Abstract
Previous experimental studies to assess the contribution of blood-borne circulating (BBC) cells to cutaneous wound healing have relied on discontinuous pulsing of labeled BBC elements or bone marrow transplant protocols. Such approaches do not permit examination of stable BBC cells that have matured in a physiologically-normal host. We have employed a parabiotic murine model for cutaneous wound healing to evaluate the relative contribution of stable populations of peripheral blood cells expressing the green fluorescent protein (GFP) transgene in otherwise normal animals. Circulating cells (mature and immature) expressing the GFP transgene were easily detected and quantified in wounds of GFP-negative parabiotic twins during all evaluated stages of the healing response. Using multiple antibody probes, the relative contribution of various subsets of BBC cells could be comparatively assessed. In early wounds, some cells expressing mesenchymal epitopes were documented to be of hematopoietic origin, indicating the utility of this model in assessing cell plasticity in the context of tissue regeneration and repair. Application of this approach enables further investigation into the contribution of peripheral blood in normal and abnormal healing responses.
Keywords: green fluorescent protein (GFP), hematopoietic, parabiotic model, regeneration, transdifferentiation, wound healing
Introduction
Wound healing is an intricate, dynamic process that includes inflammatory cells, progenitor and/or stem cells, soluble mediators (such as transforming growth factor-beta or TGF-β), and extracellular matrix (ECM) components (such as collagen and glycosaminoglycans). Wound healing is classically subdivided into three overlapping phases: inflammation, proliferation, and remodeling.1,2 It is generally accepted that circulating bone marrow (BM)-derived leukocytes play a critical role in early wound healing, namely via phagocytosis, cytokine production, and coordination of other cellular/molecular events essential to the inflammatory response. Cells that eventually repopulate the wound site have traditionally been believed to derive primarily from adjacent uninjured tissue.3 These cells include fibroblasts, endothelial cells (ECs), tissue-derived mononuclear and dendritic cells, and epithelial cells—mobilized from adjacent mature cells stimulated to divide and migrate, or from their local progenitors.4 However, recent reports have provided evidence that cells seen in the peripheral blood (PB), mainly hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs), circulating fibrocytes, and BM-derived mesenchymal stem cells (MSCs), and rare tissue-derived mesenchymal stem cells may also play an important role in the reconstitution of injured tissue.5-14 Moreover, because skin has recently been shown to harbor unexpectedly large numbers of resident leukocytes (in human skin, for example, approximately twice the number of T cells as in the entire circulation15), immunohistochemical approaches alone fail to reveal whether leukocyte derive from local or peripheral compartments.
It is thought that adult HSCs and BM-derived MSCs may have some degree of ‘plasticity,’ and thus, under specific situations (such as extensive injury), may have the capacity to ‘transdifferentiate’ into non-lineage cells, including fibroblasts,5 endothelial cells,6 smooth muscle cells,7 cardiomyocytes,8 hepatocytes,9 and GI tract epithelial cells.10 Like mature hematopoietic cells (mature HCs), hematopoietic progenitor cells (including EPCs) are also thought to be recruited to the wound microenvironment soon after injury. Tissue damage may be responsible for homing of BM-derived stem/progenitor cells to the wound site, and may also stimulate their differentiation into various solid-organ specific tissues, including skin.11-14 Reports from studies in cutaneous wound healing suggest that HSCs (and BM-derived MSCs) have the ability to transdifferentiate into fibroblasts.2,16-18 Moreover, it has been proposed that circulating fibrocytes are a transitional form in this event.19 In addition, circulating EPCs are suspected of playing an important role in regenerative responses incited by tissue ischemia and related cytokine release.16.17.20.21
