Abstract
Objective
In order to examine the mechanisms behind the beneficial effects of motion-based therapies, the hypothesis that physiologic levels of tensile stress have a beneficial effect on annulus fibrosus cells was tested.
Design
To examine the roles of mechanical forces and inflammation in the intervertebral disc, changes in gene expression in response to inflammatory stimulus (IL-1β) and tensile stress (6% stress at 0.05Hz) were examined in fibrochondrocytes isolated from the annulus fibrosus of Sprague-Dawley rats.
Results
Cells exposed to an inflammatory stimulus demonstrated an increase in catabolic gene expression, which decreased approximately 50% after exposure to both inflammatory stimulus and tensile stress. After exposure of cells to tensile stress alone, only matrix metalloprotease-13 showed a 50% decrease in expression. Collagen II showed a modest decrease in expression in response to tensile stress in the inflammatory environment. The expression of collagen I and aggrecan did not show a significant change under any of the conditions tested.
Conclusions
In this in vitro model, our data demonstrate that moderate levels of tensile stress act as a protective signal by decreasing the expression of catabolic mediators under conditions of inflammation. These data suggest that motion based therapies which create tensile stress on the annulus may exert their beneficial effects through anti-inflammatory actions.
Keywords: Anulus Fibrosus, Fibrochondrocytes, Tensile Strain, Inflammation, Metalloproteases
Promising clinical evidence continues to demonstrate the effectiveness of motion-based therapies in the treatment of low back pain1–3. However, this evidence is largely based on outcome studies, providing little insight into the mechanisms behind the beneficial effects. While biomechanical analyses can predict forces generated by movements of the spine, the effects at the cellular level are not known. Bone, tendon, and articular cartilage respond positively to controlled forces with increased cellular proliferation, matrix production, and improved biochemical profiles4–6. This beneficial effect also exists in the cartilage of the intervertebral disc. Dynamic loading of the spine in animal models has demonstrated an anabolic effect of compressive force on production of matrix structural proteins7. Similarly, physiologic levels of hydrostatic pressure stimulate production of proteoglycans and tissue inhibitors of metalloproteases, which slow matrix degradation8. As the annulus cells experiences tensile stress in vivo9, examination of changes in gene expression in response to tensile stress will lead to an understanding of how these forces have the potential to facilitate repair. However, beneficial changes in gene expression in response to tensile stress on the annulus fibrosus have not been reported previously. In vivo models applying tensile stress to the disc have demonstrated histological improvements10, though proteoglycan content has been noted to decrease in response to tension placed on the disc11. As in vivo models of dynamic compression have demonstrated an effect on catabolic and anabolic gene expression in both the anulus and nucleus which is magnitude dependent12,13, examination of gene expression response to beneficial levels of tensile force will provide the information necessary to predict levels of stress expected to be anabolic or catabolic. This improved understanding of the cellular response to tensile stress, if coupled with modeling of disc forces experienced during various activities, could lead to development of novel motion based therapies capable of slowing or reversing damaging effects on the disc.
Mechanical stress does not act on the cell in isolation. In fact, there is increasing evidence that inflammatory mediators are critical in the regulation of structural changes of the intervertebral disc, and the effects of inflammation and mechanical stress may be synergistic14. Cells from both the annulus and the nucleus respond to pro-inflammatory stimuli15, 16. Pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) have been implicated in the pathogenesis and pain of degenerative disc disease17–20. The chief mediators of matrix remodeling, matrix metalloproteases (MMPs), are regulated by these inflammatory cytokines and are increased in degenerative discs21–23. In fact, a polymorphism of the MMP-3 gene has been associated with increased incidence of degenerative disc disease in the elderly, underscoring the importance of the expression of this enzyme in disc degeneration. However, how physiologic mechanical force affects the expression of these early inflammatory mediators has not yet been established. Furthermore, as the inflammatory component is related to pain in intervertebral disc disease, an understanding of how mechanical forces interact with these inflammatory pathways will provide useful insight into the initiation of disc degeneration and low back pain.
