Abstract
The glial fibrillary acidic protein immunoreactive astroglial layout of the cerebral cortex from Albert Einstein and other four age-matched human cases lacking any known neurological disease was analyzed using quantification of geometrical features mathematically defined. Several parameters (parallelism, relative depth, tortuosity) describing the primate-specific interlaminar glial processes did not show individually distinctive characteristics in any of the samples analyzed. However, A. Einstein's astrocytic processes showed larger sizes and higher numbers of interlaminar terminal masses, reaching sizes of 15 μm in diameter. These bulbous endings are of unknown significance and they have been described occurring in Alzheimer's disease. These observations are placed in the context of the general discussion regarding the proposal – by other authors – that structural, postmortem characteristics of the aged brain of Albert Einstein may serve as markers of his cognitive performance, a proposal to which the authors of this paper do not subscribe, and argue against.
Keywords: Interlaminar astroglia, Astroglial layout, Primate cerebral cortex, Cerebral cortex organization, Human brain
1. Introduction
In spite of the demands placed by the scientific method on the generation of objective knowledge, opinions and theories in science are not immune to cultural impacts, trends, and preconceptions (see for example Gould, 1996). Such is the case with the longstanding pursuit of biological markers of human intellectual abilities, occasionally enhanced by the opportunity to analyze the brain configuration of men with outstanding talents (Amunts et al., 2004; Donaldson and Canavan, 1928). In this regard, analyses of V. Lenin's (Bentivoglio, 1998; Kreutzberg et al., 1992) and A. Einstein's (AE) (Anderson and Harvey, 1996; Diamond et al., 1985; Witelson et al., 1999) brains are two notable examples. For instance, in the first case, the size of layer III pyramidal neurons in various cortical areas was considered by O. Vogt as evidence of Lenin being a “brain athlete and association giant” (cf., Kreutzberg et al., 1992). Besides this “enthusiastic” statement, the pathological condition of Lenin's brain following his cerebrovascular episodes should raise concern regarding any possible functional interpretation of his earlier mental abilities. In the second case, glial:neuronal ratios (Diamond et al., 1985), reduced thickness of the frontal cortex (Anderson and Harvey, 1996), and an unusual variation of specific gyral and sulcal patterns (Witelson et al., 1999) in AE's brain have been argued to represent biological traits related to his special cognitive capabilities. In general, hypotheses suggesting an association between structure and function – when properly constructed – are of great value in sparking discussions on possible correlations or causal relationships, among elements placed within both levels of analysis. In fact, the history of neuroscience shows numerous examples of the fertility of such an approach (e.g., Dierig, 1994; Mountcastle, 1997). Yet, in order to become part of a sound theory of brain function – or even to draw a provisional, reasonable concept from such observations – those correlations are necessarily in need of additional, experimental proof. Since this requirement cannot be met under the conditions imposed solely by postmortem findings on a single human brain, such hypotheses may only have the heuristic value of any observation of which its functional significance1 cannot be accurately determined.
With these limitations in mind, the above considerations raised the interest to revisit AE's brain and to analyze some specific aspects of the cerebral cortex astroglia, as pivotal for this commentary. In this regard, it seems that if during his life AE was an emblematic individual, the physical remnants of his brain still contribute to the discussion of some fundamental matters in neuroscience, which is the purpose of the present note.
2. Einstein's cerebral cortex analysis
The analysis of cortical astroglia from Albert Einstein's brain was included in order to ascertain if individual and case group characteristics of glial interlaminar processes would be affected by the inclusion of cortical samples from a well-recognized scholar, among a group of four age-matched cases (H58: 67, H59: 69, H60: 77, and H61: 70 year) considered to lack any known neurological or psychiatric disease.