Thus, skin consists of endogenous epithelial and mesenchymal cellular components as well as robust populations of resident cells of bone marrow and hematopoietic origin, and blood-born circulating (heretofore BBC) are composed of both hematopoietic and mobile mesenchymal stem cells. The ability to investigate roles of mature BBC cells in cutaneous wound healing requires a model whereby cells in question: (1) circulate as stable populations within a physiological intravascular microenvironment, (2) infiltrate wounded tissues devoid of complicating factors (e.g. conditioning regimen), and (3) express endogenous markers that signify their origin from the intravascular compartment. Existing models, in which labeled circulating elements are administered via pulsing, produce transient and potentially unstable conditions, and thus, do not achieve this goal. Moreover, most transplantation approaches involve hematopoietic reconstitution and require host irradiation, which may affect relevant microenvironmental factors. In contrast, the parabiotic model consists of two genetically identical, physiologically-normal mice, surgically conjoined at the torso, resulting in stable anastomotic linkage between the two peripheral circulations.22
The parabiotic model employed herein consists of a wild-type mouse (designated GFP-) conjoined to a transgenic (GFP+) mouse (with somatic cells expressing the gene for green fluorescent protein, or GFP). After stable chimerization, experimental wounds may be created, permitting sequential evaluation of the wounds for influx and the potential differentiation of any GFP-expressing blood-borne elements.23,24 In this study, we hypothesize that (1) the parabiotic system is a novel and valuable model for studies of the contribution of the BBC cell compartment to skin healing and regeneration, and (2) BBC cells, including those with capacity for transdifferentiation, may be studied by this model in a manner to assess their participation in the early stages of the healing response. Our data indicate that BBC cells, including those with capacity for transdifferentiation, actively participate in the early stages of the healing response, and that this model system is applicable to further studies of the contribution of the circulating cellular compartment to skin healing and regeneration.
Materials and Methods
Animal model
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals [National Institute of Health (NIH) publication No. 86-23] and approved by Brigham & Women's Hospital/Harvard Medical Area Standing Committee on Animals (IACUC). The generation of parabiotic mice has been described previously by authors from this study.23 A parabiotic pair consists of an unirradiated (one month-old) wild-type mouse (C57BL/6 background), surgically conjoined to an age-matched transgenic GFP+ mouse (C57BL/Ka-EGFP, bred and maintained at the Joslin Diabetic Center) via a dermal-to-dermal anastomosis at the torso.24 After two weeks with shared circulatory systems, stable chimerization of a common blood supply (approximately 50% GFP+ and GFP- nucleated blood cells) was confirmed by flow cytometry, as previously shown.23 Thereafter, identical one cm2 full-thickness wounds (including the panniculus carnosus muscle) were created on the mid-back of each mouse of the parabiotic pair. Wounds were covered with transparent, semi-occlusive adhesive dressings (Tegaderm™). Wounds were then harvested, with early time points selected to maximize evaluation of both mature and immature hematopoietic cell participation in the early healing response.
Harvesting and processing of tissue specimens
Three and seven days after wounding, five parabiotic pairs (n=10) were sacrificed and wounds of the wild-type mice were harvested. One pair that remained conjoined was available for late harvesting (day 21), such that a total of 22 wounds were collectively evaluated. Adjacent uninjured tissues were also sampled. Once tissue samples were collected, they were immediately placed in 2% paraformaldehyde (PFH) in 1X Phosphate Buffer Saline (PBS) solution, where they remained for 4 hours at room temperature (RT). Subsequently, samples were placed in 10% sucrose in 1X PBS overnight at 4 °C. Specimens were then placed in optimum cutting temperature (OCT™, Tissue-Tek®) solution and stored at -25 °C. Frozen tissue blocks were cut at 6-7 μm and serial sections were collected onto slides for staining.
