Abstract
Background
The mechanisms whereby melanocytes populate the epidermis and developing hair follicles during embryogenesis are incompletely understood. Recent evidence implicates an intermediate mesenchymal stage in this evolutionary process in which HMB-45-positive melanocyte precursors (`melanoblasts') exist both in intradermal as well as intraepithelial and intrafollicular compartments. The melanocyte master transcriptional regulator, microphthalmia transcription factor (MITF), identifies mature melanocytes as well as melanocyte precursor stem cells that reside in the bulge region of the hair follicle.
Methods
To better define the use of MITF expression in the evaluation of melanocyte ontogeny, human embryonic and fetal skin samples (n = 28) at 6-24 weeks gestation were studied immunohistochemically for expression of MITF and Mart-1. Adjacent step sections were evaluated to correlate staining patterns with cell localization in the intraepidermal, intrafollicular and intradermal compartments.
Results
At 6-8 weeks, MITF and Mart-1-positive cells were primarily intradermal with only rare positive cells in the epidermis. By 12-13 weeks, most of these cells had migrated into the epidermis, predominantly the suprabasal layers. Between 15-17 weeks, these cells localized to the basal layer and colonized developing hair follicles. Rare intradermal MITF and Mart-1 positive cells were found as late as week 20. At 18-24 weeks, MITF and Mart-1 positive cells were identified in the outer root sheath, bulge, and follicular bulge epithelium, in addition to the epidermis. Unexpectedly, weak but diffuse nuclear MITF expression was also present in the keratinocytes of the bulge area.
Conclusions
The in situ migratory fate of MITF/Mart-1-expressing cells in fetal skin involves a well-defined progression from intradermal to intraepidermal to intrafollicular localization. Occasional intradermal melanocytes may persist after the intraepithelial stages are completed, a finding of potential significance to melanocytic proliferations that may arise de novo within the dermis. Because MITF may play a role in stem cell maintenance, the presence of MITF in bulge epithelial cells suggests that it may be a novel marker for follicular stem cells of both epithelial and melanocytic lineage.
Melanocytes are derived from neural crest cells, which acquire pigment-producing capabilities upon migration to the skin.1 The sequence of melanocyte precursor (`melanoblast') migration into the developing epidermis and hair follicles has been characterized in murine models,2-4 but little is known about these pathways in human embryonic and fetal development. HMB45-positive cells were recently identified within the human fetal dermis, potentially representing an intermediate stage in melanoblast migration.5
In this study, we investigated the localization of melanoblasts at various time points in human embryonic and fetal skin development, using microphthalmia transcription factor (MITF) in conjunction with Mart-1 (Melanoma Antigen Recognized by T cells) immunohistochemistry. MITF, a nuclear transcription factor best known as a master regulator of melanocyte development, is also found in other cell types.6 Functionally, MITF plays an anti-apoptotic role,7 and in conjunction with BRAF, may serve as a melanoma oncogene.8 Mart-1, originally identified as a melanoma antigen recognized by T cells in patients with metastatic melanoma,9 is a melanocyte-specific protein whose gene expression is regulated by MITF.10 The use of Mart-1 in addition to MITF provides enhanced specificity for detection of melanocytic differentiation, and, as a cytoplasmic protein, Mart-1 also facilitates visualization of cell shape.
Materials and methods
Discarded human fetal tissue was sampled during routine specimen processing of embryos and fetuses in the Women's and Perinatal Division of Brigham and Women's Hospital between 2004 and 2006 with the approval of the Partners Human Research Committee. Twenty-eight human embryonic and fetal skin specimens were obtained from conceptuses of 6-24 weeks estimated gestational age (EGA) (Table 1). EGA was determined from maternal history (date of last menstrual period), ultrasound measurements during pregnancy and/or fetal measurements at the time of fetopsy (foot length).
Table 1.