Interlaminar astroglial processes are only found in the cerebral cortex of species within the Primate Order (Colombo, 2001; Colombo et al., 2000; Colombo and Reisin, 2004). In other words, they constitute a marker of primate – mostly anthropoid monkeys and human – brains. Their cell somata appear to be located within layer I of the cerebral cortex, abutting to the glia limitans. These (up to 1.0 mm long) processes would contribute to the spatial management of cortical neuropil, perhaps optimizing the modular (columnar) organization of the cerebral cortex (Colombo, 2001; Reisin and Colombo, 2002a), in contrast to a conditional “mass” action of the so-called panglial syncytium (Mugnaini, 1986).
Sections at a thickness of 40 μm were obtained from celloidin-embedded blocks of cerebral cortex. After removing the celloidin (Miguel-Hidalgo and Rajkowska, 1999)2, and exposure to antigen rescue procedures (see supplementary material for more details), the sections were processed to detect immunoreactive glial fibrillary acidic protein (GFAP, an intermediate filament component of astroglial cytoskeleton) (mouse monoclonal antibody, Clone GA-5, dilution 1:5000; from Chemicon Int., Inc., Temecula, CA, USA). Control procedures used (preincubation blockade of the peptide and omission of first antibody) resulted in lack of immunolabeling (Colombo et al., 2000).
Two different cytoarchitectures were found: a mesh-like astroglial band located in the uppermost superficial laminae, and the “primate-specific” (Colombo et al., 2000; Colombo and Reisin, 2004) parallel arrangement of the interlaminar glial “palisade” penetrating deep into lamina III/IV (Figs. 1A–C). Scattered regions of astrogliosis, mostly located in laminae I–II, were found (Fig. 1 B). When signs of astrogliotic condition were absent, the collection of interlaminar processes expressed periodic changes in density, sometimes resembling the form of a “tufted” layout (Figs. 1A–C), approximately 50–80 μm apart from each other. In addition, a “fasciculated” appearance of these processes was also found. Fascicles could be formed by incoming processes from various cell somata, as suggested by the “Y”-shaped geometry occasionally seen (see Fig. 1A). The linear adjustment shown in Fig. 1D indicates almost the same relationship (for controls and AE), between the size of the superficial glial band, and the penetration of interlaminar processes. Adjacent sections were processed either for hematoxylin or for Nissl stains. The thickness of lamina I (LI) was also measured by an operator blind to the sample being analyzed, using a calibrated reticle eyepiece. In general, the superficial glial “mesh” (SGM) surpassed the deepest limits of lamina I (100–250 μm), although all samples (except by two out of four regions of AE) fall over a linear correspondence between the thicknesses of LI and SGM (Fig. 1E). Conversely, palisade's depth was uncorrelated to the thickness of lamina I in all cases (including AE) (Fig. 1F). It must be noted that mean values of AE palisade's depth, SGM, and LI thicknesses were similar to those of controls. Also, some type of “mosaic-like” alterations of the interlaminar “palisade” were observed in most samples, consisting in astrogliotic foci, disruption of “palisade” density or lack of it. This “patchy” appearance morphologically resembled the early “mosaic” alterations found in intermediate or early Alzheimer's disease (Colombo et al., 2002), and in infantile brains from Down's syndrome cases (Colombo et al., 2005), suggestive of age-linked degenerative processes. The presence of massive, enlarged “terminal masses”3 of interlaminar processes in AE's cortical samples (mostly occipital region) (Figs. 2A–C) would suggest that in fact, glial degenerative changes might have been in progress, since in our experience, they have not been typically observed in young adult individuals.
Fig. 1.
