Abstract
Formation of channel-like structures, also termed vasculogenic mimicry (VM), describes the ability of aggressive melanoma cells to form anastomosing PAS-positive anastomosing structures that correlate with tumor virulence. This phenomenon may indicate differentiation plasticity, a feature melanoma cells may share with stem cells in the developing embryo. Recent studies have indicated that VM and tumorigenicity of human malignant melanoma may depend on the signaling pathways of an embryonic morphogen, Nodal. However, given the secretory nature of Nodal protein and melanoma cell heterogeneity, it remains unclear whether the Nodal-expressing cells participate directly or indirectly in VM that is potentially related to tumorigenic growth. We have developed a humanized murine xenograft model in which developing human melanomas may be sequentially studied during early stages of tumorigenic growth within a physiological human dermal microenvironment. Nodal protein localized diffusely to melanoma cell membranes, with occasional foci of accentuated reactivity in patterns suggestive of channel formation. Similar findings were detected in a limited number of patient-derived tumors. In situ hybridization confirmed Nodal mRNA to be restricted to tumor cells within xenografts that formed arborizing networks in patterns consistent with VM. These data indicate that Nodal gene expression is associated with formation of VM-like structures in a physiologically relevant model of human melanoma tumorigenesis, and further support a key role for Nodal expression in the formation of channel-like structures. The humanized xenograft model should be useful in future studies to define the mechanistic pathways responsible for VM and melanoma progression.
Keywords: Nodal, melanoma, vasculogenic mimicry, stem cells, CD31, CD133, VE-cadherin
Introduction
Based on the pioneering contributions of Mihm and coworkers, human malignant melanoma is universally recognized as an unusually virulent neoplasm upon entering the vertical, tumorigenic phase of growth. Although one may infer that vertical growth phase melanoma cells possess a multiplicity of molecular strategies to foster virulent growth, only recently have we learned of biomarkers, growth factors, and morphogens that may mediate melanoma aggressiveness.
Nodal is a morphogen normally expressed during embryogenesis that belongs to the transforming growth factor (TGF)-β family. It plays several crucial roles in vertebrate development, including the maintenance of pluripotency, regulation of neurogenesis, the patterning of right-left asymmetry in vertebrates, induction of mesendoderm, and establishment of the anteroposterior axis1. Nodal initiates signal transduction via association with EGF-CFC co-receptors (Cripto), and the complex then binds to heterodimeric receptors comprised of type I (ALK4, ALK5, ALK7) and type II (ActRIIA, ActRIIB) activin-like kinase receptors2. The kinase activity of Nodal receptors phosphorylates receptor-regulated SMADs (SMAD2, SMAD3), which then associate with SMAD4 and translocate to the nucleus to activate transcription factors such as FoxH1 and Mixl13. Nodal exhibits both short- and long-range signals that act directly or via concentration gradients regulated by antagonists such as Lefty and Cerberus4. Recently, Nodal expression has been implicated in the regulation of melanoma tumorigenicity in the zebrafish model5. In these studies, inhibition of Nodal abrogated melanoma growth, decreased expression of VE-cadherin, and prevented the formation of vasculogenic networks or channels-like structures, a phenomenon designated as vasculogenic mimicry (VM)5.
In human cancers, VM depends on activation of the VE-cadherin (CD144) pathway, also involved in endothelial differentiation6. VM has been observed in osteosarcoma, breast cancer, colon cancer, hepatocellular carcinoma, glioma, ovarian cancer, prostate cancer, and uveal melanoma7-9. In uveal melanoma, VM in the form of PAS- and CD144-positive, CD31-negative channel formation is associated with increased mortality, mitotic activity, and cell type10. The concept of VM implies melanoma cell plasticity, a feature also observed in cancer stem cells11. While it remains controversial as to whether VM facilitates tumor virulence via formation of true vascular surrogates by tumor cells, there is increasing acceptance of its significance as a biomarker for melanoma virulence.
