Abstract
In humans, most hearing loss results from death of hair cells, the mechanosensory receptors of the inner ear. Two goals of current hearing research are to protect hair cells from degeneration and to regenerate new hair cells, replacing those that are lost due to aging, disease, or environmental challenges. One limitation of research in the auditory field has been the relative inaccessibility of the mechanosensory systems in the inner ear. Zebrafish possess hair cells in both their inner ear and their lateral line system that are morphologically and functionally similar to human hair cells. The external location of the mechanosensory hair cells in the lateral line and the ease of in vivo labeling and imaging make the zebrafish lateral line a unique system for the study of hair cell toxicity, protection, and regeneration. This review focuses on the lateral line system as a model for understanding loss and protection of mechanosensory hair cells. We discuss chemical screens to identify compounds that induce hair cell loss and others that protect hair cells from known toxins and the potential application of these screens to human medicine.
Introduction
The auditory and vestibular receptor organs of the inner ear relay mechanical information for hearing and balance, respectively, to the brain. The mechanosensory hair cells of the inner ear transduce mechanical stimuli via actin-based stereocilia into electrical impulses, which are conveyed centrally.1,2
Death of mechanosensory hair cells is a common denominator in many forms of hearing impairment.3–5 Significant progress has been made in determining the etiology of congenital forms of deafness, and mouse models are emerging at increasing rates.6 Sensorineural hearing loss accounts for profound hearing loss in approximately 1 in 1000 newborn babies.7 Many of the genes underlying hereditary deafness function during hair cell development. Hair cells in the zebrafish share many characteristics and molecular constituents with their counterparts in the mammalian inner ear,8,9 and inactivation of genes affecting human hereditary deafness also cause loss of hair cell function in zebrafish. Examples include mutants of myosins VI10 and VIIa,11 cadherin 23,12 protocadherin 15,13 and tmie.14
Hair cell loss is most commonly due to environmental insults, including exposure to excessive noise or ototoxic drugs (such as aminoglycoside antibiotics or certain chemotherapeutic drugs such as cisplatin), or progressive loss due to aging (presbycusis). Nearly 15% (29 million) of U.S. adults aged 20–69 report hearing impairment.15 Hearing loss is accompanied by many quality-of-life issues such as feelings of isolation and depression, making it a potentially devastating sensory disorder.16 Although interventions such as hearing aids and cochlear implants provide some individuals with significant benefit, the loss of sensory hair cells comes with an as of yet irrecoverable loss of sensory input. Cochlear implants, although having been of enormous importance to the population of profoundly hearing-impaired children and adults, lack the normal specificity of stimulation and are dependent on preservation of the auditory nerve axons, which are compromised to a degree that is roughly correlated to the degree of hair cell loss.17 In addition to loss of auditory hair cells, vestibular hair cells may also be lost due to aging or ototoxic drug administration, resulting in devastating vestibular deficits causing severe ataxia and oscillopsia. Many researchers are pursuing ways to induce hair cell regeneration.18–20 Unfortunately, hair cell loss in humans, as yet, is irreversible. Therefore, drugs that can prevent hair cell death offer great potential benefit for millions of people, particularly drugs that could be administered immediately before, or shortly after, exposure to an ototoxic stimulus.
In this review, we will focus on chemical screening using the lateral line of larval zebrafish as a model system for mechanosensory hair cell loss and protection. We then discuss potential clinical uses of protective drugs and drug delivery systems.
Hearing Loss and Protection
Research in the past few decades has uncovered some of the key intracellular events that can cause hair cell death.21 Several candidate protectants have been evaluated such as antioxidants, caspase inhibitors, and jun kinase inhibitors.22–26 Although a few of these candidate otoprotectants have progressed to human trials,27,28 as yet, no definitive protection has emerged for clinical use, and there appears to be disagreement among investigators with respect to their broad efficacy in laboratory animals.
Further, different cell death pathways may be triggered in response to different forms of damage29–31 and many protective molecules offer incomplete hair cell protection, hinting that polypharmacy approaches may offer the greatest benefit.32–35 Given the difficulty of assessing many putative hair cell protectants for efficacy against multiple ototoxins, the field has proceeded slowly.
Although testing individual candidate compounds in rodents has been informative, researchers have been limited to candidate approaches based on known pathways. Our goal has been to take an unbiased screening approach to identify compounds that either induce hair cell loss or protect against hair cell loss. The small size, high fecundity, and external development of zebrafish provide a robust model system for unbiased, broad chemical screening. A growing number of labs have performed chemical screens in zebrafish. The first chemical screen for small molecules that altered wild-type zebrafish development was based on direct phenotypic examination of central nervous system, ear, cardiovasculature, and pigment cells.36 Since then, phenotypic analysis has been applied to identify compounds that alter zebrafish development,37,38 heart formation,39 heart rate,40 and fin regeneration.41 Suppression of a mutant phenotype has been used to identify chemicals that attenuate angiogenesis defects42 or suppress oncogenic dysregulation.43 In contrast to direct phenotypic analysis, alternative readouts have been exploited for chemical screening, including altered antibody staining,44,45 in situ hybridization,46,47 and expression of in vivo fluorescent proteins.48–50
For the study of hair cells, the zebrafish has an additional advantage of having a mechanosensory system, called the lateral line, located externally on its body. This model system has allowed us to screen thousands of compounds against multiple ototoxins, giving us many candidate molecules that may be used individually or in combination to test for protection against mammalian inner ear damage.