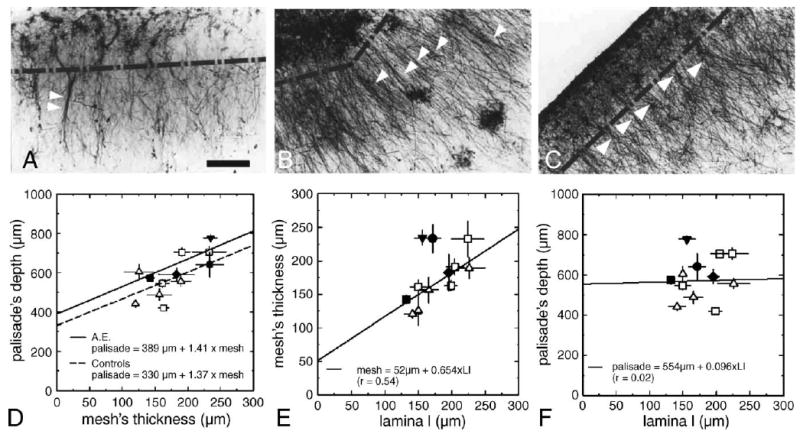
Astroglial architectures in the brain of AE and control cases: presence of the “interlaminar palisade” and of stellate (intralaminar) astrocytes (mostly in lamina I). (A–C) (A) Case AE, occipital cortex, block #185; (B) case H59, frontal cortex, area 8/46; (C) case H60, occipital cortex, area 17/18. Note periodic aggregates of interlaminar processes (single arrowheads) (B, C), and occasional fascicles (double arrowheads) within it (A). Broken line indicates extent of lamina I. Bar (A–C): 100 μm. (D) Linear regression performed on AE (continuous line) and control cases (dashed line) shows a common trend of data points in all samples. Also, the superficial glial net and the thickness of lamina I showed a good correspondence (E). On the contrary, no relation was found between the length of interlaminar processes and the thickness of lamina I (F). Analyzed regions: prefrontal cortex (Brodmann's) area 8/46 (triangle facing up); occipital cortex, area 17/18 and block #185 (AE) (square); frontal cortex, block #211 (AE) (diamond); inferior parietal cortex, block #106 (AE) (circle); parietal somatosensory cortex, block #49 (AE) (triangle facing down).
Fig. 2.
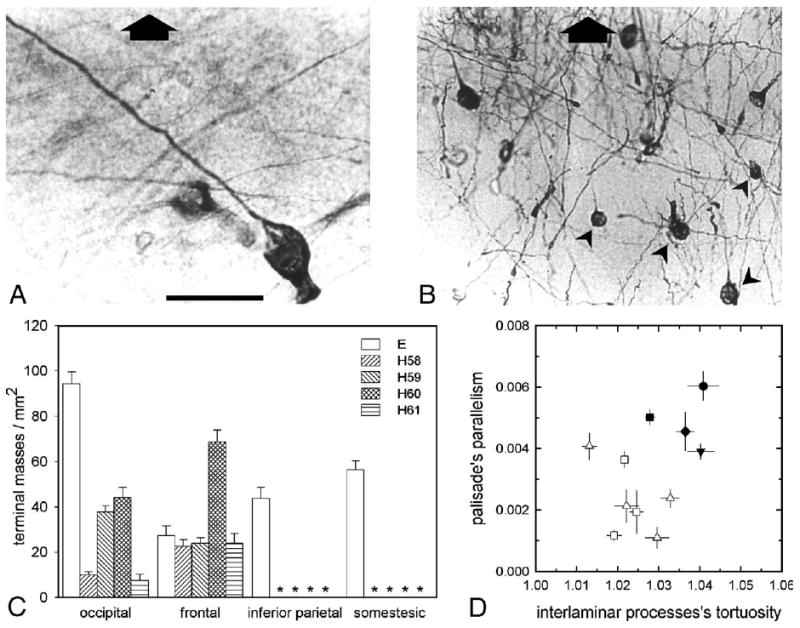
Morphological characteristics of AE's interlaminar processes. Selected samples (A, B) illustrate variations in size, and GFAP-IR density of terminal masses (arrowheads) (AE block #185). Large arrows are directed toward cortical surface. Bar: 20 μm (A); 50 μm (B). (C) Counting of GFAP-IR “terminal masses” (mean ± SE) from interlaminar processes. Differences in the number of terminal masses between cortical regions were found, suggesting inter-areal differences among individuals. Asterisks indicate lack of data. (D) Values represent the tortuosity (mean ± SE) of individual interlaminar silhouettes (values approaching to 1 denote a marked linearity (Reisin and Colombo, 2002b). The parallel arrangement of these processes was also measured (mean ± SE). Symbols are identical to those used in Fig. 1. Please note that authors apologize for the final quality of the images, which was not uniform due to residual densities following the procedures used, i.e., elimination of the embedding celloidin followed by exposure to aluminum chloride and microwave oven (Colombo et al., 2000, 2002).