Interestingly, melanoma cells have been shown to exhibit stem cell-like patterns of protein expression. Increased expression of the stem cell marker CD133—a transmembrane glycoprotein expressed in hematopoietic stem cells and endothelial progenitor cells—has been identified in melanoma cells12,13. Moreover, discrete subpopulations of melanoma stem cells capable of self-renewal and tumorigenicity that express the MDR transporter ABCB5 have recently been discovered14. In addition, studies also have shown expression of the Nodal coreceptor, Cripto-1, in a subset of slow-growing, sphere-forming malignant melanoma cells that also expressed increased levels of stem cell markers Oct4, Nanog, and MDR115. In this regard, Hendrix has speculated that Nodal expression in melanoma cells also capable of expressing VE-cadherin may correlate with a more primitive, potentially stem-like subpopulation of malignant cells within the tumor3.
Based upon these observations, we reasoned that expression of Nodal might be spatially associated with the phenomenon of VM in human melanoma. To this end, we have developed a humanized murine model for the study of VM in melanomas grown intradermally in human skin xenografted to immuncompromised mice. This model has the advantage of permitting sequential study of human melanoma nodules that develop within a physiological human dermal microenvironment that contains intact human blood vessels and related human dermal stroma. In tumors so generated, Nodal mRNA expression was intimately associated with tumor cells that in adjacent sections formed arborizing PAS- and CD144-positive, CD31- weak-to-negative channels in patterns consistent with VM. Nodal protein was diffusely expressed within the experimentally-induced tumors, as previously shown by others5, although increased staining intensity was focally associated with CD144-positive anastomosing channels. Similar patterns were seen focally in human melanoma tissue. These data show that melanoma cells selectively express Nodal mRNA in a channeled pattern typical of VM, and further support the key role of a primitive stem cell phenotype in the genesis of this phenomenon.
Materials and Methods
Cell culture and tissue sources
Human melanoma cell lines (A375, A2058, SK-MEL-5) were cultured in RPMI1640 (Invitrogen, Carlsbad, CA) with glutamine supplemented with 10% FCS and 1% Penicillin / Streptamicin until they were 70-80% confluent (exponential growth phase). Melanoma cells obtained from a patient-derived metastatic melanoma were also isolated and evaluated in the xenograft model, as previously described14. Cells were washed three times with PBS (Invitrogen) and counted. A limited number of primary human patient melanomas preserved as formalin-fixed, paraffin-embedded specimens were also evaluated for Nodal protein expression (n=12). All specimens were obtained according to human subjects research protocols approved by the IRB of Brigham and Women’s Hospital.
Human melanoma xenotransplantation
NOD/SCID and RAG2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in accordance with the institutional guidelines of Children’s Hospital Boston and Harvard Medical School. Mice were shaved on the right flank and injected subcutaneously with 100 μLs of cell suspensions at 2×106 cells/mL derived either from cultured melanoma cells or melanoma cells isolated from a patient sample. Xenografted tumors were grown for 2-6 weeks. The mice were sacrificed when tumors reached 2-6 mm in diameter and the tumors were then removed and embedded in O.C.T. or fixed in formalin and embedded in paraffin (Fisher Scientific, Pittsburgh, PA).
Immunohistochemistry and immunofluorescence
Frozen sections of xenografted tumors derived from cell lines (A375, A2058, SK-MEL-5) and freshly isolated patient melanoma cells, and patient metastatic melanoma samples (3538 and 3487) were stained with an antibody against human Nodal (4 or 6 μg/mL, Chemicon, Temecula, CA). Controls were stained with isotype-matched mouse anti-human IgG1κ antibody (4 or 6 μg/mL; BD Biosciences, San Jose, CA). Samples were incubated with primary antibodies at 4°C overnight, then with horse-radish-peroxidase-conjugated secondary antibodies for 30 minutes at room temperature, then visualized with Vector NovaRed (Vector Laboratories, Burlingame, CA) and counterstained with Gill’s Hematoxylin #1 (Fisher Scientific). For co-localization of Nodal protein and CD144 by immunofluorescence, antibodies to human Nodal (4 μg/mL; Chemicon) and VE-Cadherin (CD144; 1:40; Cell Signaling Technology, Danvers, MA) were diluted in 10% donkey serum with 1% BSA. Controls were stained with isotype-matched mouse anti-human IgG1κ antibody (4 or 6 μg/mL; BD Biosciences) and non-immune rabbit IgG (0.1 mg/mL; Invitrogen). Alexa Fluor conjugated secondary antibodies (Invitrogen) were used according to the manufacturer’s recommendations. Nuclei were stained with ProLong Gold Antifade Reagent with DAPI (Invitrogen). Images were photographed using a BX51 fluorescence microscope (Olympus, Center Valley, PA).