Zebrafish Lateral Line
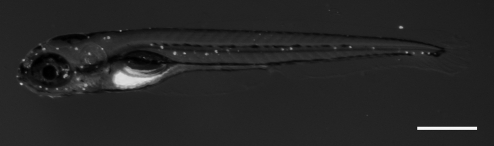
The lateral line is a series of sensory organs arrayed along the head and body of fishes and aquatic amphibians (Fig. 1). Each organ contains several sensory hair cells and surrounding supporting cells.51 The lateral line hair cells are developmentally, morphologically, and physiologically similar to the hair cells of the inner ear.52 The lateral line system enables the animal to detect nearby water currents and is important in such diverse behaviors as rheotaxis (orientation to water flow), prey detection, and predator avoidance.51,53–56
FIG. 1.
Fluorescent micrograph of a 5 days postfertilization zebrafish labeled with the mitochondrial potentiometric dye DASPEI. Each white dot is a neuromast arrayed along the head and body of the animal. Scale bar = 500 μm.
In zebrafish, the lateral line system develops from cephalic placodes that give rise to migratory primordia, which then form the anterior and posterior lateral lines.57,58 Lateral line development has been studied in detail in the posterior lateral line, where cell clusters form in the migrating primordium.59,60 Neuromasts are deposited from the trailing edge of the primordium at 5–7 somite intervals and then differentiate into mature hair cells and supporting cells. Deposition of neuromasts occurs in stereotyped positions along the head and body of the animal,61,62 making this system a tractable vertebrate model for morphogenesis studies. This stereotyped arrangement of neuromasts makes this system particularly convenient for hair cell death and protection screens, as one knows the expected location of each neuromast, allowing missing neuromasts to be quickly identified.
The lateral line possesses unique features not available in other in vivo models. The surface location of hair cells makes for easy drug delivery and in vivo imaging. These cells are permeable to several vital dyes as well as fluorescently labeled aminoglycosides, allowing for real-time assessment using fluorescent imaging techniques.63–67
Chemical Screening for Ototoxins
Zebrafish have been long used as a model for general toxicology studies.68,69 More specifically, several research groups have recognized the potential of the zebrafish lateral line for studies of hair cell toxicity.70 Hair cell sensitivity has been reported to divalent cations such as copper and other heavy metals.71–75 Like hair cells in the mammalian and avian inner ear, hair cells of the zebrafish lateral line are sensitive to aminoglycoside antibiotics and the chemotherapy agent cisplatin.63–65,76–81 Williams and Holder first observed neomycin-induced hair cell death in larval zebrafish neuromasts.77 Subsequently, our group developed assays for investigating hair cell death and regeneration in this system.63 Ton and Parng used the lateral line as a model system to look at ototoxicity and protection using five toxic and five protective compounds, and showed the potential of automated fluorescent systems for high-throughput screening.79
Most ototoxic drugs are discovered when human patients experience hearing loss or vestibular dysfunction; the aminoglycoside antibiotics are a classic example of this situation.82 Nowhere in the drug development process is there a mandatory test for ototoxic side effects, so we known very little about the ototoxic potential of approved drugs. Further, since the majority of drugs are prescribed for people over the age of 50, ototoxic drug effects may often be attributed to age-related changes. Hence, several years ago we began a screening program to identify putative hair cell toxins among compounds in clinical use.83
We began by evaluating the National Institute of Neurological Disorders and Stroke (NINDS) Custom Collection II (Microsource, Inc., Gaylordsville, CT), a library of 1040 Food and Drug Administration–approved drugs and known bioactives, many of which are in clinical use. Hair cells of 5–6 days postfertilization (dpf ) zebrafish were prelabeled with the vital nuclear dye YO-PRO-1 (Fig. 2). Individual zebrafish were placed in wells of a 96-well glass-bottom plate and treated for 1 h with a single library compound at 100 μM. The entire 96-well plate was placed on the stage of a Zeiss Axiovert inverted microscope equipped with a Marianas imaging system for observation (Intelligent Imaging Innovations, Inc., Denver, CO). Each fish was examined for the presence or absence of hair cells in every neuromast that was visible in the field of view, as well as more subtle signs of hair cell damage such as nuclear condensation or fragmentation. Although the need for the experimenter to screen each fish precludes the ability to perform true high-throughput screening, a single 96-well plate can be screened in 30–60 min by a trained observer.
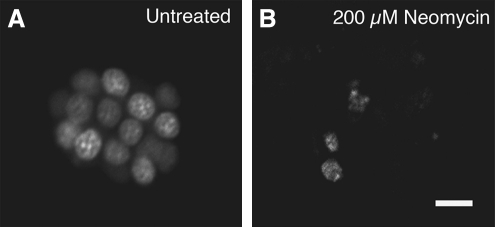
FIG. 2.
Zebrafish hair cells labeled with the fluorescent dye YO-PRO-1, which binds DNA and labels hair cell nuclei. (A) An undamaged neuromast labeled with YO-PRO-1. Approximately 15 hair cells are visible and healthy in appearance. (B) After a 1-h exposure to the ototoxic drug neomycin at a concentration of 200 μM, most of the hair cells have died. Scale bar in (B) = 10 μm and applies to both panels.
