cardiff University, Cardiff, United Kingdom) was maintained in DMEM/F12 medium (Mediatech) containing 10% fetal bovine serum and 2 mmol/L L-glutamine (28). Both TPC-1 and K2 cells have been genotyped to ensure their identity by the Fragment Analysis Facility at the Johns Hopkins University Genetics Resource Core (29).
Reagents
PD0325901, provided by Pfizer (New York, NY), was dissolved in DMSO as a 10-mmol/L stock solution and stored at −20°C for the in vitro study. For the in vivo experiments, PD0325901 was dissolved in 80 mmol/L citric buffer (pH 7). Staurosporine was purchased from EMD Chemicals.
Cell proliferation assay
PTC cells (1 × 104) were plated in 24-well plates (Costar) with 1 mL of medium for 4 days in a 37°C incubator. MEK inhibitor at varying concentrations was added to the cells in triplicate on day 0. MTT dissolved in 0.8% NaCl solution at 5 mg/mL was added to each well (0.2 mL) on day 2 to test GI50 or every day for cell growth curves. The cells were incubated at 37°C for 3 hours with MTT. The liquid was then aspirated from the wells and discarded. Stained cells were dissolved in 0.5 mL of DMSO and their absorption at 570 nm was measured using a Synergy HT multidetection microplate reader (BioTek Instruments). For GI50, cell growth was calculated as 100 × (T − T0)/(C − T0), where T is the optical density of the wells treated with inhibitors after a 48-hour period, T0 is the optical density at time zero, and C is the control optical density with DMSO only. GI50 was determined using Prism 3.0 software (GraphPad Software).
Western blot analysis
Protein extracts from PTC cell lines were prepared and analyzed as described previously (17). Tumor protein extracts were prepared by homogenizing tumor tissues from mice in radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L sodium chloride, 1% Tergitol-type NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 × PhosSTOP, and 1 × complete protease inhibitor cocktail from Roche) by a homogenizer (Pro200, Proscientific Inc.). Protein concentration was determined by Dc protein assay (Bio-Rad). The antibodies for p-ERK1/2, total ERK1/2, and poly (ADP-ribose) polymerase (PARP; Cell Signaling Technology) were used at a dilution of 1:1,000.
Cell death enzyme-linked immunosorbent assay
A cell death detection enzyme-linked immunosorbent assay (ELISAplus) was obtained from Roche Applied Science and was used per the manufacturer’s instructions. Briefly, 4 × 104 cells were plated in 24-well plates in triplicate the day before treatment. PTC cells were treated with 0.1 μmol/L PD0325901 for 96 hours. Cells treated with 1 μmol/L staurosporine served as positive controls for apoptosis (30). At the end of treatment, cells were lysed using the lysis buffer provided in the kit for 30 minutes at room temperature and then centrifuged in 24-well plates. Lysates (20 μL of supernatant) were transferred to streptavidin-coated wells and incubated for 2 hours at room temperature with two antibodies (biotin-labeled antihistone antibody and peroxidase-conjugated anti-DNA antibody). After the wells were washed three times, the samples were incubated with peroxidase substrate (ABTS) and the amount of colored product was determined spectrophotometrically at 405 nm. The background was measured at 490 nm.
Tumor growth in athymic mice using an orthotopic model
Establishment of the murine orthotopic thyroid carcinoma model was described previously (17, 31). Athymic Ncr-nu/nu mice were obtained from the National Cancer Institute at ages 6 to 8 weeks and housed for at least 1 week after arrival. All experimental procedures and care for mice were in accordance with the Institutional Animal Care and Use Committee and the Department of Veterinary Medicine of M.D. Anderson Cancer Center. Mice (10–14 per group) were anesthetized s.c. with a cocktail (100 μL/10 g body weight of 10 mg/mL keta-mine and 1 mg/mL xylazine). K2 and TPC-1 cells stably infected with a retrovirus expressing luciferase (5 × 105 cells in 5 μL RPMI1640 medium) were inoculated into the thyroid gland, and the mice were monitored weekly for tumor growth by Xenogen (IVIS 200 imaging system, Caliper Life Sciences,) using Living Image 3.0 software. One week after inoculation, PD0325901 was dissolved in 80 mmol/L citric buffer (pH 7) by sonication and given to mice daily by oral gavage (20–25 mg/kg) for 3 weeks (5 consecutive days/week). Mice were sacrificed only due to tumor burden or loss of 20% of body weight. Tumor sizes were measured with calipers and tumor volume (V) was calculated by the formula (V = length × width × depth). Control mice were given 80 mmol/L citric buffer (pH 7) alone. All in vivo experiments were done at least twice.