Immunofluorescence (IF) staining
In preparation for IF staining, slides were thawed to RT. Once dried, slides were fixed in acetone at -5 °C for 5 minutes. Slides were then rinsed twice (each time for 5 minutes) in PBS to remove OCT.™ Ten percent donkey serum (in PBS at RT) was added onto samples (on the slides) and incubated for 30 minutes. Next, primary antibody was added and allowed to incubate overnight at 4 °C. On the following day, slides were rinsed three times (each time for 5 minutes) with PBS. Subsequently, secondary antibody was added and allowed to incubate at RT for 30 minutes (to protect samples from photodamage, incubating apparatus was wrapped with foil sheets). Slides were then rinsed three more times in the same fashion. Once dried, 4’,6-diamidino-2-phenylindole (DAPI, nuclear counterstain) solution was applied and slides were mounted. All slides were also examined using hematoxylin and eosin staining by an experienced dermatopathologist in order to define the nuclear morphology of the infiltrates and to permit their initial classification (bi- or multilobed cells containing cytoplasmic granules = granulocytes [neutrophils/eosinophils], mononuclear cells = lymphocytes and monocytes).
Primary antibodies used are as follows
(1) rat monoclonal anti-CD45 (1:10 dilution, BD Pharmingen Inc., San Diego, CA), (2) anti-CD11b (1:200 dilution, Pharmingen), (3) anti-CD3 (1:50 dilution, Antigenix America Inc., Huntington Station, NY), (4) anti-collagen I (1:400 dilution, Biogenesis Inc., Kingston, NH), (5) anti-α-smooth muscle actin (α-SMA, 1:200 dilution, Epitomics Inc., Burlingame, CA), (6) rabbit polyclonal anti-VE-cadherin/CD144 (1:20 dilution, Cell Signaling Technology Inc., Danvers, MA), (7) anti-CD31 (1:50 dilution, Pharmingen), (8) anti-CD34 (1:20 dilution, Pharmingen).24 Secondary antibodies used are as follows: (1) Alexa Fluor 594 donkey anti-rat IgG (1:800 dilution, Invitrogen Corp., Eugene, OR) for anti-CD45, CD34, CD11b, CD3, and CD31, (2) Alexa Fluor 594 donkey anti-rabbit IgG (1:800 dilution, Invitrogen) for anti-CD144, collagen type I, and α-SMA antibodies. Stains for collagen and smooth muscle actin were all run with appropriate positive and negative tissue controls, as well as isotype-matched irrelevant controls to exclude non-specific bindings.
Fluorescence microscopy and evaluation of HCs
Slides were examined under fluorescence microscopy (MX51, Olympus Corp., Tokyo, Japan) with a mercury vapor light source. Photographs of live images at 10X, 40X, 60X (with oil immersion) were taken with the aid of CytoVision™ 3.7 software (utilizing serial layer stacking for superior clarity; Applied Imaging Corp., San Jose, CA). Cells were visualized as follows: nuclei—rendered blue by DAPI counterstain; cells expressing GFP transgene—green; and specific lineage and non-lineage markers—red by Alexa Fluor 594 fluorochrome. Cells co-expressing GFP and red fluorochrome were identified by (partial to circumferential) red-to-orange membrane-associated staining surrounding a zone of green cytoplasm and a variably detectable central blue nucleus.
For quantification of GFP+ cells that express surface markers of interest, at least ten high-power fields (HPFs) were randomly selected from the central zone of each wound bed (coagulum excluded). The enumerated cells, based on marker expression plus or minus associated GFP, were counted only when discrete circumferential membrane staining was apparent. For accuracy, replicate counts were made for each experimental condition. In a given field, the total number of cells was tallied for cells with DAPI-positive nuclei (blue). Cells expressing GFP (in wounds of wild-type parabionts) were taken to represent one-half of the entire hematopoietic infiltrate, based on known chimerization ratio: total HC population = 2 × GFP+ cell population. It is worth mentioning at this juncture that although the aforementioned equation does not encompass the complexity of shared circulation in a parabiotic pair (since the true total HC population, with respect to GFP+ cell population, is likely to be multifactorial and presumptively asymptotic), it does however, allow for calculation of first-order approximation. Accordingly, resident cell (RC) population (non-hematopoietic, tissue-based) for each field was deduced by the following equation: RC population = total cell population - (2 × GFP+ cell population). To facilitate analysis, raw data were normalized, such that total cell population (sum of GFP+ HCs, GFP- HCs, and GFP- RCs) is set to 100. Numeric values of tabulated subsets of day 3 and day 5 were averaged and presented as mean ± standard deviation. Ad hoc analysis with associated probability (p) of 0.05 or less was rendered significant.