This initial screen uncovered 21 confirmed hits (Table 1). Seven compounds were known ototoxins (e.g., neomycin and cisplatin), demonstrating proof of concept in our screening approach. The other 14 compounds were not identified ototoxins, although examination of the clinical literature revealed an occasional case report describing hearing loss in patients treated with a few of these drugs (e.g., chloramphenicol and estradiol valerate).84,85 Two drugs, the anticholinergic compound propantheline bromine and the antiprotozoal pentamidine isethionate, were tested in vitro in cultures of mouse utricle (a vestibular end organ in the mammalian inner ear), and both compounds demonstrated ototoxicity in this mammalian model. These findings highlight the need to establish standardized screening for hair cell toxicity during drug development, and they demonstrate the potential of the zebrafish lateral line as a model system for such studies.
Table 1.
Candidate Ototoxic Drugs Identified by Zebrafish Lateral Line Screen of National Institute of Neurological Disorders and Stroke Custom Collection II for Drugs That Cause Hair Cell Death After 1 h of Treatment83
| Ototoxic drug | Class | Mammalian testing |
|---|---|---|
| Chloramphenicol | Antibiotic | No |
| Chlortetracycline HCL | Antibiotic | No |
| Pentamidine isethionate | Antiprotozoal | Yes |
| Spermadine | Ornithine decarboxylase inhibitor | No |
| Tobramycin | Antibiotic | Yes |
| Propantheline bromide | Anticholinergic | Yes |
| Ethacrynic acid | Loop diuretic | Yes |
| Pomiferin | Antioxidant | No |
| Chlorophyllide | Antineoplastic, chlorophyll derivative | No |
| Estradiol valerate | Estrogen | No |
| Neomycin | Antibiotic | Yes |
| Pentetrazole | CNS/respiratory/circulatory stimulant | Yes |
| Guaiazulene | Antioxidant, color additive agent | No |
| Rosolic acid | Diagnostic aid | No |
| Cisplatin | Antineoplastic | Yes |
| Vincamine | Vasodilator | No |
| Kanamycin | Antibiotic | Yes |
| Demeclocycline HCL | Antibiotic | No |
| Mefloquine | Antiprotozoal | Yes |
| Candesartan | Angiotensin 1 receptor antagonist | No |
| Simvastatin | HMGCoA reductase inhibitor, antihyperlipidemic | No |
Mammalian testing denotes whether there is literature confirming ototoxic effects in mammalian tissue, in vitro or in vivo. CNS, central nervous system.
Chemical Screening for Hair Cell Protection
There are two major classes of clinically relevant drugs recognized to have known ototoxicity: the aminoglycoside antibiotics and platinum-based chemotherapeutics.86,87 Aminoglycosides are used to treat gram-negative bacterial infections. Once the ototoxicity (and nephrotoxicity) of aminoglycosides was recognized, use was curtailed in favor of alternative antibiotics in many applications. However, these drugs are still used for recalcitrant bacterial infections, particularly in life-threatening cases (e.g., with premature infants, or in patients with tuberculosis or cystic fibrosis), with use increasing due to the prevalence of multidrug-resistant bacterial strains. Use of aminoglycosides worldwide continues due to the low cost and availability of these drugs. Development of less ototoxic aminoglycosides has resulted in safer alternatives, but all have some degree of hair cell toxicity. In the case of cisplatin, although its ototoxic effects are well recognized, it remains one of the most effective chemotherapeutic treatments for solid tumors.
We became interested in identifying chemicals that can protect hair cells from drug-induced damage as potential candidates for clinical co-administration with known hair cell toxins. The two screens described below each use the lateral line system to look for compounds that could prevent hair cell loss induced by ototoxic drugs. Protective compounds and drugs identified in our screens are listed in Table 2.
Table 2.
Protective Drugs Identified by Zebrafish Lateral Line Screen for Protection Against Neomycin-Induced Hair Cell Death66,88
| Protective drug | Known activity/target | Library | Blocks uptakea | Mammal testingb |
|---|---|---|---|---|
| PROTO1 | Unknown | Diverset (Chembridge, Inc., San Diego, CA) | N | Y |
| PROTO2 | Unknown | Diverset (Chembridge) | N | Y |
| Amsacrine | Topoisomerase 2 inhibitor | NINDS Custom Collection (Microsource, Inc.) | Y | N |
| Carvedilol | Beta-2 adrenergic blocker | NINDS Custom Collection (Microsource, Inc.) | Y | N |
| Cepharanthine | Plasma membrane stabilizer | NINDS Custom Collection (Microsource, Inc.) | N | N |
| Drofenine | Acetylcholinesterase inhibitor | NINDS Custom Collection (Microsource, Inc.) | N | N |
| Hexamethyleneamiloride | Na/H exchange inhibitor | NINDS Custom Collection (Microsource, Inc.) | Y | N |
| Phenoxybenzamine | Alpha-1 adrenergic blocker | NINDS Custom Collection (Microsource, Inc.) | Y | N |
| Tacrine | Acetylcholinesterase inhibitor | NINDS Custom Collection (Microsource, Inc.) | N | Y |
All drugs were tested for blockade of uptake of fluorescently labeled aminoglycoside. A smaller subset of drugs were tested for protection against damage in mammalian (mouse) hair cells. Each of these drugs protects hair cells from acute (1 h) neomycin exposure when administered 1 h before neomycin.
Y indicates that the compound blocked aminoglycoside uptake and N indicates that uptake was not blocked.
Y indicates that testing has been performed and N indicates that experiments have not yet been performed in mammals.