The development of appropriate motion based therapies and preventative ergonomics to manage discogenic pain requires an understanding of the biochemical signaling induced by forces. Under axial compression of the disc, the annular fibers experience tensile strain, and the tensile properties are an extremely important determinant of mechanical failure of the disc. It was our goal to elucidate the biochemical response of annulus fibrosus cells to tensile stress. Fiber strains have been shown to be 6% or less in the annulus fibrosus under physiologic loading conditions25. Therefore, we tested the metabolic response of annulus fibrosus cells to 6% tensile strain to mimic physiologic loading conditions. We examined the response of cells in a homeostatic environment as well as an inflammatory environment, mimicking disc disease, to evaluate the responses to mechanical force under both conditions. We monitored expression of marker genes representing early mediators of the IL-1β-induced inflammatory response (TNF-α and inducible nitric oxide synthase (iNOS)), mediators of matrix degradation (MMP-3 and -13), and structural genes (collagen I, II, and aggrecan) in order to examine both anabolic and catabolic effects of mechanical forces. Through this, we tested the hypothesis that mechanical forces act on healthy and inflamed disc cells to generate molecular signals that regulate the early determinants of cell metabolism.
Materials and Methods
Isolation of Intervertebral Disc Fibrochondrocytes and Culture Conditions
The annulus fibrosus of the lumbar spine segment of 10–12 week old female Sprague-Dawley rats was aseptically excised immediately after sacrifice. All protocols were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Tissue was minced and digested in 0.2% trypsin followed by 0.25% collagenase to release fibrochondrocytes from the matrix. The cells were suspended in Ham’s F12 medium (Cellgro, Mediatech), 10% fetal bovine serum (Hyclone, Logan, UT), 1% penicillin/streptomycin (Cellgro, Mediatech), 2mM glutamine (Gibco, Invitrogen) and grown to 95% confluence in 5% CO2, 37°C, pH 7.2. These cells have been shown to maintain their fibrochondrocytic phenotype under these conditions26. Second passage fibrochondrocytes were plated onto 6 well culture plates with a silicone membrane coated with collagen I (Bioflex™, Hillsborough, NC) at a density of 50–60,000 cells per well and grown to 95% confluence. Three wells were dedicated to each condition, and the cell lysates were pooled from these three wells for mRNA isolation as described below. Medium was changed to Ham’s F12, 1% fetal bovine serum, 1% penicillin/streptomycin, 2mM glutamine, 18 hours before each experiment to decrease background. Cells were placed under four conditions: control (C), interleukin stimulated (IL), mechanically stressed (S), and interleukin stimulated with mechanical stress (SIL) as described below, and each condition repeated in triplicate.
Stimulation of Fibrochondrocytes
Cells in the IL and SIL group were stimulated immediately prior to initiating tensile strain by the addition of recombinant human IL-1β (CalBiochem) directly to the culture well to a final concentration of 1 ng/mL.
Application of Cyclic Tensile Force
Cells in the S and SIL groups were subjected to tensile strain using a FlexercellR Strain Unit FX-4000 (Flexcell International Corp., Hillsborough, NC). Using this system, a deformation of the surface of the plate is created via vacuum beneath the plate. The cells adherent to the membrane experienced uniform circumferential strain at 6% at a rate of 0.05 Hz for 4 hours. Minimal (less than 1%) cell detachment occurs in response to this regimen. In addition, tensile strain in this range does not result in change in cell phenotype of annulus fibrosus cells26.
Isolation of mRNA
Reactions were stopped after 4 hours by washing the plates with phosphate buffered saline and adding RLT lysis buffer (Qiagen, Valencia, CA) containing 1% β-mecaptoethanol and detaching the cells from the membrane by mechanical disruption. We selected the 4 h time point because by this time measurable differences in the control and experimental groups become pronounced and provide a reliable measure of cytokine mediated induction of proinflammatory molecules and their inhibition by tensile strain. Prior studies in articular chondrocytes demonstrated that 4 hours was the optimal timepoint for observed induction of inflammatory mediators in response to IL-1β6. The resultant solution was passed through a Qiashredder, and mRNA isolated using a RNA extraction kit (Qiagen, Valencia, CA) as recommended by the manufacturer employing a DNAse I step to remove genomic material.