Distribution of MITF and Mart-1 positive cells by EGA*
| EGA | Intradermal (per HPF) | Epidermis, suprabasal (per HPF) | Epidermis, basal (per HPF) | Hair peg | ORS | Hair matrix | Bulge area |
|---|---|---|---|---|---|---|---|
| 6-8 weeks (n = 2) | 15.2/17.2† | N/A | <0.1/<0.1 | N/A | N/A | N/A | N/A |
| 12-13 weeks (n = 3) | <0.1/<0.1 | 3.4 ± 1.28/4.6 ± 1.5 | 1.2 ± 0.8/0.9 ± 0.3 | N/A | N/A | N/A | N/A |
| 15-17 weeks (n = 4) | <0.05/<0.05 | 1.0 ± 1.0/1.3 ± 1.2 | 6.6 ± 1.1/7.5 ± 2.0 | 2.5 ± 0.7/ 2.8 ± 0.9 | N/A | N/A | N/A |
| 18-24 weeks (n = 19) | <0.2/<0.2 | <0.1/<0.1 | 5.5 ± 1.7/6.4 ± 1.5 | N/A | 4.6 ± 1.42/ 4.6 ± 1.1 | 4.2 ± 1.68/ 4.6 ± 1.65 | 1.7 ± 0.73/ 1.6 ±1.0 |
EGA, estimated gestational age; ORS, outer root sheath; N/A, not applicable.
Mean number of MITF-positive cells/Mart-1 positive cells per HPF or per hair follicle structure ± standard deviation.
Standard deviation not calculable (n = 2).
Each skin sample was examined on hematoxylin and eosin (H&E)-stained sections, and the stage of epidermal, dermal and hair follicle development was noted. Adjacent sections separated by 6 microns were stained with antibodies to MITF (clone D511) and Mart-1 (Signet Laboratories, Dedham, MA, USA; clone M2-7C10, dilution 1 : 200). Heat-induced epitope retrieval was used for both antibodies. All antigens were localized using the avidinbiotin complex method with a secondary incubation using the Envision Plus detection system (Dako, Carpinteria, CA, USA) and 3,3′-diaminobenzidine as a chromogen. Slides were counterstained with hematoxylin. Normal adult human skin and an epithelioid malignant melanoma was used as positive controls for MITF and Mart-1, and negative controls were performed by substituting phosphate-buffered saline for the primary antibody. Only nuclear staining for MITF was considered positive. The number of MITF and Mart-1 positive cells was counted per high power field (HPF) (epidermis and dermis) and per identifiable follicular structure (i.e., hair germ, outer root sheath (ORS), hair matrix, bulge) for each case. Between 5 and 10 HPF or 5 and 10 follicular structures were counted in each section, depending on the amount of tissue and number of evaluable hair follicles present. The mean was calculated for each case, and using these values, the mean for each gestational age group was calculated for each site.
Results
The distribution of melanocytes at each gestational age as determined by MITF and Mart-1 staining is summarized in Table 1. At 6-8 weeks EGA (n = 2), numerous MITF and Mart-1-positive cells were present within the dermis (15.2 per single HPF and 17.2 per single HPF, respectively; Fig. 1A). Based on cell number and distribution, these two populations appeared to be coincident, although these cells could not be distinguished from other primitive intradermal cells on routine H&E-stained sections. However, MART-1-positive cells consistently equaled or slightly exceeded MITF-positive cells in number in all samples, an observation that along with coincident spatial distribution, suggested that MITF-positive non-melanocytic cells were not significantly represented. Because MITF and Mart-1-positive cell distribution and number was qualitatively identical and quantitatively similar (correlation coefficient: r = 0.90076), the data is presented as the ratio of MITF/Mart-1 (Table 1), The labeled intradermal cells had a dendritic appearance on the Mart-1 stain, which highlighted cytoplasmic processes in addition to cell bodies (Fig. 1B). Only rare intraepidermal cells were identified at this juncture (<1/10 HPF on both MITF and Mart-1 stains).