Statistical analysis
Statistical analysis was done using the HSD (honest significant difference) posthoc test from multifactorial ANOVA (STATISTICA data analysis software system version 7.1; StatSoft Inc.) for cell growth and cell cycle analysis. The unpaired Student’s t test was used for analysis of tumor volume. P ≤ 0.05 was considered significant.
Results
GI50 of PD0325901 in PTC cells
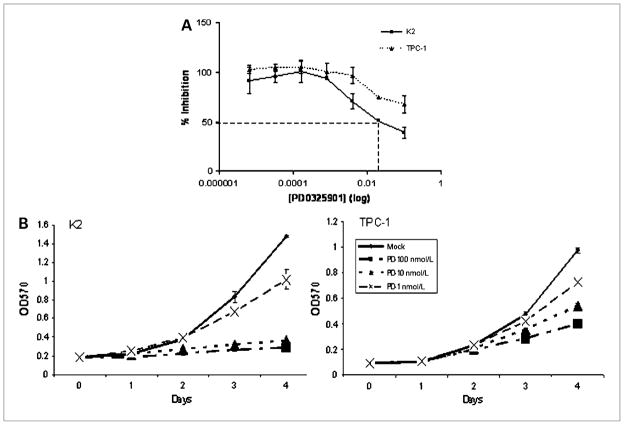
To assess the effects of PD0325901 on PTC cell growth, the GI50 was determined in PTC cell lines (TPC-1 and K2). Cells were treated in vitro with serial dilution of the PD0325901 ranging from 100 nmol/L to 0.0064 nmol/L. After 2 days of incubation with varying concentrations of PD0325901, cell growth was determined by MTT. The GI50 was 11 nmol/L for TPC-1 cells and 6.3 nmol/L for K2 cells, as determined by Prism software (Fig. 1A).
Figure 1.
PD0325901 inhibits PTC cell growth in vitro. A, PTC cells (K2 and TPC-1) were treated with PD0325901 for 2 days at 0.0064, 0.032, 0.16, 0.8, 4, 20, or 100 nmol/L. The MTT assay was used to determine the number of cells at each concentration by measuring the absorption at 570 nmol/L. The percent inhibition was calculated using Prism 3.0 software. The PD0325901 concentrations are shown in log scale (X scale). Each concentration was determined in triplicate for each type of PTC cell. This experiment was repeated twice. B, PTC cells (K2 and TPC-1) were treated with PD0325901 for 4 days at 1, 10, or 100 nmol/L. The MTT assay was used to determine the number of cells at each concentration by measuring the absorption at 570 nmol/L each day. Each concentration was determined in triplicates for each type of PTC cell. This experiment was repeated once.
Inhibition of cell growth with PD0325901 treatment
After determining the GI50 of PD0325901 in PTC cells, we treated PTC cells with PD0325901 at three different concentrations (100, 10, or 1 nmol/L) for 4 days. After 4 days of treatment, results of the MTT assay indicated that PD0325901 suppressed the cell growth by 80% (P < 0.0001), 75% (P < 0.0001), and 27% (P = 0.0015) in K2 cells, and by 58% (P < 0.0001), 40% (P = 0.0002), and 26% (P = 0.0001) in TPC-1 cells, respectively (Fig. 1B). Both K2 and TPC-1 cells showed dose-dependent growth inhibition with PD0325901. These data showed that PD0325901 significantly inhibited the growth of PTC cells harboring a BRAF mutation at very low concentration (10 nmol/L) and only moderately reduced the growth of the PTC cells carrying the RET/PTC1 rearrangement at the same concentration.