Results
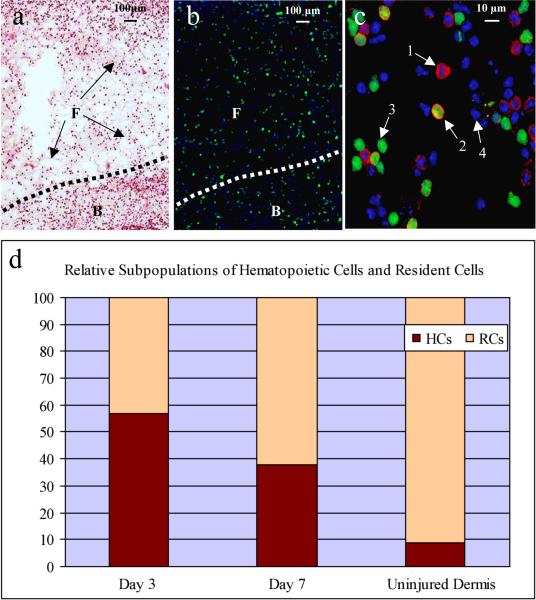
Early stages of wound healing in both wild-type and transgenic parabionts were characterized by the formation of a fibrin coagulum intimately associated with the wound bed (Fig. 1a). Both components contained predominantly rounded and occasionally elongated mononuclear cells at this juncture, without significant accumulation of cells with bi- or multi-lobed nuclei typical of granulocytes. In addition to demonstrating the efficacy of the parabiotic model for studies of the initial stages of wound healing, we also sought to test the model's efficacy for qualitative and quantitative evaluation of BBC cell participation at early timepoints in the healing response. At both the day 3 and day 7 time points, numerous GFP+ cells could be detected within both compartments of the wild-type animals (Fig. 1b). Double labeling permitted lineage determination for both the GFP+ and GFP- compartments Fig. 1c). This drop-off is verified by H&E stains that reveal parallel decrements in cells with hematopoietic cytologic features. The ratio of GFP+ versus GFP- cells declined from day 3 to day 7, indicating a more robust hematopoietic infiltrate (> 50% of all nucleated cells) at the earlier time point [Fig. 1d, Table 1 (due to small sample size, day 21 was excluded from analysis)]. As compared to control uninjured dermis, hematopoietic cells homed to wound sites in greater than 5:1 (day 3) and 3.5:1 (day 7) ratios, consistent with the trophic influence of tissue injury.
Figure 1.
Evaluation of day 3 wound in wild-type parabiont by (a) light microscopy, (b and c) fluorescent microscopy, and (d) graphic representation showing relative subpopulations of hematopoietic cells and resident cells. Note the numerous GFP+ cells infiltrating the wound bed (B) and associated fibrin coagulum (F) (panel b). Application of lineage markers (here depicted CD144) permits discrimination of four cell types: 1) CD144+, non-hematopoietic (resident) cell; 2) CD144+, hematopoietically-derived cell; 3) CD144- hematopoietically-derived cell; and 4) CD144-, non-hematopoietic (resident) cell. The graphic representation (d) of relative numbers of hematopoietic versus resident cells suggests a marked contribution of the former at day 3 (as compared to uninjured dermis at day 3), followed by a decline by day 7.
Table 1.