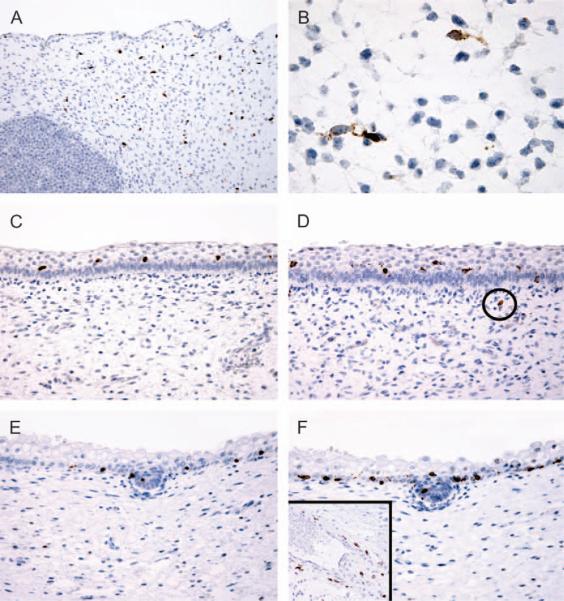
Fig. 1.
Immunohistochemical evaluation of microphthalmia transcription factor (MITF)/Mart-1 reactivity in developing fetal skin. Numerous intradermal melanoblasts are seen on MITF (A) and Mart-1 (B) stains in skin of a 6-8 week fetus; the Mart-1 stain highlights the dendritic cytoplasmic processes. Positively-labeled cells are primarily suprabasal at 12-13 weeks (MITF, C; Mart-1, D); a single intradermal melanocyte (encircled) is seen on the Mart-1 stain. By 15 weeks, MITF-positive (E) and Mart-1-positive (F) cells are primarily basally located within the epidermis and also are randomly detected in developing hair pegs. Persistent intradermal melanocytes are often clustered around blood vessels at this stage (F, inset: Mart-1 stain).
By 12-13 weeks EGA (n = 3), the majority of the MITF and Mart-1-positive cells were intraepidermal, predominantly localized to the suprabasal layers (Fig. 1C,D). Persistent intradermal MITF-and Mart-1-positive cells were identified in 2 of 3 cases, often in a perivascular distribution, but, compared to the 6-8 week skin specimens, these were markedly decreased in number (2 per 10 HPF in one case and less than 1 per 10 HPF in the other).
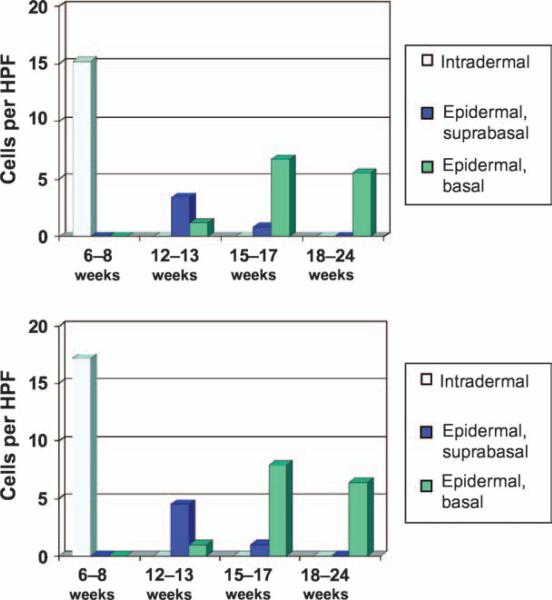
At 15-17 weeks (n = 4), the vast majority (approximately 90%) of the intraepidermal MITF and Mart-1-positive cells had localized to the basal layer (Fig. 1E,F). These cells appeared to be randomly distributed along the basal layer of the epidermis without evidence of clustering or an identifiable relationship to the developing hair follicles. MITF and Mart-1 positive cells were identified in small numbers (1-3 cells) in individual developing hair pegs at this stage. Intradermal cells positive for MITF and Mart-1 were identified in only 1 of the 4 cases and numbered less than 1 per 10 HPF. Similar to the 12-13 week specimens, these cells appeared to be preferentially located in a perivascular distribution (Fig. 1F, inset). The relative numerical trends of MITF and Mart-1-positive cells in intradermal and intraepidermal compartments over time are summarized in Fig. 2.