The methodology for our protection screens is similar to that of our toxicity screen. We screen 5–6 dpf larvae because at earlier times the hair cells show resistance to aminoglycoside effects, a common feature of developing hair cells. Lateral line hair cells are born beginning at 2 dpf and can mechanotransduce by 3–4 dpf. Full drug sensitivity begins at 5 dpf.64,78 In the studies described below, individual zebrafish with prelabeled hair cells were placed into 96-well plates, pretreated for 1 h with the library compound, and then concurrently exposed to the aminoglycoside neomycin for an additional hour.
We screened the Chembridge Diverset E small-molecule library of 10,960 compounds to identify molecules that protected lateral line hair cells from neomycin toxicity.88 These molecules were designed with chemical properties conforming to the Lipinsky “Rule of 5” to optimize for potential biological activity.89 To efficiently screen this larger library, compounds were multiplexed with five per well, with each compound at a concentration of 10 μM. If protection was observed, the five drugs were reassessed individually, and confirmed hits were explored in more detail. This screen identified two compounds that exhibited robust protection across the neomycin dose–response function (Fig. 3). Both compounds, which we named PROTO1 and PROTO2, are benzothiophene carboxamides. Due to both the nature of the Chembridge library (uncharacterized small molecules) and the phenotypic marker used for this screen (hair cell survival), several additional experiments were performed to determine how these PROTO compounds protected hair cells.
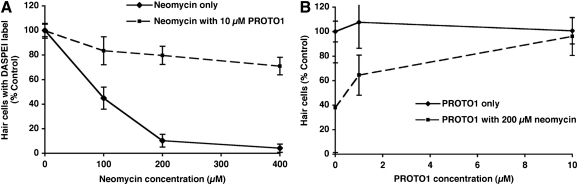
FIG. 3.
PROTO1 provides significant protection from neomycin-induced hair cell death. (A) 10 μM PROTO1 provides robust hair cell protection from all tested concentrations of neomycin. Hair cell survival was assessed with DASPEI scoring, a semiquantitative scoring measure for hair cell loss that is highly correlated with direct cell counts.30,63,75 (B) Increasing concentrations of PROTO1 provide increasing protection from a damaging 200 μM neomycin stimulus (dashed line), whereas PROTO1 alone does not affect hair cell survival (solid line). Hair cell survival was assessed by direct counts of labeled cells.
Neither compound inhibited aminoglycoside uptake, suggesting that the PROTO compounds act intracellularly during aminoglycoside exposure to attenuate hair cell toxicity. The presence of PROTO1 or PROTO2 did not inhibit the bactericidal activity of neomycin, suggesting that these compounds could be used clinically to limit ototoxicity during aminoglycoside treatment without compromising the therapeutic benefit of the aminoglycoside. Finally, experiments in cultured mouse utricles demonstrated that PROTO drugs protect mammalian hair cells from neomycin toxicity in vitro. This finding validates the zebrafish lateral line as a model for discovering drugs that can protect hair cells in mammals. One drawback to the Chembridge library is that the molecular targets of these compounds are unknown, making it difficult and time consuming to determine the mechanism(s) underlying the protective effects of the PROTO drugs. We are pursuing the molecular targets of the benzothiophene carboxamides using biochemical approaches and examining analogs for optimization of protective effects.
To maximize the chances of identifying otoprotectants that could quickly be translated into clinical trials, we have taken a second approach as well, screening chemicals with known activities. In our first study of this kind we screened the same NINDS library used in our ototoxicity screen (above), but tested for compounds that provided protection against neomycin-induced hair cell death.66 The smaller size of this library allowed us to test each drug singly and thus avoid the possibility that protective compounds were masked by toxic drugs. The NINDS screen yielded seven confirmed hits, of which three are already approved by the Food and Drug Administration. These three drugs encompass diverse uses, including a beta-2 adrenergic blocker (carvedilol), a diuretic (hexamethyleneamiloride), and an anticholinergic (tacrine). Experiments with fluorescently tagged aminoglycoside90 showed that four of the seven drugs (amsacrine, carvedilol, hexamethyleneamiloride, and phenoxybenzamine) reduced aminoglycoside uptake, whereas the other three drugs (tacrine, cepharanthine, and drofenine) did not. Presumably, these latter drugs protect hair cells by interacting with intracellular death and survival signaling pathways. Tacrine was further shown to protect mammalian hair cells of the utricle from in vitro neomycin toxicity.66 As tacrine did not significantly alter the bactericidal activity of neomycin, it is a good candidate for in vivo validation and clinical testing as a potential otoprotectant.
Ongoing Studies
We consider the hair cell toxicity and protection studies conducted to date as proof of the principle that the zebrafish lateral line can be used as a valuable model system in which to discover drugs and drug-like compounds that may have clinical utility. In addition to further studies on the drugs and small-molecule drug-like compounds identified in our screens, we are currently screening additional libraries for substances that are toxic to hair cells, drugs that can protect hair cells, and drugs that alter the regenerative potential of lateral line hair cells.
Clinical Scenarios
How could the drugs and chemicals identified by the zebrafish lateral line screens ultimately be used? From the standpoint of hearing protection, there are several medical scenarios that lend themselves to clinical intervention.
As stated previously, ototoxicity is typically not considered during drug development. Most known ototoxic drugs were identified after anecdotal reports of hearing loss led to more systematic testing. It would be difficult and costly to perform hearing tests on all patients in clinical trials with experimental drugs. It is, however, feasible to use the zebrafish lateral line to screen experimental drugs for their potential toxicity to hair cells, and to recommend audiometric testing for those drugs that have confirmed ototoxic effects in animal models. This kind of screening is not realistic in any other animal model, and would potentially have very direct effects on patient care.