Real Time Polymerase Chain Reaction
Following heat denaturation, total RNA (1ug) was reverse transcribed in 30 uL total volume containing, 2.5 mM MgCl2, 0.25mM nucleotides, 4 units/uL Rnase inhibitor, 50ug/mL oligo dT (Promega, Madison, WI), 20 units/uL M-MLV, 10mM DTT in 1X 1st strand buffer (Invitrogen, Hercules, CA). The resultant cDNA (4uL) was analyzed in duplicate via real time PCR in iQ™ SYBRR Green supermix (25uL total volume) using a Biorad iCycler IQR thermocycler (Biorad, Valencia, CA) followed by melting curve analysis to assure amplicon specificity. In addition, the specificity of the reaction was confirmed using 2% agarose gel electrophoresis. Primers were designed against rat specific sequences available in GeneBank using OligoPerfect™ Designer (Invitrogen, Valencia, CA) and screened against all available sequences in GeneBank to ensure specificity (see Table 1). A constitutively expressed housekeeping gene, glyceraldehyde phosphate dehydrogenase (GAPDH), was amplified under the same conditions with each sample to correct for any small differences in starting amounts of mRNA. For quantitative gene expression, the comparative Ct method was used as previously described27. After normalization using GAPDH, the relative gene expression was reported as fold expression compared to control, which by definition is set at one.
Table 1.
Primer sequences used for real-time PCR
| Target Gene | Primer sequence (5′–3′) | GeneBank Accession# |
|---|---|---|
| GAPDH | ATGACTCTACCCACGGCAAG GATCTCGCTCCTGGAAGATG |
X02231 |
| iNOS | CCTGTGTTCCACCAGGAGAT CGCTTTCACCAAGACTGTGA |
D44591 |
| TNF-α | TGCCTCAGCCTCTTCTCATT GAGCCCATTTGGGAACTTCT |
X66539 |
| MMP-3 | GGAAGCCAGTGGAAATGAAA ATGCAATGGGTAGGATGAGC |
NM133523 |
| MMP-13 | GCAGCTCCAAAGGCTACAAC GAAATGGCTTTTGCCAGTGT |
XM343345 |
| Col Iα1 | TTCTGAAACCCTCCCCTCTT CCACCCCAGGGATAAAAACT |
Z78279 |
| Col IIα1 | CGAGGTGACAAAGGAGAAGC AGGGCCAGAAGTACCCTGAT |
L48440 |
| Aggrecan | AGACACCCCTACCCTTGCTT AAAGTGTCCAAGGCATCCAC |
NM022190 |
Statistical Analysis
Analysis of variance was performed to evaluate differences in mean values for each condition. This was followed by pairwise comparisons using post-hoc t-test to compare each condition (IL, S, and SIL) with control (C) and SIL with IL. Using a Bonferroni correction, statistical significance was set at p < 0.0125.
Results
Gene Expression in Response to Cyclic Tensile Strain