Fig. 2.
Distribution of the microphthalmia transcription factor-positive cells (top) and Mart-1-positive cells (bottom) by gestational age.
Beginning at 18 weeks, when distinct components of the hair follicle were first recognizable (Fig. 3), MITF and Mart-1 positive cells were identifiable in the ORS, the follicular bulge region, and the follicular bulb epithelium, where they formed a cap over the follicular papilla (not shown), similar to that seen in adult skin. The number of melanocytes in these 2 components of the hair follicle was maintained in stable numbers from 18 to 24 weeks (n = 19), as the follicle matured. Intradermal melanocytes were identified in only 2 of the 19 cases at this stage: one 19-week fetus had scattered intradermal MITF and Mart-1 positive cells (mean, 5 per 10 HPF) and one 20-week fetus had numerous intradermal MITF and Mart-1 positive cells (mean, 3 per HPF). In both cases, the intradermal melanocytes were found in the lower half of the dermis, often adjacent to vessels.
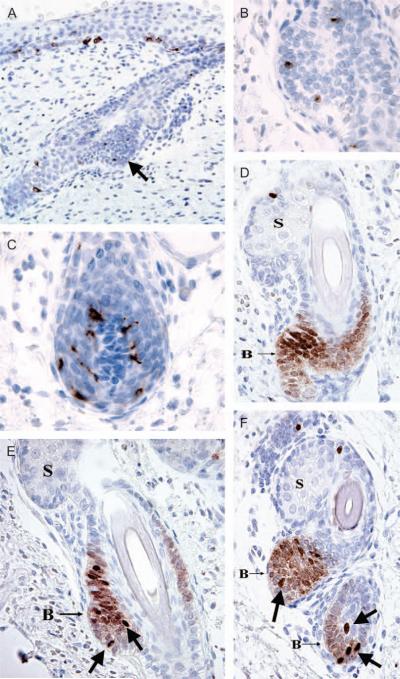
Fig. 3.
Microphthalmia transcription factor (MITF) and Mart-1 staining patterns in maturing hair follicles. Beginning at 18 weeks, the bulge area was first identified (a, arrow). The Mart-1 stain (a) showed ovoid, non-dendritic melanocyte precursors in the bulge area (b, higher magnification), in addition to dendritic melanocytes in the epidermis, outer root sheath and hair matrix (c). MITF staining showed 2 patterns (d-f): strong nuclear reactivity in epidermal and follicular melanocytes and bulge melanocyte precursors (arrows, e and f), and weaker nuclear staining in bulge keratinocytes (B). (S, developing sebaceous lobule).
The MITF and Mart-1 positive cells identified within the bulge region of the hair follicle beginning at 18 weeks were relatively ovoid and non-dendritic (Fig. 3B), in contrast to the melanocytes within the dermis, epidermis, ORS and follicular bulb (Fig. 3C), all of which showed dendritic processes on the Mart-1 stain at all stages of development. Less intense but diffuse nuclear positivity for MITF was identified in the keratinocytes of the bulge areas from 18 to 24 weeks (Fig. 3D-F). No other evidence of keratinocyte MITF expression was noted in any other epithelial compartments at any of the evaluated gestational ages.
To exclude the possibility that fetal stress (e.g. because of stillbirths) versus elective abortion) may have influenced the results, we compared these two groups for MITF and Mart-1 staining. There were four stillbirths, all in the 18-24 months EGA interval; There was no significant between these cases and the elective abortions with respect to qualitative observations or MITF and Mart-1 counts (t-test p values 0.38 and 0.34 for MITF and Mart-1, respectively).