With respect to individual processes, in general, their “stalk” portion showed a distinct lack of geometrical uniformity among cases (Figs. 2D, 3). Individual processes from cortical lamina III were manually traced with a cursor and digitally saved. The tortuosity (κ) of each process was calculated as the quotient of its length divided by the straight distance between its edges, thus providing a measure of how much twisted the cytoskeleton is (values approaching to κ = 1 indicate a marked linearity) (Reisin and Colombo, 2002b). All measurements revealed a general rectilinear profile (tortuosity < 1.1 (Reisin and Colombo, 2002b)). Collectively, these processes were predominantly aligned normal to the pial surface, tending to form a palisade or brush (Figs. 1A–C). Depending on the skew and tortuosity of individual processes, deviations from this radial disposition were found, resulting into less ordered palisades (Fig. 3). The parallelism4 of this palisade was calculated using a well described and tested procedure (Colombo et al., 2005; Reisin and Colombo, 2004) (see Supplementary material), indicating that that AE's cerebral cortex tended to show, in average, a more parallel palisade than the rest of the samples analyzed, although some variability was present (compare Figs. 3A and D).
Fig. 3.
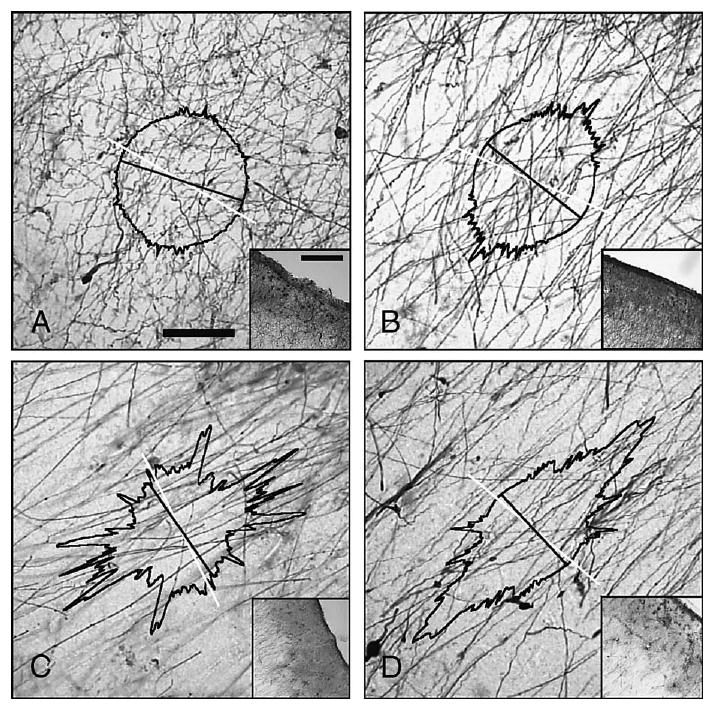
Parallelism of AE's GFAP-immunoreactive interlaminar processes. Superimposed to the analyzed sample, a polar plot represents the amount of processes running into specific directions (the magnitude is given by the distance to the center of the plot; circular grid omitted for clarity). In general, the major peak of this plot was coincident with the main direction of the palisade. This peak was also found minimally deviated from the radial direction (less than 15° for all samples), as depicted by the angle defined between the direction of the cortical surface (white straight line) and the black line (located at 90° with respect to the peak). We defined parallelism (H) for each sample as the height of this peak. Selected samples are arranged according to increasing values of parallelism. (A) Case AE, parietal somatosensory cortex, block #49 (H = 1.28 × 10−3); (B) case H59, prefrontal cortex (Brodmann's) area 8/46 (H = 3.70 × 10−3); (C) same case, occipital cortex, area 17/18 (H = 8.15 × 10−3); (D) case AE, occipital cortex, block #185 (H = 9.89 × 10−3). Bar in panel A (also B–D): 50 μm; 200 μm for insets.