Numerous ototoxic drugs are given to treat serious infections (e.g., aminoglycosides) or cancers (platinum drugs) with the expectation and acceptance that severe hearing loss may be an unfortunate consequence. In addition, doses of antibiotics and antineoplastic drugs are often limited by their ototoxic and nephrotoxic side effects. Otoprotectant delivery concomitant with therapy may attenuate ototoxic side effects without compromising therapeutic efficacy. This scenario, most closely tied to the zebrafish lateral line drug screens, is attractive because the exact timing of the damaging event is known and can be controlled. Thus, drugs that are potentially protective can be given before or concurrently with the damaging drug to prevent hair cell loss.
Noise injury is the second most common cause of hearing loss (after aging) and can be the result of single impulse noise or continuous long-term exposure.91–93 Typically, there is a variable amount of recovery after noise-induced hearing loss that may benefit from the administration of protective drugs. In the zebrafish system it remains unknown whether damage induced by drugs and noise uses similar signaling pathways, but it is conceivable that protectants for drug-induced hair cell death may be effective at reducing noise damage. On the other hand, noise-induced hearing loss can be more unpredictable. In some situations, such as a rock music concert or certain military engagements, noise exposure can be anticipated such that a protective drug could be given before or during a noise exposure. Hence, it is possible that protective drugs could play a role in the limited recovery that typically occurs after noise injuries. Antioxidants such as D-methionine, for example, appear to reduce permanent sensorineural hearing loss when given before or immediately after a noise injury in some species and some conditions.28
In addition to protective compounds, the toxic effects of newly identified compounds could be used as a pharmacological therapy. Patients who suffer from intractable vertigo from Meniere's disease have been treated with transtympanic injections of gentamicin. The gentamicin is titered to ablate the vestibular hair cells. Although efforts are made to prevent concomitant auditory hair cell loss, it is a known complication of this treatment. Identifying compounds that target vestibular and not auditory hair cells therefore has a needed role in such patients.
Finally and most importantly, age-related hearing loss (presbycusis) affects approximately 300 million people in the world, making this not only the most prevalent form of sensorineural hearing impairment,5,95 but next to the common cold, the most prevalent disease. With the increasing geriatric population, this number is expected to rise to 900 million by 2050. The most common histopathology found in age-related hearing loss is loss of hair cells, typically starting at the high-frequency coding region (basal turn) of the cochlea, and then progressing to affect lower frequencies such as those used for encoding speech. Knowledge of the death pathways involved in age-related hearing loss is poor; however, prevention of this slowly progressive hair cell loss could positively impact a large percentage of the population.
Drug Delivery
How protective drugs will be delivered to the inner ear is a question complicated by limitations in access. In contrast to organs such as the heart, lungs, and liver that are affected by systemic drugs, the hair cells of the inner ear are isolated within extracellular fluid spaces that have complex and poorly understood relations with blood and cerebrospinal fluid. As a result, many clinicians have preferred direct drug application to the inner ear through either extracochlear routes (application of drug outside the cochlea, typically at the round window membrane) or intracochlear routes (direct administration into the cochlea).94 Due to the inherent risk of inner ear injury from intracochlear application, extracochlear application is the favored method, typically involving injection through the tympanic membrane to fill the middle ear with drug, direct application of drug-impregnated gels or polymers to the round window, or osmotic pumps that slowly infuse drugs into the middle ear.96 The ability of drugs applied in this way to penetrate the inner ear is highly variable, particularly because the fluids of the inner ear have a negligible rate of flow, making diffusion of drugs slow.97
Identification of nontoxic protective drugs that can be given systemically affords the possibility of avoiding more invasive drug delivery methods. It is important to note that the inner ear fluid spaces are sealed by a tight barrier via the capillaries within the lateral wall of the cochlea comparable to the blood–brain barrier.97 Thus, the ability of a systemically administered drug to penetrate the inner ear and affect hair cells is difficult to predict. Nevertheless, the prospect of an orally administered protective drug is preferable to a surgically placed gel or infusion pump, particularly for slowly progressive forms of hair cell loss (e.g., presbycusis).
Conclusion
Our current and future screening studies incorporate multiple time parameters and a broader range of ototoxins. Beyond the translational aspects of identifying ototoxins and protectants, the molecules that induce hair cell death or promote hair cell survival provide information about the pathways involved in these processes. We have also undertaken a parallel genetic screen for zebrafish mutations that alter hair cell sensitivity to aminoglycosides.88 This genetic approach complements our chemical screening studies, particularly the ability to examine epistatic interactions between protective drugs and protective genes. A better understanding of the pathways involved in drug-induced hair cell death will allow greater ability to predictively design drugs or select targets to optimize protective effects. This will provide tools that could be used to evaluate the similarities and distinctions between drug-induced hair cell death and noise- or age-related hair cell damage.