Discussion
Melanocytes of haired lower mammals, located principally in hair follicles and intradermally, function to determine hair color, which may vary in response to melatonin levels regulated by seasonal light cycles.12 In contrast, human melanocytes are more concentrated in the epidermal layer where they provide photoprotection in response to the ultraviolet light regulated p53-melanocyte-stimulating hormone pathway.13 Accordingly, the ability of melanocytes to migrate effectively to epidermal as well as follicular destinations during human embryogenesis may be of importance in establishing normal post-natal photoprotective function.
Human hair follicle development can be classified into four stages.14 The first stage is marked by formation of the hair germ, which is followed in the second stage by development of the hair peg, a cord of epidermal cells growing from the basal layer of the epidermis into the dermis. During the third stage, known as the bulbous hair peg stage, the ORS differentiates into the primordial sebaceous gland, and the bulge area of the hair follicle can first be identified. The final stage of hair follicle maturation is characterized by differentiated lanugo hair follicles, which are formed late in the second trimester. Although the development of the follicular epithelial component from the epidermal layer is well described, the sequence of melanocyte colonization of the epidermis and developing human hair follicle has received little attention.
Studies of melanoblast migration during murine embryonic development reported a consistent progression from intradermal to suprabasal intraepidermal to basal intraepidermal localization followed by follicular colonization.3 While substantial structural and functional differences exist between the murine and human integument, our data showed a similar pattern in human cutaneous embryogenesis. Dendritic melanocyte precursors were intradermal at 6-8 weeks. At 12-13 weeks, these melanoblasts penetrated the epidermis and were localized primarily to the suprabasal layers. By 15-17 weeks, however, these cells were predominantly basally located within the epidermis. In this regard, a recent report implicated the matricellular protein CCN3 and its receptor DDR1 as critical mediators of the basal localization of mature melanocytes.15 Although expression of CCN3 and DDR1 has not been examined in immature melanocytes, it is possible that one or both proteins are upregulated at 13-15 weeks, which then stabilizes and anchors maturing melanocytes to the basal layer. No clustering of melanocyte precursors within the basal layer was noted prior to the development of the hair germ. In addition, no consistent pattern of melanocyte colonization of the follicle was identified: some hair germs and hair pegs lacked melanocyte precursors entirely, whereas others contained between 1 and 3 melanocytic cells. The lack of association between melanocytes precursors and the developing hair follicles suggests that melanocyte migration and epithelial hair follicle development are independent events at this juncture. As seen in Fig. 2, at certain time-points the overall number of intradermal MITF and Mart-1-positive cells tends to outnumber those that ultimately localized within the epidermal layer. This raises possibilities that include cell attrition during migration because of apoptosis, or loss of marker expression by a subset of the intradermal melanoblasts. The numbers of cases available for evaluation was limited, however, and additional confirmatory studies are therefore required to further elucidate and understand the mechanism(s) responsible for these migratory events.
Beginning at 18 weeks, dendritic melanocytes colonized the ORS and the matrical epithelium of the hair bulb. In addition, to their expected MITF and Mart-1 positivity, scattered round to ovoid cells positive for both markers were identified in the follicular bulge area beginning at 18 weeks. This pattern of MITF expression is consistent with that previously documented in murine melanocyte stem cells and their putative human counterparts.16,17 Similar putative melanocyte precursors have been documented in the bulge areas of developing murine follicles by high-resolution light microscopy and c-kit immunohistochemistry.2 The consistent absence of dendritic contours of the MITF-positive cells in the bulge area, as opposed to those in the dermis, epidermis, hair matrix and ORS raises questions regarding the origin of the bulge melanocyte precursors, for which two hypotheses may be postulated. First, the bulge melanocyte precursors may originate from the same dendritic melanoblasts that migrate from dermis to epidermis, but they take on an ovoid shape when reaching the bulge region because of as yet unknown factors in the niche microenvironment. Second, the bulge melanoblasts may arise from a separate and currently unrecognized population of MITF- and Mart-1-negative precursor cells in the developing hair follicle.