Decreased phosphorylation of ERK1/2 in PTC cells following PD0325901 treatment
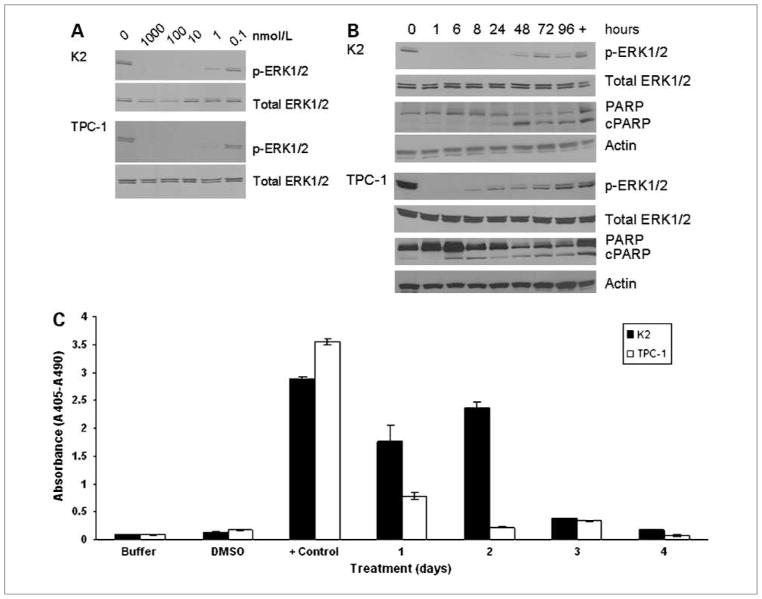
PD0325901 is a specific MEK1/2 inhibitor and the downstream effectors of these kinases are ERK1/2. The ability of PD0325901 to decrease the phosphorylation of ERK1/2 (p-ERK1/2) was examined by Western blot analysis. Following treatment with different concentrations of PD0325901 for 1 hour, the phosphorylation of ERK1/2 was reduced at 1 nmol/L and was completely abolished at 10 nmol/L in both K2 and TPC-1 cells (Fig. 2A). At 100 nmol/L, the dephosphorylation of ERK1/2 in K2 cells was detected as early as 1 hour, lasted up to 48 hours, and did not approach levels of untreated cells until 72 hours later (Fig. 2B). In TPC-1 cells, p-ERK1/2 signal became detectable again as early as 8 hours after treatment with PD0325901 and did not approach levels of untreated cells until 48 to 72 hours later (Fig. 2B). Five other PTC cell lines developed in our laboratory were also tested with 100 nmol/L PD0325901. These cell lines also exhibited decreased p-ERK1/2 levels after 1 hour of treatment (data not shown). These data showed that PD0325901 could effectively suppress the phosphorylation of ERK1/2 in multiple PTC cell lines.
Figure 2.
PD0325901 suppresses the expression of p-ERK1/2 and induces apoptosis in PTC cells. A, K2 cells (top) or TPC-1 cells (bottom) were treated for 1 hour at 37°C with 1,000, 100, 10, 1, or 0.1 nmol/L of PD0325901. B, K2 (top) or TPC-1 (bottom) cells were treated with 100 nmol/L of PD0325901 once at 0 hour and cells were harvested at 1, 6, 8, 24, 48, 72, or 96 hours. Western blot analysis showed the expression of p-ERK1/2 and cleaved PARP (cPARP). Total ERK1/2 and actin were used as loading controls. Cells treated with DMSO (0 hour) were used as a positive control for the expression of p-ERK1/2, and cells treated with 1 μmol/L staurosporine (+) for 8 hours were used as a positive control for apoptosis. The Western blot analysis was repeated twice. C, K2 or TPC-1 cells were treated with 100 nmol/L PD0325901 for 4 days. The Cell Death Detection ELISAPLUS photometric enzyme immunoassay was used to detect DNA fragmentation with absorbance at 405 nm and subtracted from a background fluorescence at 490 nm. Each data point was determined in duplicate and the assay was repeated twice. Cells treated with DMSO (DMSO) and lysis buffer only (buffer) in ELISA were used as negative controls. Cells treated with 1 μmol/L staurosporine for 24 hours (+ control) were used as a positive control for apoptosis.
Apoptosis detected in PTC cells after PD0325901 treatment
To determine the mechanism of cell-growth inhibition in PTC cells by PD0325901, we used Western blot analysis to evaluate the expression and cleavage of caspase 3 substrate PARP as an indicator of apoptosis. K2 cells showed cleaved PARP after 48 hours of treatment with PD0325901, and expression remained detectable for up to 4 days (Fig. 2B). Cleaved PARP was also detected in TPC-1 cells especially at 6 and 8 hours after treatment (Fig. 2B). It was not clear from the Western blot analysis if cleaved PARP was detected in other time points for TPC-1 cells, because a trace amount of cleaved PARP was present in untreated cells (0 h).
To confirm apoptosis in PTC cells, a cell death ELISAplus assay was used to quantitatively measure DNA fragmentation during apoptosis. PTC cells were treated with 100 nmol/L PD0325901 for up to 4 days. DNA fragmentation was detected in 1 and 2 days of PD0325901 treatment in K2 cells and in 1 day for TPC-1 cells (Fig. 2C).