Overall, it was found that the inclusion of AE cortical samples within the group of aged brains expanded the morphological diversity of the interlaminar processes of the studied group. It should not go unnoticed that the reported measurements (i.e., tortuosity of interlaminar processes, enlarged terminal masses) could be taken as indirect estimates of astroglial membrane exposure (Reisin and Colombo, 2002b) and, hence, suggest a potential increase in the local numbers of glial channels and receptors. This, in turn, may represent – in healthy conditions – a functional upgrading of the cortical neuropil. Yet, suggestive as they might be, these observations may be only a random finding within the sampling conditions of a particular aging population, with variable degrees of maladaptive – or neurodegenerative – cellular changes, as observed for example in Alzheimer's cases (Colombo et al., 2002). At any rate, its order represents a variant interpretation with respect to the “glial case” made by previous authors. In this regard, variations in terms of relative numbers of neurons and glia in Brodmann's area 39 (considered to include circuital elements for mathematical processing) of the cerebral cortex of AE, were proposed as a biological ‘trait’ of his special mental performance (Diamond et al., 1985). That report has already received some methodological criticisms (Hines, 1998), which should also include its limitations in terms of providing a distinct characterization of glial cell types, since it was performed with routine nuclear staining procedures. Furthermore, the basis for such a proposal would imply that classical stellate astroglia represent a rather uniform – structurally and chemically – cell type, associated among their constituents in a sort of panglial syncytium (according to current concepts on cerebral cortex astroglial architecture), or rather, syncytial-like (Mugnaini, 1986). Yet, such astroglial cell uniformity is no longer a tenable assumption, as it has been demonstrated during recent years to express multiple cell types and brain regional differences, as they are able to generate and receive chemical signals, thus participating in neurono:glial intercellular signaling and brain circuit organization and function (Araque et al., 1999; Hertz, 1965; Le Roux and Reh, 1994; Monard et al., 1973; Nedergaard, 1994; Pfrieger, 2002; Retzius, 1894; Tsacopoulos and Magistretti, 1996; Ullian et al., 2001). These dynamic functions go beyond a role restricted to management of the extracellular space composition and that one based on a ‘mass’ effect. Hence, a general, relative astroglial cell number – which may be affected by age and repair – cannot be considered a parameter to account for the efficiency of astroglial management of cortical neuropil in the primate brain (see, e.g., Senitz et al., 1995). Moreover, there is no available evidence that a relative increase in the number of glial cells – argued to represent a response to enhanced neuronal activity in Diamond et al. (1985) – is positively correlated with a more efficient information processing. Besides, as pointed out by Hines (1998), at the moment of his death, Albert Einstein was 76 years old, while the control group selected by Diamond et al. (1985) ranged between 47 and 80 years old. This rather inclusive age group introduced a potential source of variability in the comparative numbers of astroglial cells. In our own preparations, a moderate increase of immunolabeled astroglial cells in superficial layers was found in AE's brain, not different from what can generally be observed in age-matched controls. Even considering that cell nuclear counts (used in Diamond et al., 1985) and immunoreactivity are two different indicators of cell populations, results caution on the relative increment of glial cells in area 39 found by Diamond et al. (1985). In fact, such increase in cell number may not represent a footprint of special intellectual aptitudes but, rather, an acquisition that took place during aging, associated to individual variations in the continuous cell death and tissue repair process, or a modification subsequent to a brain lesion, as mentioned by Kantha (1992).