An unexpected and provocative finding in this study was the consistent, diffuse intranuclear immunoreactivity for MITF in bulge keratinocytes. The bulge region of the hair follicle has been established to be a niche for stem cells in which epithelial progenitors predominate.18 Bulge epithelial stem cells are believed to be important for normal hair cycling19,20 as well as for epidermal regeneration.21 The primitive keratinocytes that form the bulge have been shown to express distinctive and occasionally unexpected biomarkers, including cytokeratin 1522 and CD34,23 the latter associated predominantly with cells of hematopoietic lineage. MITF expression is not confined to melanocytes, but is also found in osteoclasts and mast cells, where it is required for normal development.6 A subset of normal smooth muscle cells, Schwann cells, and fibroblasts may also stain for MITF, but normal epithelial cells, to our knowledge, have heretofore been considered to be negative for this marker.27 While in melanocytes, MITF regulates both melanogenesis-related differentiation and cell survival pathways, in other nonpigmented cell types, its dominant effect appears to be direct regulation of expression of Bcl-2,7,24a protein that plays a key role in protection from apoptosis.25,28-30 Bcl-2 also is required for the survival and maintenance of melanocyte precursors in the stem cell niche (the bulge area of the hair follicle)17 and has been hypothesized to be a marker of follicular epithelial stem cells.31,32 It is therefore conceivable that MITF may be involved in the maintenance of the epithelial stem cells within the bulge area via induction of Bcl-2 expression; further studies will be required to investigate this possibility.
Although MITF plays a functional role other cells in addition to melanocytes, Mart-1 is a transmembrane protein-specific for melanocyte lineage. In most instances in our study, the number of Mart-1-positive cells either equaled or slightly exceeded the number of MITF-positive cells. MITF also drives melanocytic differentiation, and the MITF pathway and Mart-1 expression are probably to be linked. Accordingly, the apparent slight increased sensitivity of Mart-1 in detecting and enumerating melanocytes may be related to spatial factors, namely that Mart-1 defines a greater volume of an individual melanocyte in a 6 micron thick section than does MITF, and accordingly, Mart-1-positive cells may be slightly better represented due to this technical consideration.
One aim of our study was to document presence and migratory fate of melanocyte precursors within the fetal dermis. Although previous studies have documented the existence of epidermal melanocytes beginning at approximately 40-50 days of gestation,26,33,34 to our knowledge only three studies have previously described melanocyte precursors within the human fetal dermis. Zimmerman and Becker35 identified melanocyte-like cells in the mesenchymal tissue of a 10-week fetus using a silver impregnation method, and Hashimoto36 documented melanoblasts in the perifollicular mesenchyme of a 12-week fetus by electron microscopy. Recently, Suder et al.5 found HMB45 positive cells in the fetal dermis at unspecified gestational ages. In our study, MITF and Mart-1 positive cells were identified predominantly in the dermis at 6-8 weeks and persisted, albeit in lower numbers, up to 20 weeks. While we cannot rule out that some of the MITF-positive cells were mast cells, which are known to express MITF37 and have been reported in the fetal dermis,38 the correspondence of these cells to Mart-1-positive cells on serial sections, the roughly equal number of MITF- and Mart-1 positive cells in each case, and the lack of cytoplasmic granules on sections prepared using histochemical reagents conducive to granule visualization support a melanocytic lineage.
The finding of intradermal melanocytic cells that persist into the second trimester may be of potential significance to melanocytic proliferations that may arise de novo within the prenatal and postnatal dermis, such as predominantly intradermal congenital nevi and blue nevi and their variants, respectively. Some have questioned Unna's concept of Abtropfung (cells dropping into the dermis from the epidermis)39 as the universal mechanism for the formation of common acquired nevocellular nevi. Cramer, in particular, has proposed the opposite (Hochsteigerung): that acquired melanocytic nevi derive from pluripotential melanocytic cells during their migration from the neural crest.40,41 In this model, the variable appearances of common acquired nevi (junctional, compound and intradermal, with A, B and C subtypes of dermal morphology) are a reflection of different stages of migration and/or differentiation induced by the particular cutaneous microenvironment in which the migrating melanocytes are arrested. While our study does not directly address this debate, it does suggest that human skin, like that of lower mammals, may harbor dermal melanocytes, and these cells are potentially capable of giving rise to de novo intradermal melanocyte neoplasia.
