With the increased incidence of obesity in our population, the metabolic syndrome is frequently encountered. This syndrome is comprised of obesity, hypertension, dyslipidemia (mostly manifest as hypertriglyceridemia and low plasma HDL cholesterol) and insulin resistance. As long as the pancreatic islets retain the capacity for compensatory increase in insulin secretion to counter the peripheral insulin resistance, mostly manifest at the adipose tissue and muscle, frank diabetes will not ensue (1). The participation of triglycerides and fatty acids in the development of this syndrome has been examined (2). Do the lower plasma HDL levels also contribute?
The paper that appears in this issue of the Journal by Fryirs and colleagues provides an important putative link between HDL components, especially the major apoproteins apoprotein A-I and apoprotein A-II, and insulin secretion by beta cells. Over the last five years, there have been a number of studies that point to the role of beta cell cholesterol homeostasis on insulin secretion. Cholesterol homeostasis is dependent on the uptake of cholesterol by the LDL receptor, endogenous cholesterol biosynthesis and cholesterol efflux via ABCA1 and related transporters. Hayden and collaborators have studied beta cell specific ABCA1 knockout mice (3, 4). The beta cells from these mice accumulate total and free cholesterol and insulin secretion is impaired upon glucose stimulation without significant changes in insulin mRNA, suggesting that cholesterol accumulation interferes with insulin exocytosis. Glucose tolerance is reduced in these animals without peripheral insulin resistance. Interestingly there was no impairment in basal insulin secretion in these deficient cells, but in these experiments isolated islets were incubated in buffer without addition of apolipoproteins. Patients with loss of function mutations in ABCA1 exhibit impaired glucose tolerance (5). Other approaches to the modification of islet cholesterol homeostasis e.g. in apoE deficient mice or incubation with methyl-β-cyclodextrin also influence insulin secretion (6). The inhibition of endogenous cholesterol synthesis also impairs insulin secretion by both unstimulated and stimulated islets (7). Taken together these data suggest that insulin secretory capacity is sensitive to a fairly finely regulated cholesterol content in beta cells.
ABCA1 interacts with extracellular amphipathic apolipoproteins to initiate efflux of cholesterol from cells. Most studies have been performed with apoprotein A-I but this can be mediated also by apoprotein A-II. The latter apoprotein competes very effectively for the binding of apoprotein A-I to ABCA1 expressing cells (8).
In the study reported in this issue (9), isolated beta cells, either MIN6 cells or primary rat beta cells, responded to either lipid free apoprotein A-I or A-II as well as discoid reconstituted HDL containing either of the two apoproteins with an increase in insulin secretion. This was observed under basal conditions (2.8mM glucose) or with glucose stimulation (25 mM). The response was rapid being demonstrable as early as 15 minutes under basal conditions and five minutes upon glucose stimulation. Also human HDL at physiological concentration increased insulin secretion under basal conditions. The mechanisms by which lipid free apoprotein promotes insulin secretion is different under basal and glucose stimulated conditions. Under basal conditions lipid free apoprotein accentuates insulin secretion by a process that is independent of the potassium ATP channel and cell glucose metabolism (low temperature). This is not the case for the glucose stimulated secretion. However under both basal and stimulated conditions, extracellular calcium and intracellular calcium homeostasis is required for optimal insulin secretion promoted by the two apoproteins.
The optimal response to lipid-free apoprotein stimulating effect in this cell culture system is dependent upon the availability of the transporters/receptors ABCA1, SRB1 and for discoid rHDL on ABCG1. This might lead one to expect that an influence on cholesterol homeostasis is involved in the stimulated insulin secretion. Yet no change in total cholesterol or free cholesterol was noted in the cells when insulin secretion was stimulated either under basal or high glucose conditions. Given the molecules involved this is somewhat surprising, though a change in the distribution of cholesterol among cell membranes and compartments cannot be excluded. It has been found recently that the engagement of ABCA1 by apoproteins activates JAK2 and STAT3 phosphorylation (10), independent of effects on cholesterol homeostasis, which influences cytokine production and perhaps apoptosis. Lipoproteins may modulate apoptosis in beta cells (11). No apoptosis was noted in the MIN6 cells incubated for 16 hours with the lipid free apoproteins. However activation of JAK2 and STAT3 phosphorylation, mediated by engagement of the leptin receptor, reduces insulin secretion (12). Also apoprotein A-I may promote cAMP formation (13). Whether these signaling pathways participate in the stimulation of insulin secretion as studied in this paper remains to be established.