Relative Percentages of GFP+ cell, Total Hematopoietic Cell (HC), and Resident Cell (RC) Subpopulations at Day 3 and Day 7 (as well as adjacent uninjured dermis) (p <0.01)
| Day 3 (n=5) | Day 7 (n=5) | Adjacent uninjured dermis (n=10) | |
|---|---|---|---|
| GFP+ Cells | 29% ± 1% | 19% ± 1% | 4% ± 1% |
| Total Hematopoietic Cells (HCs, 2 × GFP+ Cells) | 57% ± 2% | 38% ± 1% | 9% ± 1% |
| Resident Cells (RCs) | 43% ± 2% | 62% ± 1% | 91% ± 1% |
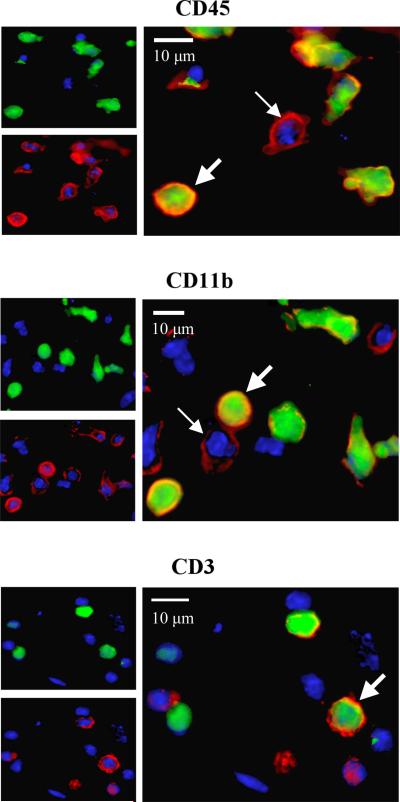
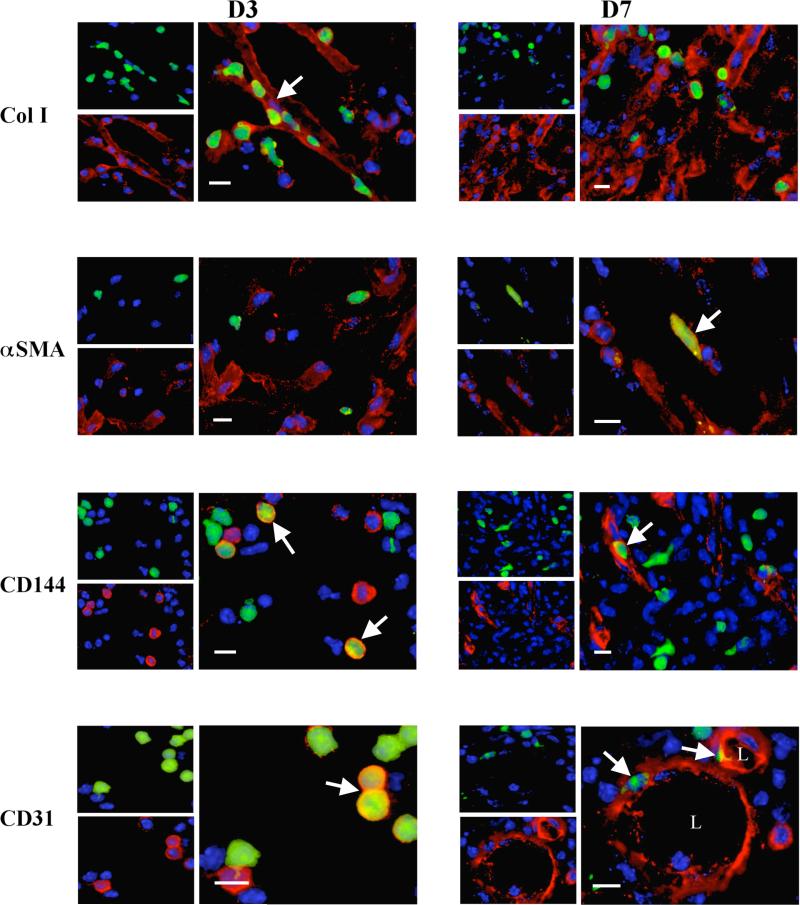
With respect to hematopoietic lineage markers, between 31% and 38% of GFP+ BBC cells infiltrating day 3 wounds co-expressed leukocyte-associated markers CD45, CD11b, and CD3 (Fig. 2, Table 2). By day 7, only about one-third to one-half of these cells maintained lineage markers. Regarding non-hematopoietic, mesenchymal marker (collagen I, α-SMA, and CD144) expression in day 3 wounds, 29-36% of GFP+ cells fit this criteria (Figure 3, Table 2). A marked decrease in co-expression of these markers was also noted among GFP+ cells by day 7. Nonetheless, at both day 3 and day 7 time points, GFP+ cells were detected in intimate association with sites of collagen type I reactivity, and individual GFP+ cells were clearly noted to express α-SMA and CD144 or CD31, consistent with local myofibroblastic and endothelial differentiation from hematopoietically-derived precursors at the wound site. These findings were not observed in GFP+ cells that trafficked in non-wounded perilesional skin of the wild-type parabionts.