Preparation of DIG-labeled Nodal RNA probe for in situ hybridization
PCR-derived RNA probe templates were synthesized by introducing the T7 promoter into the antisense strand and the SP6 promoter into the sense strand. The primer pair, NodalT7AS (5′-taatacgactcactatagggagatgtatgcatggttggtcgg) and NodalF (5′-agtggggcaagaggcaccgt) was used to generate the DNA template for the antisense NodalSP6S (5′-gatttaggtgacactatagaagtggggcaagaggcaccgt) and NodalR (5′-gatgtatgcatggttggtcgg) sense RNA probes spanning 200 base pairs of human Nodal cDNA. The sequence specificity for Nodal was checked with the BLAST program in the Genbank database13. This sequence encodes Nodal amino acids 229-296 (gi #85397850). PCR was performed as follows: 94°C for 3 min., 94°C for 45 seconds, 55°C for 30 seconds, 72°C for 1 min. for 35 cycles, and 72°C 10 min. The PCR product was separated on an agarose gel and purified with the QIAquick gel extraction kit (Qiagen, Chatsworth, CA). The template was used to synthesize antisense and sense digoxigenin-labeled RNA probes with a DIG RNA labeling kit (Roche, Indianapolis, IN) according to the manufacturer’s instructions.
In situ hybridization
8 μM frozen tissue sections of tumor xenografts were baked at 50°C for 15 min., then fixed in 4% paraformadehyde at room temperature (RT) for 20 min. The sections were treated with 1 mg/mL proteinase K/PBS at 37°C for 20 min., inactivate proteinase K with 0.2% glycine/PBS at RT for 5 min., and washed in PBS twice for 2 min. The tissue was then fixed in 4% paraformadehyde at RT for 15 min., washed in PBS twice for 5 min., and treated with 0.25% acetic anhydride/0.1M triethanolamine at RT for 10 min. Sections were placed in 2x Standard Sodium Citrate until ready for hybridization. They were then hybridized with 500 ng/mL Nodal antisense or sense probe in hybridization buffer (0.3M NaCl, 10 mM Tris-HCl pH 7.6, 5 mM EDTA, 1x Denharts solution, 50% formamide, 100 mg/mL tRNA and 10% dectran sulphate) at 42°C overnight. Post hybridization washes were carried out as follows: two 20-min. 0.2xSSC at 57°C washes; 20 mg/mL RNase A in 0.5M NaCl, 10 mM Tris-HCl pH 7.5 at 37°C for 30 min.; and 0.2xSSC at 57°C for 20 min. The hybridized probes were immunodetected with DIG Detection Kit (Roche, Indianapolis, IN) and a Tyramide Signal Amplification (TSA) Kit (Perkin Elmers, Boston, MA) as follows: 1xDIG block buffer for 30 min., 1:100 anti-DIG Ab peroxidase conjugate at RT for 1 hour, three 5-min. 1xDIG washes, TSA reagent 8 min. at RT, two 5-min. PBS washes, 1:100 streptavidin-horseradish peroxidase 30 min. at RT, three 5-min. PBS washes, incubation in NovaRed substrate (Vector Labs) for 1 min., then immediate counterstaining with Gill’s Hematoxylin #1 (Fisher Scientific).
Results
Melanoma growth and channel formation in humanized xenografts
Intradermal injection of human melanoma cells into xenografted human skin resulted in visible tumors within 2 to 3 weeks for all cell lines employed. Histological examination revealed nodular melanoma growth within the human dermis in a pattern similar to the vertical growth phase of patient melanomas (Figures 1a and 1b), and as previously described17. Occasionally, nested and pagetoid intraepidermal growth identical to the radial growth phase pattern of human melanoma was also observed. PAS-D staining revealed anastomosing channels within the intradermal tumor nodules (Figure 1c) that were structurally identical to those previously described in VM10. Immunohistochemistry for human CD31 revealed these channels to be either negative or weakly stained, in contrast to human vessels that populated the surrounding human dermis at the perimeter of the melanoma nodules (Figures 1d and 1e). In the regions of PAS-D-positive, CD31-negative channel formation, staining for VE-cadherin, a marker for VM, was present in an identical anastomosing pattern (Figures 1f and 1g).