Other parameters of AE's brain anatomy and cytoarchitecture received special attention too. In a study by Anderson and Harvey (1996), reduced thickness of AE's frontal cortex was considered to provide shorter processes which would decrease interneuronal conduction times, thus enhancing operative efficiency of the cerebral cortex. A main flaw of this argument is that it fails to provide an unambiguous linkage between structure and function, as demonstrated by the fact that an increased neuronal density was also found in the prefrontal cortex of schizophrenic patients (Selemon et al., 1998). In the latter case, the reduced interneuronal distances were related not to an enhanced mental skill as in Anderson and Harvey (1996), but to the psychiatric condition of the patients (Selemon and Goldman-Rakic, 1999), suggesting the need for a more critical judgment of such type of observations (see discussion in Colombo, 2000).
At any rate, it seems reasonable to critically assess which pathophysiological events might have produced the reported reduction of cortical volume. Neuronal density could have been increased because of a reduction in the number or complexity of connective elements and not only due to the length of their processes. In all probability, reduction of cortical thickness would not have involved a reduction of the extracellular space (because of the hindrance, it could imply to the ionic/molecular mobility in this compartment), nor a decrease of glial processes, which in fact would tend to expand with advanced age (astrogliosis). Thus, it is reasonable to ask if the particular cortical thickness, neuronal density and neurono:glial ratios reported for the cortex of AE at the time of death would correspond to those of a younger A. Einstein. This specific question cannot be answered, except by making inferences based in the general population, an assumption that would be contradictory with the pretended uniqueness of AE's brain. According to recent reports based on MRI techniques, cortical gray – and white – matter volume positively correlates with “general intelligence” (g) (Posthuma et al., 2002). These results would also contradict the hypothetical significance attributed to the reduced cortical thickness found in AE's brain.
3. Concluding remarks
Some proposals on this subject of special minds (and brains) remind the old localizationist theory of cortical brain function (Galaburda, 1999). Considering the current paradigms of modular organization of the cerebral cortex and distributed processing of complex cognitive functions (McCleland et al., 1989), it appears that attempts to link complex mental performance – not just elementary units of cognitive processing – to local structural characteristics of a singular cortical area would be ill supported. Evidence from postmortem findings could provide more meaningful data to possible structure:function relationships if performed as a follow-up to functional magnetic resonance studies. Occasionally, in those individuals with a more specific skill, perhaps such as language proficiency (Amunts et al., 2004), a more productive correlation could be found using morphometrical analysis of specific brain regional organization.
The abovementioned incongruities between the supposedly special structural ‘attributes’ of AE's brain and current interpretations of their meaning raise doubts as to the exact contribution of these types of analyses, besides spurring a provocative discussion in scientific and laymen literature. In a species with a heavily socially molded brain and mind, such as human, the full expression of an individual special aptitude depends on multiple genetic and environmental factors—which could cancel or potentiate the former. Perhaps individuals with “special” brains (and minds) are more frequent than suspected. They just may go unnoticed due to sociocultural conditions or their early potential being cancelled following exposure to unwanted health or child rearing hazards during gestation and/or early childhood, or lack of an adequate child-raising environment. In this context, brain biological variability would represent an additional characteristic of the human species, which ought to be protected from devastation—as produced by famine or lack of challenging environmental stimuli. If brain (and mind) variability is a human ‘trait’, then searching for the biological substrate of individual cognitive abilities or talents would prove to be even a harder task to meet. Following this conceptual frame, the structural ‘features’ of Albert Einstein's brain should perhaps be placed within the enormous variability of micro- and macro-strategies of human brain population. Only further investigation, a multidisciplinary effort and the dialectic nature of scientific knowledge may place these findings into an adequate perspective.