Inhibition of tumor growth by PD0325901 in a murine orthotopic xenograft model
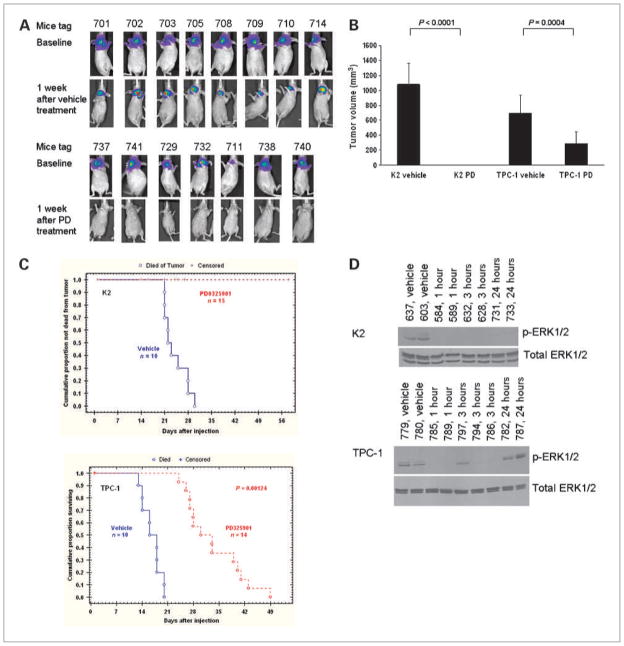
The inhibitory effects of PD0325901 were evaluated in vivo using a murine orthotopic xenograft model. PTC cells expressing luciferase were inoculated in situ into the right thyroid lobe of Ncr-nu/nu mice. A group of 10 mice was randomly selected after Xenogen luciferase bioimaging and used for treatment with vehicle, and 14 to 16 mice were used for the PD0325901 treatment group. One week after inoculation, PD0325901 was given to these mice (see Materials and Methods) for 3 weeks at 20 to 25 mg/kg. Tumor growth was monitored weekly by Xenogen luciferase bioimaging. Tumor sizes were measured and tumor volume was calculated if mice were sacrificed due to loss of 20% initial body weight or tumor burden. No tumors were detected by Xenogen in mice inoculated with K2 cells after 1 week of PD0325901 treatment, whereas mice treated with vehicle still showed intense luciferase expression (Fig. 3A). The treated mice remained tumor free during the 3-week treatment period (data not shown). At the end of 3 weeks, all mice inoculated with K2 and treated with vehicle were sacrificed with average tumor volume of 1,078.7 ± 285.3 mm3 (Fig. 3B). Tumor regression was apparent in 100% of mice harboring K2 tumors and treated with PD0325901, and therefore no mice in the treatment group died by 30 days due to tumor burden, whereas nearly 100% of mice treated with vehicle died within the same period (Fig. 3C). In mice inoculated with TPC-1 cells, the average tumor volume after 3 weeks of PD0325901 treatment was reduced by 58.3% (from 699 to 291.3 mm3; P = 0.0004) when compared with tumors from untreated (vehicle) mice (699 ± 241.8 mm3). In addition, the survival of the mice inoculated with TPC-1 cells was increased significantly compared with mice treated with vehicle (Fig. 3C). Median survival was 16 to 18 days in vehicle-treated animals and 30 to 33 days in PD0325901-treated mice (P = 0.00124). Similar to CI-1040, the solubility of PD0325901 remains an issue. The solubility of PD0325901 at pH 7 is only at 0.39 mg/mL. The poor solubility of PD0325901 resulted in a high percentage of censored deaths in mice inoculated with K2 cells (47–93%; Fig. 3C), although necropsy of these mice showed that no tumor was present in the thyroid. These data showed that PD0325901 suppressed tumor growth completely in mice inoculated with PTC cells carrying a BRAF mutation (K2) and significantly decreased tumor growth in mice inoculated with PTC cells carrying the RET/PTC1 rearrangement (TPC-1).
Figure 3.