Figure 1. Melanoma Growth and Channel Formation in Humanized Xenografts.

(a) Intradermal injection of human melanoma cells into xenografted human skin resulted in nodular melanoma growth within the human dermis in a pattern similar to the vertical growth phase of patient melanomas. Arrows indicate the junction between xenograft and mouse skin. (b) High-power view of melanoma tumor nodules growing in the human dermis (D). (c) PAS-D staining revealed anastomosing channels within the intradermal tumor nodules. (d and e) Immunohistochemical examination with CD31 showed negative or weak staining of these anastomosing channels, in contrast to strong staining of human vessels populating the surrounding human dermis at the perimeter of the melanoma nodules. (f and g) Immunofluorescence staining for VE-cadherin, a marker for VM, in the regions of PAS-D-positive, CD31-negative channel formation, showed an identical anastomosing pattern. VE-cadherin stains in red; nuclei in blue.
Nodal protein and mRNA expression in humanized xenografts
Immunohistochemistry for Nodal protein revealed focally diffuse patterns of reactivity localized to melanoma cell cytoplasm and cell membranes (Figures 2a and 2b), as previously described5. In some fields, however, the staining intensity was variable, with accentuation of Nodal reactivity in a spatial distribution suggestive of patterned networks of channel formation (Figures 2a, 2c, and 2d). . In situ hybridization for Nodal mRNA disclosed striking localization to anastomosing networks spatially identical to those forming VM channels (Figures 2e-2i). Focally, the networks formed lumen-like spaces consistent with true channel formation. Foci where Nodal protein appeared to localize to intra-tumoral channels were also detected in patient-derived melanomas (Figures 2j and 2k), although in general, the ability to detect channel formation and Nodal localization was enhanced in the melanoma xenografts over patient-derived tumors. Further support for the association of Nodal protein with channel formation was obtained using a double-labeling immunofluorescence approach whereby Nodal protein, in addition to a more diffuse pattern, was confirmed to co-localize with VE-cadherin-positive channels (Figures 2l-2n).
Figure 2. Nodal Protein and mRNA Expression in Humanized Xenografts and Patient-Derived Melanomas.
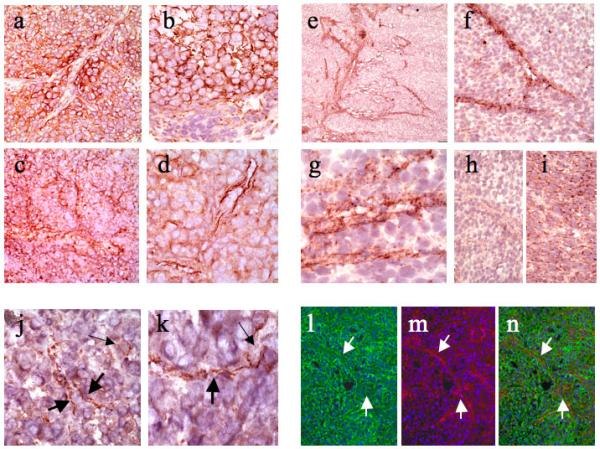
(a and b) Immunohistochemistry for Nodal protein revealed focally diffuse patterns of reactivity localized to melanoma cell cytoplasm and cell membranes. Note in Figure 2a, however, that in the center of the field Nodal stains channel-like structures. (c and d) In some fields, Nodal reactivity is accentuated in a spatial distribution suggestive of patterned networks of channel formation. (e and f) In situ hybridization for Nodal mRNA disclosed striking localization to anastomosing networks spatially identical to those forming VM channels. (g) High-power view of Nodal mRNA expression in a network forming lumen-like spaces consistent with true channel formation. (h) Negative control with Nodal RNA sense probe. (i) Positive control with housekeeping genes. (j and k) Nodal staining of patient-derived melanomas highlighted similar delicate intratumoral channels. (l) Immunofluorescence with Nodal (in green) showed localization to cell membranes and a channel-like pattern. (m) Immunofluorescence with VE-Cadherin (in red) highlighted VM channels. (n) Superimposed images showed colocalization (in yellow) of Nodal and VE-cadherin in the VM channels.