Supplementary Material
Acknowledgments
The authors are thankful to Drs. T. Harvey (McMaster University, Hamilton, Ontario, Canada) and E. Krauss (University of Princeton, NJ, USA) for making available (J.A.C.) brain samples from Albert Einstein, and to C. Stockmeier, PhD. (University of Mississippi Medical Center, MS, USA), and the Cuyahoga County Coroner's Office, Cleveland, OH, USA for making postmortem brain tissue available (J.J.M-H., G.R.) from four subjects. Secretarial and technical assistance by Beatriz Stuto, Carolina Bentham, Virginia Puissant, and Fernando Poletta is gratefully acknowledged. This work was supported in part by grants (J.A.C.) from Fondation Jerome K. Lejeune (Paris, France) and FONCYT (PICT #01-03465, 5-14109)(Argentina), San Jorge Emprendimientos S.A., Chevron-San Jorge S.A., Fundación Conectar, Corpomédica S.A., Fundación René Baron and CONICET, and PHS grants MH63187, RR17701, and MH61578.
Footnotes
The emergence of new, powerful functional imaging tools may prove to be of paramount importance on this matter.
Brain sections from AE were originally embedded in celloidin following formalin fixation (Anderson and Harvey, 1996) within 7 h postmortem delay (Dr. T. Harvey, personal communication to J.A.C.); all control samples were processed accordingly.
Terminal masses (or bulbous endings) refer to spherical, bulbous endings of the interlaminar processes with diameters ranging up to 10–15 μm (Colombo et al., 2002).
In Geometry, the term parallelism indicates the property of constant separation between two lines; here, it is used to signify the degree of ordering of a set of processes slanted in a given direction.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version at, doi:10.1016/j.brainresrev.2006.03.002.
References
- Amunts K, Schleicher A, Zilles K. Outstanding language competence and cytoarchitecture in Broca's speech region. Brain Lang. 2004;89:346–353. doi: 10.1016/S0093-934X(03)00360-2. [DOI] [PubMed] [Google Scholar]
- Anderson B, Harvey T. Alterations in the cortical thickness and neuronal density in the frontal cortex of Albert Einstein. Neurosci Lett. 1996;21:161–164. doi: 10.1016/0304-3940(96)12693-8. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–214. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M. Cortical structure and mental skills: Oskar Vogt and the legacy of Lenin's brain. Brain Res Bull. 1998;47:291–296. doi: 10.1016/s0361-9230(98)00124-5. [DOI] [PubMed] [Google Scholar]
- Colombo JA. Comentarios a propósito del cerebro de Albert Einstein. Medicina. 2000;60:530–532. [PubMed] [Google Scholar]
- Colombo JA. A columnar-supporting mode of astroglial architecture in the cerebral cortex of adult primates? Neurobiology. 2001;9:1–16. doi: 10.1556/neurob.9.2001.1.1. [DOI] [PubMed] [Google Scholar]
- Colombo JA, Reisin HD. Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res. 2004;1006:126–131. doi: 10.1016/j.brainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Colombo JA, Fuchs E, Hartig W, Marotte IR, Puissant V. “Rodent-like” and “primate-like” types of astroglial architecture in the adult cerebral cortex of mammals. A comparative study Anat Embryol (Berl) 2000;201:111–120. doi: 10.1007/pl00008231. [DOI] [PubMed] [Google Scholar]
- Colombo JA, Quinn B, Puissant V. Disruption of astroglial interlaminar processes in Alzheimer's disease. Brain Res Bull. 2002;58:235–242. doi: 10.1016/s0361-9230(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Colombo JA, Reisin HD, Jones M, Bentham C. Development of interlaminar astroglial processes in the cerebral cortex of control and Down's Syndrome human cases. Exp Neurol. 2005;193:207–217. doi: 10.1016/j.expneurol.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Diamond M, Scheibel AC, Greer MM, Harvey T. On the brain of a scientist: Albert Einstein. Exp Neurol. 1985;88:198–204. doi: 10.1016/0014-4886(85)90123-2. [DOI] [PubMed] [Google Scholar]
- Dierig S. Extending the neuron doctrine: Carl Ludwig Schleich (1859–1922) and his reflections on neuroglia at the inception of the neural-network concept in 1894. Trends Neurosci. 1994;17:449–452. doi: 10.1016/0166-2236(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Donaldson HH, Canavan MM. A study of the brain of three scholars. J Comp Neurol. 1928;46:1–95. [Google Scholar]