Figure 2.
Expression of hematopoietic lineage markers CD45 (leukocytes), CD11b (monocytes), and CD3 (T-cells) in day 3 wounds. Small panels at top left depict GFP+ and GFP- cells, while small panels at bottom left indicate respective lineage marker positive and negative populations in same field. The larger panel to the right shows a merged image with thick arrows indicating GFP+/lineage marker(+) cells (hematopoietically-derived from transgenic parabiont), and thin arrows indicating GFP-/lineage marker(+) cells (either hematopoietically or locally derived from wild-type parabiont). All scale bars = 10 μm.
Table 2.
Percent of GFP+ Cells Co-expressing Hematopoietic Lineage and Non-lineage Markers at Day 3 and Day 7 (p <0.01)
| GFP+ Cells Day 3 (n=5) | GFP+ Cells Day 7 (n=5) | |
|---|---|---|
| CD45 (all leukocytes, lineage) | 34% ± 3% | 23% ± 2% |
| CD11b (monocytes/macrophage, lineage) | 38% ± 3% | 14% ± 2% |
| CD3 (T-cells, lineage) | 31% ± 5% | 10% ± 4% |
| Collagen I (fibroblasts, non-lineage) | 36% ± 5% | 10% ± 3% |
| α-SMA (myofibroblasts, non-lineage) | 33% ± 4% | 19% ± 3% |
| CD144 (vascular endothelial cells, non-lineage) | 29% ± 2% | 13% ± 2% |
| CD31** (vascular endothelial cells) | 30% ± 4% | 13% ± 1% |
| CD34** (vascular endothelial cells) | 27% ± 2% | 14% ± 1% |
CD31 and CD34 are not non-lineage markers, but are used in conjunction with CD144 to confirm endovascular presence—see Materials and Methods
Figure 3.
Documentation of mesenchymal lineage marker expression in wounds at days 3 and 7. At both time points, GFP+ cells are focally associated with these markers (arrows indicate GFP+ cells co-expressing respective markers). By day 7, some GFP+ cells that retain the lineage markers appear to be more elongated, and cells expressing CD144 and CD31 are focally associated with arcuate and rounded structures with lumen-like centers (L) resembling forming blood vessels.
Discussion
In this study, a parabiosis model of cutaneous wound healing was employed with the primary goal of determining the utility of this model in studies designed to examine the participation of BBC cells in cutaneous wound healing. Our data indicate that the model is indeed useful, revealing that: (1) infiltrating mature and immature BBM cells are easily detected and quantified by fluorescent transgene expression in the parabiosis model, (2) BBC cells predominate over resident skin cells in the early stages of wound healing (day 3), (3) potential for BBC cell expression of mesenchymal markers exists during this interval.