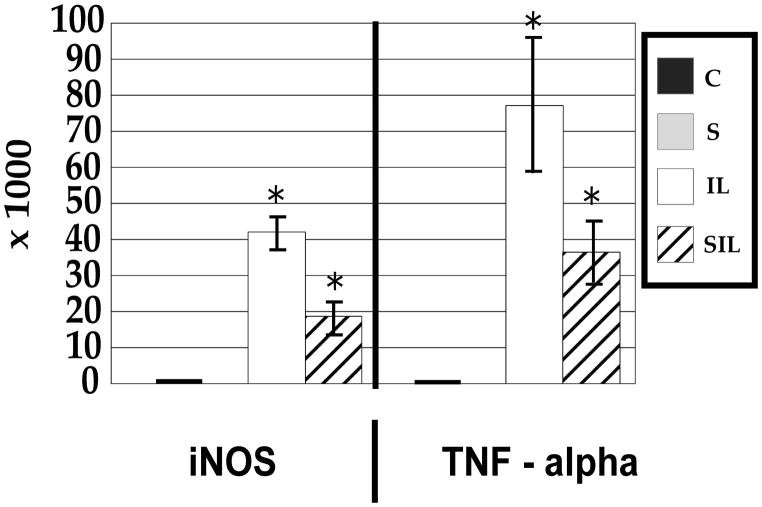
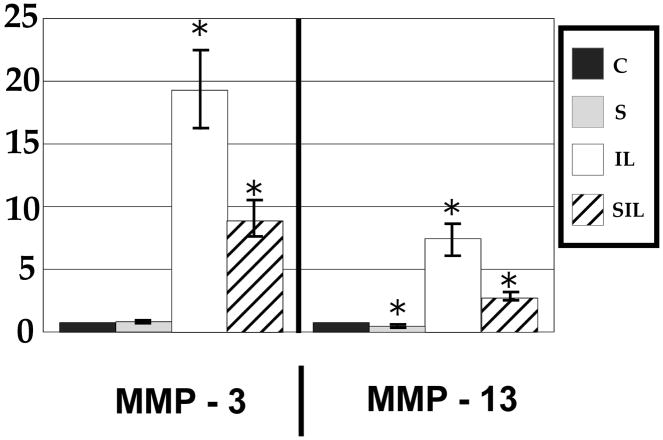
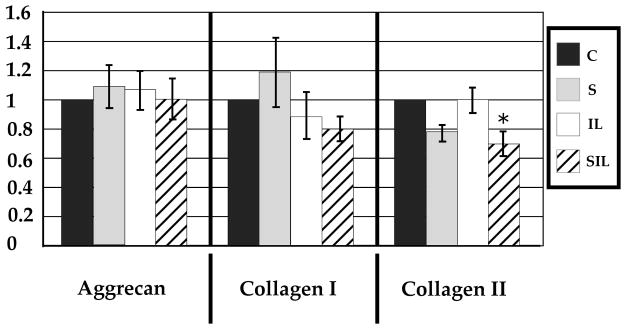
Inducible inflammatory gene expression (iNOS and TNF-α) and MMP-3 did not show a statistically significant change in expression in response to tensile strain (Figures 1,2). Interestingly, MMP-13 did show an approximately 50% decrease in expression after exposure to tensile strain (Figure 2). Expression of structural genes aggrecan and collagen I showed no change, and expression of collagen II showed a trend toward decreased expression, but this did not reach statistical significance (p=0.06) (Figure 3).
Figure 1.
mRNA expression of inflammatory mediators demonstrates an increase in response to interleukin-1 and modulation of this response after exposure to tensile stress. *P<0.01. Data are normalized against GAPDH and reported as fold gene expression. Quantitative relative gene expression profile after exposure for 4 hours to inflammatory stimulus or mechanical stimulus. Values are reported as means (n=3) +/− standard error compared to control. Conditions include control (C), interleukin stimulated (IL), mechanically stressed (S), and interleukin stimulated with mechanical stress (SIL).
Figure 2.
mRNA expression of catabolic matrix metalloproteases demonstrate an increase in response to interleukin-1 and modulation of this response after exposure to tensile stress. Note that MMP-13 demonstrated a protective effect of tensile stress both in the presence and absence of inflammatory stimulus.
Figure 3.
mRNA expression of structural genes demonstrated a relative lack of effect in response to inflammatory or mechanical stimulus, with the exception of decreased expression of collagen II after exposure to both inflammatory stimulus and tensile stress.
Gene Expression in Response to Inflammatory Stimulus
Inducible inflammatory gene expression demonstrated a large increase in expression in response to IL-1β. A greater than 40,000 fold increase in the expression of iNOS was observed, and TNF-α demonstrated a greater than 75,000 fold increase (Figure 1). Similarly, catabolic gene expression demonstrated an increase with MMP-3 increasing by 20 fold and MMP-13 by 7 fold (Figure 2). The relative expression of structural genes collagen I, collagen II, and aggrecan was unchanged in response to inflammatory stimuli (Figure 3).