Acknowledgments
We thank Mr. Glen MacDonald for help with imaging and two anonymous reviewers for their critique. Work in the laboratories of H.O., E.W.R., J.A.S., and D.W.R. are supported by grants from the National Institute of Deafness and Communication Disorders, the Royal National Institute for Deaf People, the Deafness Research Fund, and V.M. Bloedel Hearing Research Center. A.B.C., K.N.O, and F.S. have been supported by Ruth Kirschstein NRSA fellowships.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hudspeth AJ. How the ear's works work: mechanoelectrical transduction and amplification by hair cells. C R Biol. 2005;328:155–162. doi: 10.1016/j.crvi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie PG. Müller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br Med Bull. 2002;63:25–38. doi: 10.1093/bmb/63.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Holley MC. The auditory system, hearing loss and potential targets for drug development. Drug Discov Today. 2005;10:1269–1282. doi: 10.1016/S1359-6446(05)03595-6. [DOI] [PubMed] [Google Scholar]
- 5.Van Eyken E. Van Camp G. Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol. 2007;12:345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- 6.Dror AA. Avraham KB. Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. doi: 10.1146/annurev-genet-102108-134135. [DOI] [PubMed] [Google Scholar]
- 7.Smith RJ. Bale JF., Jr. White KR. Sensorineural hearing loss in children. Lancet. 2005;365:879–890. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- 9.Nayak GD. Ratnayaka HS. Goodyear RJ. Richardson GP. Development of the hair bundle and mechanotransduction. Int J Dev Biol. 2007;51:597–608. doi: 10.1387/ijdb.072392gn. [DOI] [PubMed] [Google Scholar]
- 10.Seiler C. Ben-David O. Sidi S. Hendrich O. Rusch A. Burnside B, et al. Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev Biol. 2004;272:328–338. doi: 10.1016/j.ydbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Ernest S. Rauch GJ. Haffter P. Geilser R. Petit C. Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- 12.Söllner C. Rauch GJ. Siemens J. Geisler R. Schuster SC. Müller U. Nicolson T Tübingen 2000 Screen Consortium. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 13.Seiler C. Finger-Baier KC. Rinner O. Makhankov YV. Schwarz H. Neuhauss SC. Nicolson T. Duplicated genes with split functions: independent roles of protocadherin15 orthologues in zebrafish hearing and vision. Development. 2005;132:615–623. doi: 10.1242/dev.01591. [DOI] [PubMed] [Google Scholar]
- 14.Gleason MR. Nagiel A. Jamet S. Vologodskaia M. López-Schier H. Hudspeth AJ. The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc Natl Acad Sci USA. 2009;106:21347–21352. doi: 10.1073/pnas.0911632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal Y. Platz EA. Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey 1999–2004. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- 16.Hallam R. Ashton P. Sherbourne K. Gailey L. Acquired profound hearing loss: mental health and other characteristics of a large sample. Int J Audiol. 2006;45:715–723. doi: 10.1080/14992020600957335. [DOI] [PubMed] [Google Scholar]
- 17.Hardie NA. Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 18.Bermingham-McDonogh OM. Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 19.Oesterle EC. Stone JS. Hair cell regeneration: mechanisms guiding cellular proliferation and differentiation. In: Salvi RJ, editor; Popper AN, editor; Fay RR, editor. Hair Cell Regeneration, Repair and Protection. New York, NY: Springer; 2008. pp. 141–197. [Google Scholar]
- 20.Brigande JV. Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12:679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng AG. Cunningham LL. Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- 22.Kopke RD. Liu W. Gabaizadeh R. Jacono A. Feghali J. Spray D, et al. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997;18:559–571. [PubMed] [Google Scholar]
- 23.Liu W. Staecker H. Stupak H. Malgrange B. Lefebvre P. Van De Water TR. Caspase inhibitors prevent cisplatin-induced apoptosis of auditory sensory cells. Neuroreport. 1998;9:2609–2614. doi: 10.1097/00001756-199808030-00034. [DOI] [PubMed] [Google Scholar]
- 24.Yamasoba T. Schacht J. Shoji F. Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999;815:317–325. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- 25.Matsui JI. Ogilvie JM. Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugahara K. Cunningham LL. Rubel EW. Protection of adult vestibular hair cells against aminoglycoside toxicity by inhibition in the JNK signaling pathway. Hear Res. 2006;221:128–135. doi: 10.1016/j.heares.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sha SH. Qiu J. Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 28.Campbell KC. Meech RP. Klemens JJ. Gerberi MT. Drystad SS. Larsen DL, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Le Prell CG. Yamashita D. Minami SB. Yamasoba T. Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens KN. Coffin AB. Hong LS. Bennett KO. Rubel EW. Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear Res. 2009;253:32–41. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sha SH. Chen FQ. Schacht J. Activation of cell death pathways in the inner ear of the aging CBA/J mouse. Hear Res. 2009;254:92–99. doi: 10.1016/j.heares.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McFadden SL. Ding D. Salvemini D. Salvi RJ. M40403, a superoxide dismutase mimetic, protects cochlear hair cells from gentamicin, but not cisplatin toxicity. Toxicol Appl Pharmacol. 2003;186:46–54. doi: 10.1016/s0041-008x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham LL. Matsui JI. Warchol ME. Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- 34.Wang J. Ladrech S. Pujol R. Brabet P. Van De Water TR. Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- 35.Eshraghi AA. Wang J. Adil E. He J. Zine A. Bublik M, et al. Blocking c-Jun-N-terminal kinase signaling can prevent hearing loss induced by both electrode insertion trauma and neomycin ototoxicity. Hear Res. 2007;226:168–177. doi: 10.1016/j.heares.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Peterson RT. Link BA. Dowling JE. Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khersonsky SM. Jung DW. Kang TW. Walsh DP. Moon HS. Jo H, et al. Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J Am Chem Soc. 2003;125:11804–11805. doi: 10.1021/ja035334d. [DOI] [PubMed] [Google Scholar]