PD0325901 inhibits tumor growth in mice. A, in the control group, images from Xenogen showed 8 mice inoculated with PTC cells carrying a BRAF mutation before treatment (baseline, 6 days after inoculation, top) and images after treatment with vehicle for 5 days (2nd panel). In the treatment group, 7 mice inoculated with K2 cells before treatment (baseline, 6 days after inoculation, 3rd panel) and images after treatment with 25 mg/kg/day of PD0325901 (PD) for 5 days (bottom). B, tumor volume (mm3) was calculated after mice were sacrificed and the tumor size was measured by a caliper using the formula: length (mm) × width (mm) × depth (mm). Both mice treated with PD0325901 (PD) and vehicle are shown here. C, Kaplan-Meier survival curves are shown for mice inoculated with K2 (top) or TPC-1 (bottom) and treated with vehicle or PD0325901. The in vivo study was repeated twice. D, mice (each identified by its ear tag number) inoculated with K2 cells (top) or TPC-1 cells (bottom) were treated with 20 mg/kg PD0325901 for 1, 3, or 24 hours. Tumors were harvested at the indicated time points and protein extracts were prepared. The expression of p-ERK1/2 was detected by Western blot analysis. Total ERK1/2 was used as a loading control. Mice treated with vehicle were used as positive controls for the expression of p-ERK1/2. Each mouse is identified by its tag number.
To confirm the molecular mechanism underlying PD0325901 suppression of tumor growth in vivo, mice were inoculated with K2 or TPC-1 cells. When tumor reached visible size as monitored by Xenogen imaging (between 10 and 14 days), the mice were treated with PD0325901. Tumor was harvested at 1, 3, or 24 hours after PD0325901 treatment and the expression of p-ERK1/2 was detected by Western blot analysis (Fig. 3D). Phosphorylation of ERK1/2 was suppressed as early as 1 hour following PD0325901 treatment in mice inoculated with either K2 or TPC-1 cells, and this suppression lasted at least 24 hours in mice inoculated with K2 cells. In mice inoculated with TPC-1 cells, the phosphorylation of ERK1/2 was detectable in one of three mice after 3 hours of PD0325901 treatment and returned to pretreatment level by 24 hours (Fig. 3D). These data verify PD0325901 inhibition of the ERK1/2 phosphorylation in vivo.
Discussion
The MEK/ERK signal transduction pathway has been a therapeutic target for many different types of human cancers because mutations leading to constitutive activation of this pathway result in malignant and aggressive tumors. BRAF mutations have been detected in >60% of melanoma (32), and at lower frequencies in breast cancer (33), NSCLC (34), and colon cancer (4, 35). In PTC, the rate of BRAF mutation varies from 29% to 83%, depending on the cohort being studied (4, 36). RET/PTC rearrangements are unique in thyroid carcinoma, representing 2.5% to 67% of PTC, depending on the cohort being studied (36–38). Both types of mutations are able to activate the MEK/ERK signal transduction pathway.
Several specific MEK1/2 inhibitors have been tested in PTC, including PD98059 (16), U0126 (16), and CI-1040 (15, 17). Both PD98059 and U0126 are available for in vitro study only. We found that the GI50 of CI-1040 in PTC cells carrying a BRAF mutation was 52 nmol/L (17), with the value much higher in PTC cells with the RET/PTC1 rearrangement (1.1 μmol/L). CI-1040 treatment at 300 mg/kg/day reduced the mean tumor volume by 31.3% in mice inoculated with PTC cells carrying a BRAF mutation and by 47.5% in mice inoculated with PTC cells carrying the RET/PTC1 rearrangement (17), compared with mice treated with vehicle.
The second-generation MEK1/2 inhibitor PD0325901 seems to be more potent. The GI50 of PD0325901 was 6.3 nmol/L (K2 cells in this study) or 131 nmol/L (BCPAP by Liu et al.; ref. 39) in PTC cells carrying a BRAF mutation, and 11 nmol/L (TPC-1 cells in this study), 66 nmol/L (TPC-1 cells reported by Leboeuf et al.; ref. 22), or 13 μmol/L (TPC-1 cells reported by Liu et al.; ref. 39) in PTC cells with the RET/PTC1 rearrangement. At a PD0325901 dose of 20 to 25 mg/kg/day, complete suppression of tumor growth was observed in mice inoculated with PTC cells carrying a BRAF mutation within 1 week of PD0325901 treatment, and tumor volume was reduced by 58.3% in mice inoculated with PTC cells carrying the RET/PTC1 rearrangement compared with mice treated with vehicle in this study. Although PD0325901 did not completely suppress the tumor growth in mice inoculated with PTC cells carrying the RET/PTC1 rearrangement, significant improvement in survival was observed in these mice, with survivals of 16 to 18 days in the vehicle group and 30 to 33 days in the PD0325901-treated group. The increased potency of PD0325901 has also been observed in other types of cancer. In human melanoma cell lines with a BRAF mutation, the IC50 of PD0325901 was 20 to 50 nmol/L and tumor growth in xenografts was suppressed by 60% to 65% in mice treated with 50 mg/kg/day of PD0325901 compared with vehicle control (40). PD0325901 was able to completely suppress hepatocellular carcinoma tumor growth in mice after 20 or 35 weeks of treatment at 1 mg/kg/day (41). In a NSCLC cell line carrying a BRAF mutation, the IC50 of PD0325901 was 10 nmol/L and various degrees of tumor growth suppression were observed at 20 mg/kg/day (34). Our study showed differential inhibition of PTC depending upon the activating mutation present within the tumor. PTC with a BRAF mutation showed increased response to PD0325901, as compared with PTC with the RET/PTC1 rearrangement.