Parabiotic mice have been used previously to evaluate the migratory fates of BBC cells in wound healing.23,24 Labeling of BBC cells and their progeny (with a GFP) permitted the observation that these cells do not contribute substantially to stable chimerization of normal non-hematopoietic tissues via transdifferentiation.26 Moreover, even in situations where tissue stress and injury exists, as with myocardial ischemia, resident tissue regeneration from blood-borne hematopoietic elements does not appear to occur.27 Authentic transdifferentiation is further complicated by technical difficulties in complex tissues including possible: (1) cell fusion events, (2) antigen or marker shedding, (3) phagocytosis, (4) contamination, (5) inductive stimuli for gene expression, and (6) the nature and severity of tissue injury.28
However, recent data relating to circulating fibrocytes adds potential credibility to the role of direct contribution of BBC cells to tissue repair under certain conditions.19,29 These blood-borne cells express a Col I+/CD11b+/CD13+/CD34+/CD45-RO+/MHC class II+/CD86+ phenotype and have been shown to rapidly enter sites of tissue injury in a CCR7 chemokine-dependent manner. In vitro, they have the capacity to differentiate into α-SMA+/TGF-β1-responsive cells contracting fibrin, suggesting a potential role in wound healing and tissue repair.19
Since the earlier phases of cutaneous wound healing involves inflammation,30 it is not surprising that a majority of cells infiltrating day 3 wounds are of hematopoietic origin. While an active acute inflammatory reaction, mediated by neutrophils (as incited classically by bacterial antigens or foreign material), is generally regarded as counterproductive to wound healing, the participation of mononuclear cells (monocytes/macrophages and lymphocytes) is regarded as potentially having a favorable influence, at least indirectly, via local production of mediators such as platelet-derived growth factor (PDGF) and TGF-β (both essential stimuli for the subsequent migration and proliferation of resident fibroblasts and endothelial cells that typifies the proliferative phase).31,32 Because neutrophils were excluded by conventional histology in the present study, the proportion of total BBC cell population at day 3 is largely accounted for by undifferentiated leukocytes (CD45) co-expressing both markers of monocytes (CD11b) and T-lymphocytes (CD3). Interestingly, in previous studies using rodent models to impede formation of contractile scars—via the use of biodegradable collagen-glycosaminoglycan (collagen-GAG) matrices—the early participation of mononuclear cells was one discriminating factor that favored a regenerative as opposed to a scarring response.33
One noteworthy finding in this study was the extent to which GFP-positive hematopoietic cells expressed mesenchymal markers (Col I and α-SMA: associated with fibroblasts and myofibroblasts, respectively; CD144 and CD31: associated with vascular endothelial cells) in the early wound. While we cannot exclude factors that may mimic or complicate interpretation of authentic transdifferentiation (as listed above), the robust expression of these markers by BBC cells (as defined by GFP transgene expression) raises the possibility that some degree of hematopoietic plasticity may manifest in the early stages of cutaneous wound healing. Future studies are now possible using a variety of technologies, including in situ hybridization and laser capture microdissection coupled with real time RT-PCR analysis, in order to better define transdifferentiation of BBC cells in healing wounds at gene expression levels. Interestingly, because these markers significantly dissipate, along with relative hematopoietic contribution, by day 7, the expression of mesenchymal epitopes could easily be overlooked unless very early time points are examined. The question of whether transdifferentiation of hematopoietic elements occurs during early wound healing cannot be resolved definitively based upon the present study. However, the parabiosis model should provide a useful, and potentially powerful, model for studies examining this possibility further.
The rapid dissipation of hematopoietic elements and related hematopoietic and mesenchymal markers, subsequent to day 3, suggests that these cells are not directly or permanently incorporated into new tissue during wound healing. The loss of GFP-positive cells may be the result of apoptosis34 or simply the kinetics within the proliferative phase, whereby resident skin cells encroach upon and dominate the nucleated constituents of the repair process. The possibility of some degree of degeneration and cell death of hematopoietic elements is potentially supported by the observation of an attendant decrease in lineage marker expression by GFP-positive cells over time—possibly the result of biosynthetic impairment. The consequential implication is that participants that restore fibroblast/myofibroblast and endothelial cell population in dermal wounds in physiologically-normal hosts are primarily derived from local, nonhematopoietic subpopulations. Nonetheless, the finding of a robust and potentially plastic hematopoietic response raises the possibility that strategies could be devised to experimentally or therapeutically manipulate hematopoietic cells during early stages of skin repair in a manner that promotes their potential contribution to accelerated healing or even regeneration.