Modulation of the IL-1β Induced Change in Gene Expression in Response to Cyclic Tensile Strain
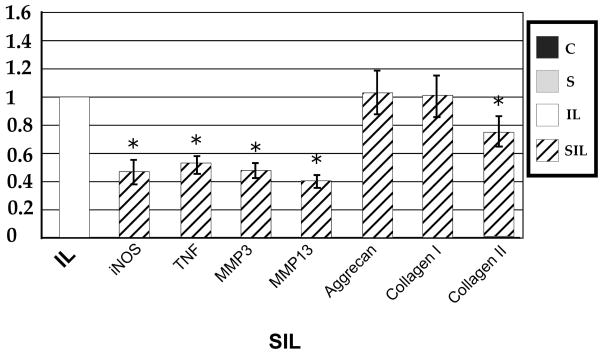
The influence of mechanical strain on the gene expression of annular cells in an inflammatory environment can best be appreciated when the data is analyzed relative to gene expression in interleukin activated cells (Figure 4). Cyclic tensile strain was able to modulate the IL-1β induced up-regulation of iNOS and TNF-α, resulting in a decrease in gene expression by approximately 50% when compared to inflammatory stimulus alone. In examining catabolic gene expression, again tensile strain was able to modulate this response, with an approximate 50% decrease in gene expression of both MMP-3 and -13 compared to inflammatory stimulus alone. While the gene expression of inflammatory and catabolic mediators was still increased over control cells in a homeostatic environment, tensile strain modulated the response of cells in an inflammatory environment.
Figure 4.
Quantitative relative gene expression profile after tensile stress in an inflammatory environment (SIL) compared to cells in an inflammatory environment alone (IL). Note the protective effect of tensile stress on inflammatory and catabolic gene expression, and the decrease in expression of collagen II.
The expression of structural genes collagen I and aggrecan did not show a significant change when compared to control or IL-1β stimulated cells. Collagen II, however, did show a modest, though statistically significant, decrease in expression when compared to control and IL-1β stimulated cells (P<0.01).
Discussion
The clinical effectiveness of activity and lack of efficacy of bed rest in patients with low back pain support a beneficial effect of motion on the spine28. Further support of this exists in the observation that the most physically active individuals have the strongest discs, as measured by mechanical property testing in cadaveric specimens29. These clinical observations are supported by our model system, in which we observed a protective effect of mechanical force. The positive effect of motion has previously been attributed to increased diffusion, resulting in enhanced nutrition and waste exchange30. However, short term exercise does not appear to affect transport into the disc31. Therefore, with evidence that mechanical forces affect cell metabolism, an effect on the signaling pathways of remodeling and prevention of matrix degeneration of the disc must be considered. In fact, our data demonstrate that low levels of tensile stress actually act as a potent anti-inflammatory and anti-catabolic signal by decreasing the expression of pro-inflammatory mediators and degenerative proteases. This suggests that during conditions of acute inflammation that accompany painful flares, mechanical forces of physiologic magnitude may exert their beneficial effect through decreasing early inflammatory mediators and catabolic proteases. However, additional mechanisms are likely involved in the response of the intervertebral disc to such mechanical strain32, and include not only the gene expression changes noted here, but also changes in the regulation of protein translation and post-translational modifications, which were beyond the scope of this study. Importantly, enzyme activation and post-translational modifications such as glycation and collagen crosslinking33,34 have implications in the mechanical properties of the tissue and may be altered in response to mechanical stress. In addition, our data examine the gene expression result at only one timepoint, and do not take into account the kinetics of the mRNA expression changes, which could affect the resultant protein expression levels. We selected a 4 hour time point because in similar cell types, measurable differences in the control and experimental groups become pronounced and provide a reliable measure of cytokine mediated induction of proinflammatory molecules and their inhibition by tensile strain6,35 and our goal was to examine the earliest changes associated with the cellular response to inflammatory and mechanical stimuli. Understanding how the magnitudes of stress used in this in vitro study compare to those experienced by the cells in vivo through different physiologic motions will be necessary to facilitate clinical translation of these effects.