The differences in response of PTC cells with a BRAF mutation (K2) and PTC cells carrying the RET/PTC1 rearrangement (TPC-1) to PD0325901 were noticeable either in vitro or in vivo, as well as in our previous studies using MEK1/2 inhibitors (16, 17). In vitro studies showed the extended suppression of p-ERK1/2 expression in K2 cells after treating cells with 100 nmol/L PD0325901 (up to 48 hours) and only limited suppression of p-ERK1/2 in TPC-1 cells (6–8 hours); we observed higher suppression of K2 cell growth at 10 and 100 nmol/L of PD0325901 (75 and 80%, respectively) and lower suppression of TPC-1 cell growth (40 and 58%); and finally, PD0325901 completely suppressed tumor growth in mice inoculated with K2 cells after just 5 days of treatment, and mice were tumor free for weeks in our in vivo studies. PD0325901 decreased TPC-1 tumor growth to a lesser degree, although both tumor volume and animal survival were statistically improved in TPC-1 mice treated with PD0325901 compared with vehicle. These differences suggest that although both BRAF mutation and the RET/PTC1 rearrangement are able to activate the MEK/ERK signal transduction pathway (2, 13, 42), other pathways not affected by PD0325901 may be involved in PTC cells with the RET/PTC rearrangements. Further exploration of other signal transduction pathways is needed to fully understand the molecular characterization of these PTC cells.
PD0325901 has been tested in phase I and II clinical trials in patients with breast cancer, colon cancer, NSCLC, or melanoma. The drug was well tolerated by patients, with dose-dependent skin, gastrointestinal dysfunction, and retinal side effects (23, 24, 43, 44). However, the clinical trials were terminated early due to ocular and neurologic toxicity and lack of responses. Based on our findings, a clinical trial of PD0325901 in treating PTC may have a better response rate because a higher percentage of PTC patients carry a BRAF mutation as compared with other tumors. Although a high rate of BRAF mutation (60%) was detected in melanoma (45), PTC cells carrying a BRAF mutation seemed to be more sensitive to PD0325901 than human melanoma cells; the rate of tumor suppression was 60% to 65% in melanoma xenografts (40) and 100% in our orthotopic mouse model. A lower dose of PD0325901 may also be used in humans to reduce those side effects observed in phase I and II clinical trials (24). In our in vivo mouse study, tolerance seemed to be better when we reduced the dose of PD0325901 from 25 mg/kg/day to 20 mg/kg/day (data not shown).
Our study was the first examination of the MEK1/2 inhibitor PD0325901 in PTC cells in vivo. PD0325901 effectively inhibited PTC cell growth in vitro by suppressing the expression of p-ERK1/2 and potently suppressed or delayed PTC tumor growth in mice. PD0325901 seemed to be more effective in PTC cells carrying a BRAF mutation than in PTC cells with the RET/PTC1 rearrangement. Any potential clinical trial for PD0325901 in PTC should incorporate the screening of PTC patients for a BRAF mutation, as those patients may have tumors more responsive to PD0325901.
Acknowledgments
We thank Dr. Carole Klingerman and Dr. Kathleen Zandi from Pfizer (New York, NY) for providing PD0325901 and information about PD0325901; Dr. Jerome Hershman from VA Greater Los Angeles Healthcare System (Los Angeles, CA) for providing TPC-1 cells; Dr. D. Wynford-Thomas from Cardiff University (Cardiff, United Kingdom) for providing K2 cells; Dr. Ge Zhou for providing retrovirus carrying luciferase gene; Samar Jasser, Kelli Brown-Cottingham, Dr. Maria Gule, and Dr. Tongwei Xie for technical support; and Michael Worley for text editing.