In conclusion, we have preliminarily evaluated a parabiosis model for experimental study of hematopoietic contribution to cutaneous wound healing. Our data support the applicability and utility of this novel approach and indicate that an early and brisk BBC cell response, mediated by mononuclear cells with transdifferentiation potential, typifies early stages of skin repair. Further studies are now possible using parabiosis models to more precisely define the molecular and cellular mechanisms whereby hematopoietic cells contribute to normal and pathologic healing responses.
Acknowledgements
The authors would like to thank Robin M. Schanche for her contribution and hard work in the final phase of manuscript submission.
This study was funded by (1) NIH 5 T32 HL007627-22 Physician-Scientist Training Grant, (2) Brigham and Women's Hospital's Program in Dermatopathology core grants (SPORE and SDRC), and (3) NIH/NIDDK (5 P30 DK36836-20) to AJW.
Footnotes
The authors state that there are no conflicts of interest.
References
- 1.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Diegelmann RF, Evans MC. Wound Healing: and overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6(11):1082–93. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 4.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126(7):1459–68. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 5.Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19(11):1561–3. doi: 10.1096/fj.04-2978fje. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Takakura N. Physiological pathway of differentiation of hematopoietic stem cell population into mural cells. J Exp Med. 2006;203(4):1055–65. doi: 10.1084/jem.20050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh ET, Zhang S, Wu HD, Körbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108(17):2070–3. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 8.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104(45):17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa M, LaRue AC, Drake AC. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108(9):2893–6. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 10.Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346(10):738–46. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 11.Mansilla E, Marín GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzmán A, Nuñez A, Gil MA, Piccinelli G, Ibar R, Soratti C. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967–9. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 12.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 13.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196(2):245–50. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 14.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow- derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22(5):812–22. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark RA. Skin-resident T cells: The ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, Huh NH. Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol. 2003;163(4):1227–31. doi: 10.1016/S0002-9440(10)63482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(7146):41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 18.Ishii G, Sangai T, Sugiyama K, Ito T, Hasebe T, Endoh Y, Magae J, Ochiai A. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells. 2005;23(5):699–706. doi: 10.1634/stemcells.2004-0183. [DOI] [PubMed] [Google Scholar]
- 19.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 20.Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 2005;20(1):36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 22.Finerty JC. Parabiosis in physiologic studies. Physiol Rev. 1952;32(3):277–98. doi: 10.1152/physrev.1952.32.3.277. [DOI] [PubMed] [Google Scholar]
- 23.Pietamaggiori G, Scherer SS, Alperovich M, Chen B, Orgill DP, Wagers AJ. Improved cutaneous healing in diabetic mice exposed to healthy peripheral circulation. J Invest Dermatol. 2009;129:2265–74. doi: 10.1038/jid.2009.60. [DOI] [PubMed] [Google Scholar]
- 24.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–6. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 25.The National Institutes of Health Hematopoietic stem cells. Stem Cells: Scientific Progress and Future Research Directions. 2001:43–58. Available from: http://stemcells.nih.gov/info/scireport/chapter5.asp.
- 26.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297(5590):2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 27.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 28.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639–48. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 29.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36(4):598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen DT, Orgill DP, Murphy GF. The pathophysiologic basis for wound healing and cutaneous regeneration. In: Orgill DP, Blanco C, editors. Biomaterials for Treating Skin Loss. Woodhead Publishing Limited; Cambridge: 2009. pp. 25–57. [Google Scholar]
- 31.Van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170(3):818–29. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3578–85. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 33.Murphy GF, Origill DP, Yannas IV. Partial dermal regeneration is induced by biodegradable collagen-glycosaminoglycan grafts. Lab Invest. 1990;62(3):305–13. [PubMed] [Google Scholar]
- 34.Ogawa R, Fujimura J, Mizuno H, Hyakusoku H, Shimada T. Tissue engineering using adipose-derived stem cells harvested from GFP transgenic animals. In: Grier EV, editor. New Developments in Stem Cell Research. Nova Science Publishers; New York: 2007. pp. 149–72. [Google Scholar]