- 38.Sachidanandan C. Yeh JR. Peterson QP. Peterson RT. Identification of a novel retinoid by small molecule screening with zebrafish embryos. PLoS ONE. 2008;3:e1947. doi: 10.1371/journal.pone.0001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson RT. Mably JD. Chen JN. Fishman MC. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr Biol. 2001;11:1481–1491. doi: 10.1016/s0960-9822(01)00482-1. [DOI] [PubMed] [Google Scholar]
- 40.Milan DJ. Peterson TA. Ruskin JN. Peterson RT. MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 41.Mathew LK. Sengupta S. Kawakami A. Andreasen EA. Löhr CV. Loynes CA, et al. Unraveling tissue regeneration pathways using chemical genetics. J Biol Chem. 2007;282:35202–35210. doi: 10.1074/jbc.M706640200. [DOI] [PubMed] [Google Scholar]
- 42.Peterson RT. Shaw SY. Peterson TA. Milan DJ. Zhong TP. Schreiber SL, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 43.Yeh JR. Munson KM. Elagib KE. Goldfarb AN. Sweetser DA. Peterson RT. Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat Chem Biol. 2009;5:236–243. doi: 10.1038/nchembio.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stern HM. Murphey RD. Shepard JL. Amatruda JF. Straub CT. Pfaff KL, et al. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol. 2005;1:366–370. doi: 10.1038/nchembio749. [DOI] [PubMed] [Google Scholar]
- 45.Murphey RD. Stern HM. Straub CT. Zon LI. A chemical genetic screen for cell cycle inhibitors in zebrafish embryos. Chem Biol Drug Des. 2006;68:213–219. doi: 10.1111/j.1747-0285.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 46.North TE. Goessling W. Walkley CR. Lengerke C. Kopani KR. Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaufman CK. White RM. Zon L. Chemical genetic screening in the zebrafish embryo. Nat Protoc. 2009;4:1422–1432. doi: 10.1038/nprot.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molina GA. Watkins SC. Tsang M. Generation of FGF reporter transgenic zebrafish and their utility in chemical screens. BMC Dev Biol. 2007;7:62. doi: 10.1186/1471-213X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran TC. Sneed B. Haider J. Blavo D. White A. Aiyejorun T, et al. Automated, quantitative screening assay for antiangiogenic compounds using transgenic zebrafish. Cancer Res. 2007;67:11386–11392. doi: 10.1158/0008-5472.CAN-07-3126. [DOI] [PubMed] [Google Scholar]
- 50.Kitambi SS. McCulloch KJ. Peterson RT. Malicki JJ. Small molecule screen for compounds that affect vascular development in the zebrafish retina. Mech Dev. 2009;126:464–477. doi: 10.1016/j.mod.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coombs S. Görner P. Münz H. The Mechanosensory Lateral Line: Neurobiology and Evolution. New York, NY: Springer-Verlag; 1989. [Google Scholar]
- 52.Coffin A. Kelley M. Manley GA. Popper AN. Evolution of sensory hair cells. In: Manley GA, editor; Popper AN, editor; Fay RR, editor. Evolution of the Vertebrate Auditory System. New York, NY: Springer; 2004. pp. 55–94. [Google Scholar]
- 53.Dijkgraaf S. The functioning and significance of the lateral line organs. Biol Rev. 1963;38:51–105. doi: 10.1111/j.1469-185x.1963.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 54.Montgomery JC. MacDonald JA. Sensory tuning of lateral line receptors in Antarctic fish to the movements of planktonic prey. Science. 1987;235:195–196. doi: 10.1126/science.235.4785.195. [DOI] [PubMed] [Google Scholar]
- 55.Montgomery JC. Baker CF. Carton AG. The lateral line can mediate rheotaxis in fish. Nature. 1997;389:960–963. [Google Scholar]
- 56.New JG. Fewkes LA. Khan SN. Strike feeding behavior in the muskellunge, Esox masquinongy: contributions of the lateral line and visual sensory systems. J Exp Biol. 2001;204:1207–1221. doi: 10.1242/jeb.204.6.1207. [DOI] [PubMed] [Google Scholar]
- 57.Ghysen A. Dambly-Chaudière C. Development of the zebrafish lateral line. Curr Opin Neurobiol. 2004;14:67–73. doi: 10.1016/j.conb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Ma EY. Raible DW. Signaling pathways regulating zebrafish lateral line development. Curr Biol. 2009;19:R381–R386. doi: 10.1016/j.cub.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 59.Nechiporuk A. Raible DW. FGF-dependent mechanosensory organ pattering in zebrafish. Science. 2008;320:1774–1777. doi: 10.1126/science.1156547. [DOI] [PubMed] [Google Scholar]
- 60.Nuñez VA. Sarrazin AF. Cubedo N. Allenda ML. Dambly-Chaudière C. Ghysen A. Postembryonic development of the posterior lateral line in the zebrafish. Evol Dev. 2009;11:391–404. doi: 10.1111/j.1525-142X.2009.00346.x. [DOI] [PubMed] [Google Scholar]
- 61.Metcalfe WK. Kimmel CB. Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol. 1985;233:377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- 62.Raible DW. Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. [PubMed] [Google Scholar]
- 63.Harris JA. Cheng AG. Cunningham LL. MacDonald G. Raible DW. Rubel EW. Neomycin-induced hair cell death and regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos F. MacDonald G. Rubel EW. Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 65.Owens KN. Cunningham DE. MacDonald G. Rubel EW. Raible DW. Pujol R. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol. 2007;502:522–543. doi: 10.1002/cne.21345. [DOI] [PubMed] [Google Scholar]