- Galaburda AM. Albert Einstein's brain. Lancet. 1999;354:1821. doi: 10.1016/s0140-6736(05)70590-0. [DOI] [PubMed] [Google Scholar]
- Gould SJ. The Mismeasure of Man. W.W. Norton Co. Inc.; New York: 1996. [Google Scholar]
- Hertz L. Possible role of neuroglia: a potassium-mediated-neuronal–neuroglial–neuronal impulse transmission system. Nature. 1965;206:1091–1094. doi: 10.1038/2061091a0. [DOI] [PubMed] [Google Scholar]
- Hines T. Further on Einstein's brain. Exp Neurol. 1998;150:343–344. doi: 10.1006/exnr.1997.6759. [DOI] [PubMed] [Google Scholar]
- Kantha SS. Albert Einstein's dislexia and the significance of Brodmann Area 39 of his left cerebral cortex. J Hist Neurosci. 1992;37:119–122. doi: 10.1016/0306-9877(92)90052-e. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW, Klatzo I, Kleihues P. Oskar and Cecile Vogt, Lenin's brain and the bumble-bees of the Black Forest. Brain Pathol. 1992;2(4):363–371. doi: 10.1111/j.1750-3639.1992.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Le Roux D, Reh TA. Regional differences in glial-derived factors that promote dendritic outgrowth from mouse cortical neurons in vitro. J Neurosci. 1994;14:4639–4655. doi: 10.1523/JNEUROSCI.14-08-04639.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleland JL, Rumelhart DE, Hinton GE. In: Parallel Distributed Processing, vol 1. Rumelhart DE, McCleland JL, PDP Research Group, editors. The MIT Press; London, England: 1989. pp. 3–44. [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska GJ. Immunohistochemistry of neural markers for the study of the laminar architecture in celloidin sections from the human cerebral cortex. J Neurosci Methods. 1999;93(1):69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Monard D, Solomon F, Rentsch M, Gysin R. Glia-induced morphological differentiation in neuroblastoma cells. Proc Natl Acad Sci U S A. 1973;70:1894–1897. doi: 10.1073/pnas.70.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. Cell junctions of astrocytes, ependyma, and related cells in the mammalian central nervous system, with emphasis on the hypothesis of a generalized functional syncytium of supporting cells. In: Fedoroff S, Vernadakis A, editors. Astrocytes: Development, Morphology, and Regional Specialization of Astrocytes, vol 1. Academic Press; London: 1986. pp. 329–371. [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Role of glia in synapse development. Curr Opin Neurobiol. 2002;12(5):486–490. doi: 10.1016/s0959-4388(02)00358-6. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJC, Baaré WFC, Hulshof Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 2002;5(2):83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Reisin HD, Colombo JA. Considerations on the astroglial architecture and the columnar organization of the cerebral cortex. Cell Mol Neurobiol. 2002a;22:633–644. doi: 10.1023/A:1021892521180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisin HD, Colombo JA. Astroglial interlaminar processes in human cerebral cortex: variations in cytoskeletal profiles. Brain Res. 2002b;937:51–57. doi: 10.1016/s0006-8993(02)02464-2. [DOI] [PubMed] [Google Scholar]
- Reisin HD, Colombo JA. Long term disruption of glial interlaminar processes following transection of the dorsal spinal cord in adult monkeys. Brain Res. 2004;1000:179–182. [Google Scholar]
- Retzius G. Die neuroglia des gehirns beim menschen und bei saugethieren Biologische Unterschungen. Verlag, Jena; 1894. [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereological counting method. Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- Senitz D, Reichenbach A, Smith TG., Jr Surface complexity of human neocortical astrocytic cells: changes with development, aging and dementia. J Brain Res. 1995;4:531–537. [PubMed] [Google Scholar]
- Tsacopoulos MT, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Kristopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Witelson S, Kigar DL, Harvey T. The exceptional brain of Albert Einstein. Lancet. 1999;353:2149–2153. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