In summary, we have described the redistribution of melanocyte precursors during the stages of cutaneous development in the human fetus and have confirmed and expanded prior reports of melanocytic precursors in the fetal dermis. Our unanticipated finding of nuclear MITF positivity in bulge keratinocytes suggests that MITF may represent a novel and functionally relevant marker for follicular stem cells of both epithelial as well as melanocytic lineage.
References
- 1.Perris R, von Boxberg Y, Lofberg J. Local embryonic matrices determine region-specific phenotypes in neural crest cells. Science. 1988;241:86. doi: 10.1126/science.3388022. [DOI] [PubMed] [Google Scholar]
- 2.Peters EM, Tobin DJ, Botchkareva N, Maurer M, Paus R. Migration of melanoblasts into the developing murine hair follicle is accompanied by transient c-Kit expression. J Histochem Cytochem. 2002;50:751. doi: 10.1177/002215540205000602. [DOI] [PubMed] [Google Scholar]
- 3.Jordan SA, Jackson IJ. MGF (KIT ligand) is a chemokinetic factor for melanoblast migration into hair follicles. Dev Biol. 2000;225:424. doi: 10.1006/dbio.2000.9856. [DOI] [PubMed] [Google Scholar]
- 4.Botchkareva NV, Botchkarev VA, Gilchrest BA. Fate of melanocytes during development of the hair follicle pigmentary unit. J Investig Dermatol Symp Proc. 2003;8:76. doi: 10.1046/j.1523-1747.2003.12176.x. [DOI] [PubMed] [Google Scholar]
- 5.Suder E, Bruzewicz S. Melanocytes of fetal dermis - studies with anti-HMB-45 antibody. Med Sci Monit. 2004;10:BR229. [PubMed] [Google Scholar]
- 6.Moore KJ. Insight into the microphthalmia gene. Trends Genet. 1995;11:442. doi: 10.1016/s0168-9525(00)89143-x. [DOI] [PubMed] [Google Scholar]
- 7.McGill GG, Horstmann M, Widlund HR, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 8.Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994;91:3515. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, Fisher DE. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol. 2003;163:333. doi: 10.1016/S0002-9440(10)63657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King R, Weilbaecher KN, McGill G, Cooley E, Mihm M, Fisher DE. Microphthalmia transcription factor. A sensitive and specific melanocyte marker for melanoma diagnosis. Am J Pathol. 1999;155:731. doi: 10.1016/S0002-9440(10)65172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rust CC, Meyer RK. Hair color, molt, and testis size in male, short-tailed weasels treated with melatonin. Science. 1969;165:921. doi: 10.1126/science.165.3896.921. [DOI] [PubMed] [Google Scholar]
- 13.Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 14.Holbrook KA, Odland GF. Structure of the human fetal hair canal and initial hair eruption. J Invest Dermatol. 1978;71:385. doi: 10.1111/1523-1747.ep12556818. [DOI] [PubMed] [Google Scholar]
- 15.Fukunaga-Kalabis M, Martinez G, Liu ZJ, et al. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J Cell Biol. 2006;175:563. doi: 10.1083/jcb.200602132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 18.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- 20.Wilson C, Cotsarelis G, Wei ZG, et al. Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: heterogeneity and functional differences of various hair cycles. Differentiation. 1994;55:127. doi: 10.1046/j.1432-0436.1994.5520127.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 22.Lyle S, Christofidou-Solomidou M, Liu Y, et al. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci. 1998;111:3179. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- 23.Trempus CS, Morris RJ, Bortner CD, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenblick A, Levy C, Razin E. Immunological trigger of mast cells by monomeric IgE: effect on microphthalmia transcription factor, STAT3 network of interactions. J Immunol. 2005;175:1450. doi: 10.4049/jimmunol.175.3.1450. [DOI] [PubMed] [Google Scholar]