Interestingly, our results show no evidence of catabolic effects in response to this level of tensile stress. This differs from previous studies on mechanical forces in the annulus fibrosus. A previous study of rabbit anulus fibrosus fibrochondrocytes exposed to cyclic tensile stress demonstrated a decrease in proteoglycan production and increase in nitric oxide production at 5% elongation36, suggesting a catabolic effect of this magnitude of strain. However, the frequency used in this study was 1 Hz, which is 20 fold higher than that used in our study. Similarly, vibratory loading of cells from the annulus fibrosus results in catabolic activity and disruption of the disc matrix in the annulus fibrosus37. It can be expected that such a high frequency force may be traumatic, whereas lower frequency force, as used in our study, protective. Similarly, a magnitude-dependence appears to exist as well. Tensile stress at 20% results in increased expression of cyclooxygenase-2 and phospholipase A238. This magnitude dependence also occurs in compressive studies of the nucleus pulposus, where traumatic levels of force are associated with deleterious matrix changes39 and lower magnitudes of load result in an anabolic response40,41. This experimental data has a clinical correlate in that the most serious spine pathology relates to the highest and lowest degrees of physical activity, with moderate physical work loading of the spine providing a relative protective effect42. It is becoming increasingly clear that mechanical forces applied to the intervertebral disc can produce both tissue trauma and beneficial adaptive changes43. This point warrants further study.
Tensile stress alone demonstrated minimal effect on inducible inflammatory mediators. This is somewhat intuitive, as in a non-inflammatory environment, these mediators are not present in an appreciable amount. Therefore, an anti-inflammatory therapeutic intervention would not be expected to have an effect. In examining markers of matrix homeostasis, MMP-3 gene expression was not affected by tensile strain. However, the decreased expression of MMP-13 in response to tensile stress suggests that it may be exquisitely sensitive to mechanical force. Thus, MMP-13 may be one of the molecules responsible for the protective effect of mechanical force through decreasing the baseline catabolic response on the matrix. Certainly, MMP-13 has been shown previously to be involved with disc degeneration23 but our findings are unique because they suggest that MMP-13 has greater sensitivity to mechanical forces, even under conditions of homeostasis. In addition, the lack of an observed effect of mechanical force on structural gene regulation (collagen and aggrecan) suggests that physiologic levels of stress do not directly modulate structural gene expression, but may instead act through altering expression of metalloproteases responsible for matrix turnover. This is consistent with prior studies which demonstrated no effect on aggrecan or biglycan mRNA expression after exposure to tensile stress for up to 24 hours36.
The robust increase in expression of inducible inflammatory and catabolic genes in response to IL-1β was expected as the annulus fibrosus is known to be responsive to the effects of IL-1β16. In addition, this result is consistent with previous work demonstrating increases in these catabolic enzymes under conditions of injury44. Interestingly, our study demonstrated no effect on the expression of structural genes in response to IL-1β after 4 hours. This suggests that under conditions of inflammation, the earliest mediators of damage include cytokines and metalloproteases, as opposed to direct regulation of structural gene expression. While it is possible that translational control of matrix proteins is also affected, these data demonstrate that the earliest upstream signals affected by mechanical stress include the catabolic mediators. This is valuable information in considering therapeutic targets to reverse the destructive effects of the inflammatory response. Certainly, after prolonged exposure to either mechanical strain or inflammatory stimulus, it is likely that structural gene expression may be altered. However, our data suggest that this likely involves signaling through upstream pathways.
Our data demonstrate a decrease in collagen II expression after exposure to tensile strain in an inflammatory environment. Interestingly, in the absence of tensile strain, as is seen in experimental denucleation, collagen II is increased in the anulus45. Because the proportion of collagen I/collagen II is important for the material properties of the annulus and is altered in disc degeneration, maintanence of a collagen I predominant matrix may be required for optimal functioning. It is not clear why collagen II may be more sensitive to mechanical strain than collagen I or aggrecan, and this warrants further investigation. As collagen II is a more significant contributor in human annulus fibrosus compared to quadruped annulus fibrosus46, this may be even more relevant in humans.
Conclusions regarding our results are limited to an in vitro environment, and responses may differ in cells within their native matrix. However, this model provides us with a powerful experimental platform to test the effects of various magnitudes and frequencies of tensile strain on cell metabolism. The use of a cell culture system has the advantage of allowing for careful control of the mechanical stimuli as well as manipulation of the local environment, such as addition of inflammatory stimulus. Our data are the first to demonstrate an anti-inflammatory and protective effect of tensile strain on the annular cells of the intervertebral disc, and contributes to the understanding of the beneficial effects of motion on the spine. Future studies which compare measurements of intervertebral disc strains experienced during different movements with changes in cell metabolism will provide the information necessary to begin to consider rational design of therapeutice exercise. The goal of such work would be to assist the clinician in predicting movements that could be expected to be reparative versus traumatic based on appropriate models. Such an approach would have the potential to lead to extremely cost effective and broadly applicable approaches to tissue healing in intervertebral disc disease. These data represent intial building blocks to lead to an improved understanding of how mechanical forces can be exploited to initiate repair.