Grant Support
NIH Independent Award R01 DE-13954, NIH Specialized Program of Research Excellence grant in Head and Neck Cancer 1P50-CA-97007, M.D. Anderson Cancer Center Support grant 5P30 CA 16672, the Michael A. O’Bannon Endowment for Cancer Research, the Betty Berry Cancer Research Fund, and National Cancer Institute Cancer Center Support (CORE) Grant CA 16672 for media production.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ain KB. Papillary thyroid carcinoma. Etiology, assessment, and therapy. Endocrinol Metab Clin North Am. 1995;24:711–60. [PubMed] [Google Scholar]
- 2.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 3.Wojciechowska K, Lewinski A. BRAF mutations in papillary thyroid carcinoma. Endocr Regul. 2006;40:129–38. [PubMed] [Google Scholar]
- 4.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 5.Bongarzone I, Monzini N, Borrello MG, et al. Molecular characterization of a thyroid tumor-specific transforming sequence formed by the fusion of ret tyrosine kinase and the regulatory subunit RI α of cyclic AMP-dependent protein kinase A. Mol Cell Biol. 1993;13:358–66. doi: 10.1128/mcb.13.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60:557–63. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- 7.Jhiang SM, Mazzaferri EL. The ret/PTC oncogene in papillary thyroid carcinoma. J Lab Clin Med. 1994;123:331–7. [PubMed] [Google Scholar]
- 8.Santoro M, Melillo RM, Carlomagno F, Vecchio G, Fusco A. Minireview: RET: normal and abnormal functions. Endocrinology. 2004;145:5448–51. doi: 10.1210/en.2004-0922. [DOI] [PubMed] [Google Scholar]
- 9.Santoro M, Dathan NA, Berlingieri MT, et al. Molecular characterization of RET/PTC3; a novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–16. [PubMed] [Google Scholar]
- 10.Jhiang SM. The RET proto-oncogene in human cancers. Oncogene. 2000;19:5590–7. doi: 10.1038/sj.onc.1203857. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21 (Suppl 2):S37–43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 14.Ji H, Wang Z, Perera SA, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007;67:4933–9. doi: 10.1158/0008-5472.CAN-06-4592. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Liu Z, Jiang D, Dackiw AP, Xing M. Inhibitory effects of the mitogen-activated protein kinase kinase inhibitor CI-1040 on the proliferation and tumor growth of thyroid cancer cells with BRAF or RAS mutations. J Clin Endocrinol Metab. 2007;92:4686–95. doi: 10.1210/jc.2007-0097. [DOI] [PubMed] [Google Scholar]
- 16.Henderson YC, Fredrick MJ, Clayman GL. Differential responses of human papillary thyroid cancer cell lines carrying the RET/PTC1 rearrangement or a BRAF mutation to MEK1/2 inhibitors. Arch Otolaryngol Head Neck Surg. 2007;133:810–5. doi: 10.1001/archotol.133.8.810. [DOI] [PubMed] [Google Scholar]
- 17.Henderson YC, Ahn S-H, Clayman GL. Inhibition of the growth of papillary thyroid carcinoma cells by CI-1040. Arch Otolaryngol Head Neck Surg. 2009;135:347–54. doi: 10.1001/archoto.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson YC, Ahn S-H, Kang Ya, Clayman GL. Sorafenib potently inhibits papillary thyroid carcinomas harboring RET/PTC1 rearrangement. Clin Cancer Res. 2008;14:4908–14. doi: 10.1158/1078-0432.CCR-07-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett SD, Bridges AJ, Dudley DT, et al. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg Med Chem Lett. 2008;18:6501–4. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Thompson N, Lyons J. Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol. 2005;5:350–6. doi: 10.1016/j.coph.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Wang JY, Wilcoxen KM, Nomoto K, Wu S. Recent advances of MEK inhibitors and their clinical progress. Curr Top Med Chem. 2007;7:1364–78. doi: 10.2174/156802607781696837. [DOI] [PubMed] [Google Scholar]
- 22.Leboeuf R, Baumgartner JE, Benezra M, et al. BRAFV600E mutation is associated with preferential sensitivity to mitogen-activated protein kinase kinase inhibition in thyroid cancer cell lines. J Clin Endocrinol Metab. 2008;93:2194–201. doi: 10.1210/jc.2007-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorusso P, Krishnamurthi S, Rinehart JR, et al. A phase 1–2 clinical study of a second generation oral MEK inhibitor, PD 0325901 in patients with advanced cancer. J Clin Oncol (Meeting Abstracts) 2005;23:3011. [Google Scholar]