- 66.Ou HC. Cunningham LL. Francis SP. Brandon CS. Simon JA. Raible DW. Rubel EW. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J Assoc Res Otolaryngol. 2009;10:191–203. doi: 10.1007/s10162-009-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Q. Steyger PS. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J Assoc Res Otolaryngol. 2009;10:205–219. doi: 10.1007/s10162-009-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill AJ. Teraoka H. Heideman W. Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 69.Yang L. Ho NY. Alshut R. Legradi J. Weiss C. Reischl M, et al. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod Toxicol. 2009;28:245–253. doi: 10.1016/j.reprotox.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 70.Froehlicher M. Liedtke A. Groh KJ. Neuhauss SC. Segner H. Eggen RI. Zebrafish (Danio rerio) neuromast: promising biological endpoint linking developmental and toxicological studies. Aquat Toxicol. 2009;95:307–319. doi: 10.1016/j.aquatox.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Hernández PP. Morena V. Olivari FA. Allende ML. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio) Hear Res. 2006;213:1–10. doi: 10.1016/j.heares.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 72.Linbo TL. Stehr CM. Incardona JP. Scholz NL. Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ Toxicol Chem. 2006;25:597–603. doi: 10.1897/05-241r.1. [DOI] [PubMed] [Google Scholar]
- 73.Johnson A. Carew E. Sloman KA. The effects of copper on the morphological and functional development of zebrafish embryos. Aquat Toxicol. 2007;84:431–438. doi: 10.1016/j.aquatox.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Olivari FA. Hernández PP. Allenda ML. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008;1244:1–12. doi: 10.1016/j.brainres.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 75.Coffin AB. Reinhart KE. Owens KN. Raible DW. Rubel EW. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253:42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seiler C. Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol. 1999;41:424–434. [PubMed] [Google Scholar]
- 77.Williams JA. Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 78.Murakami SL. Cunningham LL. Werner LA. Bauer E. Pujol R. Raible DW. Rubel EW. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio) Hear Res. 2003;186:47–56. doi: 10.1016/s0378-5955(03)00259-4. [DOI] [PubMed] [Google Scholar]
- 79.Ton C. Parng C. The use of zebrafish for assessing ototoxic and oroprotective agents. Hear Res. 2005;208:79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Ou HC. Raible DW. Rubel EW. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res. 2007;233:46–53. doi: 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim CH. Kang SU. Pyun J. Lee MH. Hwang HS. Lee H. Epicatechin protects auditory cells against cisplatin-induced death. Apoptosis. 2008;13:1184–1194. doi: 10.1007/s10495-008-0242-5. [DOI] [PubMed] [Google Scholar]
- 82.Hinshaw HC. Feldman WH. Streptomycin in treatment of clinical tuberculosis: a preliminary report. Mayo Clin Proc. 1945;20:313–318. [Google Scholar]
- 83.Chiu LL. Cunningham LL. Raible DW. Rubel EW. Ou HC. Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol. 2008;9:178–190. doi: 10.1007/s10162-008-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iqbal SM. Srivatsav CB. Chloramphenicol ototoxicity: a case report. J Laryngol Otol. 1984;98:523–525. [PubMed] [Google Scholar]
- 85.Hanna GS. Estrogen-sudden deafness and the contraceptive pill. J Laryngol Otol. 1986;100:701–706. doi: 10.1017/s0022215100099928. [DOI] [PubMed] [Google Scholar]
- 86.Rizzi MD. Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007;15:352–357. doi: 10.1097/MOO.0b013e3282ef772d. [DOI] [PubMed] [Google Scholar]
- 87.Rybak LP. Whitworth CA. Mukherjea D. Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Owens KN. Santos F. Roberts B. Linbo T. Coffin AB. Knisely AJ, et al. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4:e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lipinski CA. Lombardo F. Dominy BW. Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 90.Steyger PS. Peters SL. Rehling J. Hordichok A. Dai CF. Uptake of gentamicin by bullfrog saccular hair cells in vitro. J Assoc Res Otolaryngol. 2003;4:565–578. doi: 10.1007/s10162-003-4002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohlemiller KK. Recent findings and emerging questions in cochlear noise injury. Hear Res. 2008;245:5–17. doi: 10.1016/j.heares.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Byl FM. Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope. 1984;94:647–661. [PubMed] [Google Scholar]
- 93.Conlin AE. Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Arch Otolaryngol Head Neck Surg. 2007;133:573–581. doi: 10.1001/archotol.133.6.573. [DOI] [PubMed] [Google Scholar]
- 94.Haynes DS. O'Malley M. Cohen S. Watford K. Labadie RF. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2007;117:3–15. doi: 10.1097/01.mlg.0000245058.11866.15. [DOI] [PubMed] [Google Scholar]
- 95.Vio MM. Holme RH. Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov Today. 2005;19:1263–1265. doi: 10.1016/S1359-6446(05)03594-4. [DOI] [PubMed] [Google Scholar]
- 96.Swan EE. Mescher MJ. Sewell WF. Tao SL. Borenstein JT. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60:1583–1599. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salt AN. Plantke SK. Local inner-ear drug delivery and pharmacokinetics. Drug Discov Today. 2005;10:1299–1306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]