Acknowledgments
The authors wish to thank Drs. Lars Gilbertson, PhD, Rebecca Studer, PhD, and James Kang, MD for critical review of the data, Dr. Haikady N. Nagaraja, PhD for assistance with statistical analysis, and Ms. Mary Synnott for assistance with manuscript preparation.
Footnotes
Disclosures: This work was supported by NIH Grant #2K12HD01097-06, Medical Rehabilitation Scientist Training Program, Association of Academic Physiatrists. Data previously presented at the Association of Academic Physiatrists Annual Meeting, Daytona, FL, 2006 for the Electrode Store Best Paper Presentation.
References
- 1.Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine. 2004;29(23):2593–602. doi: 10.1097/01.brs.0000146464.23007.2a. [DOI] [PubMed] [Google Scholar]
- 2.Rainville J, Hartigan C, Martinez E, Limke J, Jouve C, Finno M. Exercise as a treament for chronic low back pain. Spine J. 2004;4(1):106–15. doi: 10.1016/s1529-9430(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 3.van Deursen DL, Lengsfeld M, Snijders CJ, Evers JJ, Goossens RH. Mechanical effects of continuous passive motion on the lumbar spine in seating. J Biomech. 2000;33(6):695–9. doi: 10.1016/s0021-9290(99)00231-6. [DOI] [PubMed] [Google Scholar]
- 4.Hirukawa K, Miyazawa K, Maeda H, Kameyama Y, Goto S, Togari A. Effect of tensile force on the expression of IGF-I and IGF-I receptor in the organ-cultured rat cranial suture. Arch Oral Biol. 2005;50(3):367–72. doi: 10.1016/j.archoralbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum free conditions. J Biomech. 2004;37(10):1543–50. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagoist of IL-1beta actions in chondrocytes. J Immunol. 2000;165:453–60. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37(3):329–37. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 8.Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22(10):1085–91. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Panjabi M, White A. Clinical Biomechanics of the Spine. 2. Philadelphia: JB Lippincott Company; 1990. [Google Scholar]
- 10.Kroeber M, Unglaub F, Guehring T, Nerlich A, Hadi T, Lotz J, Larstens C. Effects of controlled dynamic disc distraction on degenerated intervertebral discs: an in vivo study on the rabbit lumbar spine model. Spine. 2005;30(2):181–7. doi: 10.1097/01.brs.0000150487.17562.b1. [DOI] [PubMed] [Google Scholar]
- 11.Hutton W, Yoon ST, Elmer W, Li J, Murakami H, Minamide A, Akamaru T. Effect of tail suspen sion (or simulated weightlessness ) on the lumbar intervertebral disc: study of proteoglycans and collagen. Spine. 2002;27(12):1286–90. doi: 10.1097/00007632-200206150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Maclean JJ, Lee CR, Grad S, Ito K, ALini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28(10):973–981. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 13.MacLean JJ, Lee CR, Alini M, Iatridis JC. Anabilic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Lotz J, Ulrich JA. Innervation, inflammation and hypermobility may characterize pathologic disc degneration: review of animal model data. J Bone Joint Surg Am. 2006;88(2):76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- 15.Burke JG, Watson RWG, Conhyea D, et al. Human nucleus pulposus can respond to a pro-inflammatory stimulus. Spine. 2003;28(24):2685–93. doi: 10.1097/01.BRS.0000103341.45133.F3. [DOI] [PubMed] [Google Scholar]
- 16.Rannou F, Corvol MT, Hudry C, et al. Sensitivity of anulus fibrosus cells to interleukin 1 beta. Comparison with articular chondrocytes. Spine. 2000;25(1):17–23. doi: 10.1097/00007632-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DG, Izzo MW, Hall DJ, et al. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27(12):1291–6. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 19.Kang JD, Stefanovic-Racic M, McIntyre L, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–73. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T, Kikuchi S, Shubayev V, Myers MM. Volvo award winner in basic science studies: Exongenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–80. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Nemoto O, Yamagishi M, Yamada H, Kikuchi T, Takaishi H. Matrix metalloproteinase-3 production by human degenerated intervertebral disc. J Spinal Disord. 1997;10(6):493–8. [PubMed] [Google Scholar]