Discussion
In the present study, we have shown that 1) Nodal, the embryonic morphogen, is expressed in melanoma nodules at an mRNA level in association with anastomosing channels with features consistent with the phenomenon of vasculogenic mimicry; and that 2) the humanized immunocompromised mouse model whereby melanoma tumors develop in the context of a relevant dermal microenvironment is an informative system for the study of this association. As such, it is the first to demonstrate Nodal expression in human melanoma cells involved in the dynamic formation of patterned channel networks consistent with vasculogenic mimicry in human skin. A potential association between Nodal expression and VM was previously suggested in the zebrafish model, where Nodal protein was shown to be expressed by injected human melanoma cells and to have biological effects on both embryonic morphogenesis and the molecular pathway implicated in the VM response5. Specifically, it was found that metastatic melanoma cells transplanted into the blastula-stage embryo of the zebrafish caused formation of either a duplicate body axis or an ectopic outgrowth. Nodal produced by the melanoma cells was identified as the agent responsible for initiating these anomalies, and overexpression of its antagonist Lefty-1 or the presence of Nodal anti-sense oligonucleotides prevented their formation. The investigators then used a soft agar bioassay to assess melanoma aggressiveness, showing that inhibition of Nodal decreased invasiveness. Finally, inhibiting Nodal expression also decreased the expression of VE cadherin—previously found to play a critical role in vasculogenic mimicry—and prevented vasculogenic network formation by melanoma cells6. Consistent with these observations, Postovit et al. showed that exposure of metastatic melanoma cells to the extracellular microenvironment of the human embryonic stem cells caused re-expression of Melan-A, reduced expression of VE-cadherin, and decreased tumorigenicity, which was reversed by the addition of recombinant Nodal18. Nodal expression by aggressive melanoma cells participating in VM, as well as its potential role in maintaining melanoma cell pluripotency, suggests that it may promote tumor cell reversion to an embryonic, more plastic phenotype.
One possible mechanism whereby Nodal expression and VM pathways may intersect is through hypoxia-induced upregulation of VEGF, described by Sun19. Their experiments indicate that melanoma cells exposed to a hypoxic environment increase expression of hypoxia-induced factor 1-α (HIF-1α), which activates VEGF and induces the formation of VM networks. It is reasonable that in the early stage of tumorigenesis, as melanomas begin to outgrow their blood supply, hypoxia induces VEGF, which signals to VE-cadherin and induces VM channel formation20. Concurrently, HIF-1α may upregulate Notch, which in turn induces Nodal expression21,22. Once activated, Nodal signals to melanoma cells via ALK 4/5/7-mediated phosphorylation of Smad-2, maintaining the transdifferentiated, pluripotential phenotype of aggressive melanoma cells in an autocrine manner (Figure 3).
Figure 3. Proposed Nodal signaling pathway in vasculogenic mimicry.
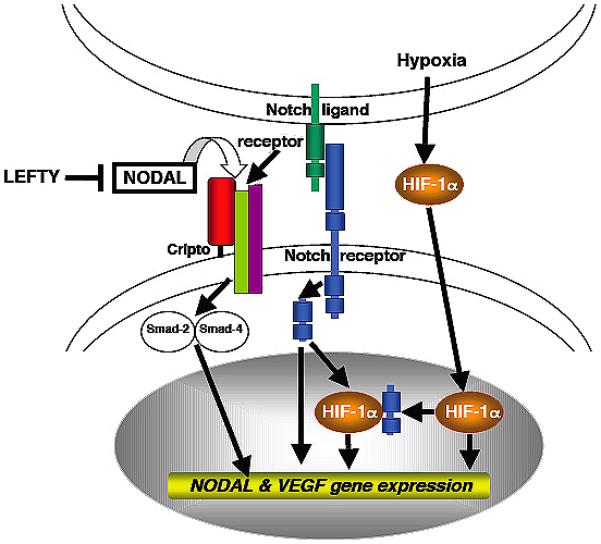
Rapid growth of early melanoma tumor nodules causes them to outgrow their blood supply and creates hypoxia. This stimulates expression of hypoxia-induced factor 1-α (HIF-1α), which activates VEGF. VEGF induces the formation of VM networks. Concurrently, HIF-1α signals to the intracellular domain of Notch. Once activated, the intracellular component of Notch translocates to the nucleus and activates transcription of Nodal. Nodal is then secreted, where it induces Nodal expression by melanoma cells. Melanoma cells signal to one another in an autocrine manner via Nodal, maintaining a stem cell-like state capable of continued endothelial differential and VM formation.