- 24.Wang D, Boerner SA, Winkler JD, LoRusso PM. Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta. 2007;1773:1248–55. doi: 10.1016/j.bbamcr.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Ohta K, Pang XP, Berg L, Hershman JM. Antitumor actions of cytokines on new human papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1996;81:2607–12. doi: 10.1210/jcem.81.7.8675585. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka J, Ogura T, Sato H, Hatano M. Establishment and biological characterization of an in vitro human cytomegalovirus latency model. Virology. 1987;161:62–72. doi: 10.1016/0042-6822(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 27.Ohta K, Pang XP, Berg L, Hershman JM. Growth inhibition of new human thyroid carcinoma cell lines by activation of adenylate cyclase through the β-adrenergic receptor. J Clin Endocrinol Metab. 1997;82:2633–8. doi: 10.1210/jcem.82.8.4136. [DOI] [PubMed] [Google Scholar]
- 28.Challeton C, Branea F, Schlumberger M, et al. Characterization and radiosensitivity at high or low dose rate of four cell lines derived from human thyroid tumors. Int J Radiat Oncol Biol Phys. 1997;37:163–9. doi: 10.1016/s0360-3016(96)00449-x. [DOI] [PubMed] [Google Scholar]
- 29.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–41. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertrand R, Solary E, O’Connor P, Kohn KW, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994;211:314–21. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
- 31.Ahn SH, Henderson Y, Kang Y, et al. An orthotopic model of papillary thyroid carcinoma in athymic nude mice. Arch Otolaryngol Head Neck Surg. 2008;134:190–7. doi: 10.1001/archoto.2007.36. [DOI] [PubMed] [Google Scholar]
- 32.Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 2008;68:5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- 33.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5:195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 34.Legrier ME, Yang CP, Yan HG, et al. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67:11300–8. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- 35.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev. 2004;4:937–47. doi: 10.1038/nrc1503. [Review] [110 refs] Cancer. [DOI] [PubMed] [Google Scholar]
- 36.Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15:485–91. doi: 10.1158/1078-0432.CCR-08-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–41. doi: 10.1210/en.2006-0921. [DOI] [PubMed] [Google Scholar]
- 38.Cinti R, Yin L, Ilc K, et al. RET rearrangements in papillary thyroid carcinomas and adenomas detected by interphase FISH. Cytogenet Cell Genet. 2000;88:56–61. doi: 10.1159/000015485. [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Xing M. Potent inhibition of thyroid cancer cells by the MEK inhibitor PD0325901 and its potentiation by suppression of the PI3K and NF-κB pathways. Thyroid. 2008;18:853–64. doi: 10.1089/thy.2007.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciuffreda L, Del Bufalo D, Desideri M, et al. Growth-inhibitory and antiangiogenic activity of the MEK inhibitor PD0325901 in malignant melanoma with or without BRAF mutations. Neoplasia. 2009;11:720–31. doi: 10.1593/neo.09398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wentz SC, Wu H, Yip-Schneider MT, et al. Targeting MEK is effective chemoprevention of hepatocellular carcinoma in TGF-α-transgenic mice. J Gastrointest Surg. 2008;12:30–7. doi: 10.1007/s11605-007-0396-4. [DOI] [PubMed] [Google Scholar]
- 42.Knauf JA, Kuroda H, Basu S, Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003;22:4406–12. doi: 10.1038/sj.onc.1206602. [DOI] [PubMed] [Google Scholar]
- 43.Sebolt-Leopold JS. Advances in the development of cancer therapeutics directed against the RAS-mitogen-activated protein kinase pathway. Clin Cancer Res. 2008;14:3651–6. doi: 10.1158/1078-0432.CCR-08-0333. [DOI] [PubMed] [Google Scholar]
- 44.Hersey P, Bastholt L, Chiarion-Sileni V, et al. Small molecules and targeted therapies in distant metastatic disease. Ann Oncol. 2009;20(Suppl 6):vi35–40. doi: 10.1093/annonc/mdp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sosman JA, Margolin KA. Inside life of melanoma cell signaling, molecular insights, and therapeutic targets. Curr Oncol Rep. 2009;11:405–11. doi: 10.1007/s11912-009-0054-y. [DOI] [PubMed] [Google Scholar]