- 25.Arita Y, Santiago-Schwarz F, Coppock DL. Survival mechanisms induced by 12-O-tetradecanoylphorbol-13-acetate in normal human melanocytes include inhibition of apoptosis and increased Bcl-2 expression. Melanoma Res. 2000;10:412. doi: 10.1097/00008390-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi A, Shimizu H, Nishikawa T. Epidermal melanocytes in normal and tyrosinase-negative oculocutaneous albinism fetuses. Arch Dermatol Res. 1995;287:529. doi: 10.1007/BF00374071. [DOI] [PubMed] [Google Scholar]
- 27.Busam KJ, Iversen K, Coplan KC, Jungbluth AA. Analysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanoma. Am J Surg Pathol. 2001;25:197. doi: 10.1097/00000478-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Klein-Parker HA, Warshawski L, Tron VA. Melanocytes in human skin express bcl-2 protein. J Cutan Pathol. 1994;21:297. doi: 10.1111/j.1600-0560.1994.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamamura K, Kamada S, Ito S, Nakagawa K, Ichihashi M, Tsujimoto Y. Accelerated disappearance of melanocytes in bcl-2-deficient mice. Cancer Res. 1996;56:3546. [PubMed] [Google Scholar]
- 30.Cohen-Saidon C, Nechushtan H, Kahlon S, Livni N, Nissim A, Razin E. A novel strategy using single-chain antibody to show the importance of Bcl-2 in mast cell survival. Blood. 2003;102:2506. doi: 10.1182/blood-2002-12-3921. [DOI] [PubMed] [Google Scholar]
- 31.Polakowska RR, Piacentini M, Bartlett R, Goldsmith LA, Haake AR. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- 32.Gho CG, Braun JE, Tilli CM, Neumann HA, Ramaekers FC. Human follicular stem cells: their presence in plucked hair and follicular cell culture. Br J Dermatol. 2004;150:860. doi: 10.1111/j.1365-2133.2004.05862.x. [DOI] [PubMed] [Google Scholar]
- 33.Holbrook KA, Underwood RA, Vogel AM, Gown AM, Kimball H. The appearance, density and distribution of melanocytes in human embryonic and fetal skin revealed by the anti-melanoma monoclonal antibody, HMB-45. Anat Embryol (Berl) 1989;180:443. doi: 10.1007/BF00305119. [DOI] [PubMed] [Google Scholar]
- 34.Haake AR, Scott GA. Physiologic distribution and differentiation of melanocytes in human fetal and neonatal skin equivalents. J Invest Dermatol. 1991;96:71. doi: 10.1111/1523-1747.ep12515868. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman A, Becker S., Jr. Precursors of epidermal melanocytes in the negro fetus. Academic Press; New York: 1959. p. 159. [Google Scholar]
- 36.Hashimoto K. The ultrastructure of the skin of human embryos. 8. Melanoblast and intrafollicular melanocyte. J Anat. 1971;108:99. [PMC free article] [PubMed] [Google Scholar]
- 37.Hodgkinson CA, Moore KJ, Nakayama A, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 38.Breathnach AS. Development and differentiation of dermal cells in man. J Invest Dermatol. 1978;71:2. doi: 10.1111/1523-1747.ep12543601. [DOI] [PubMed] [Google Scholar]
- 39.Unna P. Naevi und Naevocarcinoma. Berl Klin Wochenschr. 1893;30:14. [Google Scholar]
- 40.Cramer SF. The histogenesis of acquired melanocytic nevi. Based on a new concept of melanocytic differentiation. Am J Dermatopathol. 1984;6(Suppl. 1):289. [PubMed] [Google Scholar]
- 41.Cramer SF. The origin of epidermal melanocytes. Implications for the histogenesis of nevi and melanomas. Arch Pathol Lab Med. 1991;115:115. [PubMed] [Google Scholar]