In summary, we have confirmed that Nodal-expressing melanoma cells are spatially associated with formation of channel-like networks with features of the phenomenon of vasculogenic mimicry. This association provides additional validation of the notion advanced by Hendrix and co-workers for a key role for Nodal expression in the induction of channels that correlate with and putatively support tumorigenic growth. The ability of a subset of melanoma cells to transdifferentiate and form a vasculogenic phenotype may be due to Nodal’s role as an embryonic morphogen23. Further studies are now indicated to explore the relationship of Nodal gene and protein expression to melanoma subpopulations implicated in tumor initiation and stem cell behavior. Given the potential nutrient function played by melanoma cells engaging in vasculogenic channel formation, as well as the apparent restriction of Nodal expression to a subpopulation of more virulent tumor cells, Nodal expression may provide a useful target to enhance the anti-tumor effect of antiangiogenic therapy in this deadly disease.
Acknowledgements
This work was supported by the following grant from National Institutes of Health: 5R01CA138231; 5P50CA093683; and 5P30AR042689. We would like to thank Dr. Qian Zhan and Shuang Xu for their superb technical assistance and support.
Abbreviations
- VM
vasculogenic mimicry
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- 1.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 2.Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44:253–265. [PubMed] [Google Scholar]
- 3.Hart AH, Willson TA, Wong M, Parker K, Robb L. Transcriptional regulation of the homeobox gene Mixl1 by TGF-beta and FoxH1. Biochem Biophys Res Commun. 2005;333:1361–1369. doi: 10.1016/j.bbrc.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 5.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA. 2001;98:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi H, Shirakawa K, Kawamoto S, Saga T, Sato N, Hiraga A, et al. Rapid accumulation and internalization of radiolabeled herceptin in an inflammatory breast cancer xenograft with vasculogenic mimicry predicted by the contrast-enhanced dynamic MRI with the macromolecular contrast agent G6-(1B4M-Gd)(256) Cancer Res. 2002;62:860–866. [PubMed] [Google Scholar]
- 8.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Zhang D, Sun B. Vasculogenic mimicry: current status and future prospects. Cancer Lett. 2007;254:157–164. doi: 10.1016/j.canlet.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanoma. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 12.Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 13.Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, et al. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res. 2004;77:475–486. doi: 10.1002/jnr.20151. [DOI] [PubMed] [Google Scholar]
- 14.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strizzi L, Abbott DE, Salomon DS, Hendrix MJ. Potential for cripto-1 in defining stem cell-like characteristics in human malignant melanoma. Cell Cycle. 2008;7:1931–1935. doi: 10.4161/cc.7.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Juhacz I, Albelda SM, Elder DE, Murphy GF, Adachi K, Herlyn D, et al. Growth and invasion of human melanomas in human skin grafted to immunodeficient mice. Am J Pathol. 1993;143:528–537. [PMC free article] [PubMed] [Google Scholar]
- 18.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, et al. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA. 2008;105:4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun B, Zhang D, Zhang S, Zhang W, Guo H, Zhao X. Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett. 2007;249:188–197. doi: 10.1016/j.canlet.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Science. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Raya A, Yasuhiko K, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes and Dev. 2003;17:1213–1218. doi: 10.1101/gad.1084403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postovit LM, Margaryan NV, Seftor EA, Hendrix MJ. Role of nodal signaling and the microenvironment underlying melanoma plasticity. Pigment Cell Melanoma Res. 2008;21:348–357. doi: 10.1111/j.1755-148X.2008.00463.x. [DOI] [PubMed] [Google Scholar]