- 22.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11(4):308–20. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H, Shinomiya K. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83(4):491–5. doi: 10.1302/0301-620x.83b4.11617. [DOI] [PubMed] [Google Scholar]
- 25.Stokes IA. Surface strain on human intervertebral discs. J Orthop Res. 1987;5(3):348–55. doi: 10.1002/jor.1100050306. [DOI] [PubMed] [Google Scholar]
- 26.Rannou F, Poiraudeau S, Foltz V, Bioteux M, Corvol M, Revel M. Monolayer annulus fibrosus cell cultures in a mechanically active environment: Local culture condition adaptations and cell phenotype study. J Lab Clin Med. 2000;136:412–21. doi: 10.1067/mlc.2000.109755. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Hagen K, Hilde G, Jamtvedt G, Winnem M. Bed rest for actue low back pain and sciatica. Cochrane Database Syst Rev. 2004;4:CD001254. doi: 10.1002/14651858.CD001254.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Porter RW, Adams MA, Hutton WC. Physical activity and the strength of the lumbar spine. Spine. 1989;14(2):201–3. doi: 10.1097/00007632-198902000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Holm S, Nachemson A. Variations in the nutrition of the canine intervertebral disc induced by motion. Spine. 1983;8(8):866–74. doi: 10.1097/00007632-198311000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Urban J, Holm S, Maroudas A. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop. 1982:296–302. [PubMed] [Google Scholar]
- 32.Iatridis JC, Maclean JJ, Roughley PJ, Alini M. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88(2):41–46. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experiemntal measurements and theoretical predictions. J Biomech. 2005;39(6):1021–9. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Pokharna H, Phillips F. Collagen crosslinks in human lumbar intervertebral disc aging. Spine. 1998;23(15):1645–8. doi: 10.1097/00007632-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Ferretti M, Madhavan S, Deschner J, Rath-Deschner B, Wypasek E, Agarwal S. Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol. 2006;290(6):C1610–5. doi: 10.1152/ajpcell.00529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rannou F, Richette P, Benallaoua M, et al. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc annulus fibrosus cells through production of nitrite oxide. J Cell Biochem. 2003;90:148–57. doi: 10.1002/jcb.10608. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki S, Banes A, Weinhold P, Tsuzaki M, Kawakami M, Minchew J. Vibratory loading decreases extracellular matrix and matrix metalloproteinase gene expression in rabbit annulus cells. Spine J. 2002;2:415–20. doi: 10.1016/s1529-9430(02)00427-8. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto H, Doita M, Nishida K, Yamamoto T, Sumi M, Kurosaka M. Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine. 2006;31(1):4–9. doi: 10.1097/01.brs.0000192682.87267.2a. [DOI] [PubMed] [Google Scholar]
- 39.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23(23):2493–506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 40.Ohshima H, JPU, Bergel D. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. J Orthop Res. 1995;13:22–9. doi: 10.1002/jor.1100130106. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Yan W, Setton L. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573–83. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Videman T, Nurminen M, Troup JD. 1990 Volvo Award in clinical sciences. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation, and physical loading. Spine. 1990;15(8):728–40. [PubMed] [Google Scholar]
- 43.Stokes IA, Iatridis J. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29(23):2724–2732. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts S, Caterson B, Menage J, Evans E, Jaffray D, Eisenstein S. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25(23):3005–13. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 45.Takaishi H, Nemoto O, Shiota M, et al. Type-II collagen gene expression is transiently upregulated in experimentally induced degeneration of rabbit intervertebral disc. J Orthop Res. 1997;15(4):528–38. doi: 10.1002/jor.1100150408. [DOI] [PubMed] [Google Scholar]
- 46.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochimica et Biophysica Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]