There is a suggestion that the apoproteins A-I and A-II stimulation of insulin secretion may have different quantitative dependencies on the intracellular calcium balance, especially in glucose stimulated cells. Preincubation with EDTA eliminates the stimulatory effect of apoprotein A-I but not apoprotein A-II. A similar trend is seen when intracellular calcium redistribution is inhibited. This highlights our relative ignorance of the physiological functions of apoprotein A-II. In addition, the physiological existence of lipid free apoprotein A-II in tissue fluid and plasma is uncertain, as this is a highly hydrophobic apolipoprotein.
The insulin resistance associated with metabolic syndrome and low HDL may progress to frank diabetes. Is it possible that apoprotein stimulated insulin secretion is attenuated during this progression? First, the stimulation of insulin secretion is apoprotein concentration dependent over a physiologically relevant range. So with low HDL in vivo especially if this HDL is dysfunctional, as is likely the case in diabetes and the metabolic syndrome, the optimal cofactors for insulin secretion and compensatory function of the beta cell may be limited. Furthermore in the prediabetic and diabetic state, many proteins may become glycated or modified by carbonyls derived from carbohydrate oxidation such as glycolaldehyde, glyoxal, and methylglyoxal. Apoprotein A-I modified by these carbonyls is still able to mediate ABCA1 dependent cholesterol efflux (14). However these carbohydrate derived aldehydes have the capacity to influence ABCA1 function and particularly stabilization so that the functioning of this interacting couple of proteins may well be impaired in diabetes or prediabetes (15).
Thus it is clear that the interaction of HDL and its constituent apoproteins with beta cell ABC transporters yields a complex series of responses affecting insulin production that may well be pathophysiologically relevant. The precise mechanisms of these interactions and how they signal to influence beta cell function has yet to be explored in detail. The paper by Fryirs and colleagues should certainly stimulate a good deal of further work to elucidate in detail the mechanisms by which HDL interacts with and modulates the function of the beta cell.
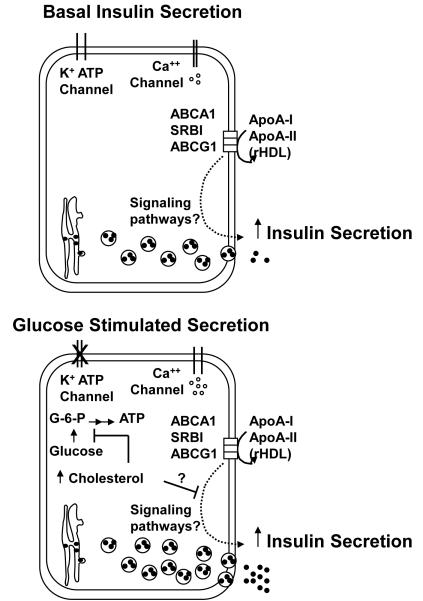
Figure.
Apoproteins A-I and A-II, as lipid free proteins and in rHDL, stimulate insulin secretion from pancreatic beta cells under basal and glucose stimulated conditions. The apoprotein stimulated secretion is dependent upon their interaction with ABCA1, SBRI or ABCG1. Whether or which signaling pathways are involved is not clear. Increased cellular cholesterol inhibits insulin secretion via inhibition of glucokinase activity, preventing the phosphorylation of glucose, and perhaps also by the inhibition of signaling pathways initiated by the interaction of the apoproteins with the transporters or receptor on the cell surface.
Acknowledgement
The work was supported by NIH grant HL092969.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus - a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 2.Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235–241. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- 3.Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasnji Z, Marsh BJ, Bodriques R, Johnson JD, Parks JS, Verchere CB, Hayden MR. B-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 4.Brunham LR, Kruit JK, Verchere B, Hayden MR. Cholesterol in islet dysfunction and type 2 diabetes. J Clin Invest. 2008;118:403–408. doi: 10.1172/JCI33296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruit JK, Brunham LR, Verchere CB, Hayden MR. HDL and LDL cholesterol significantly influence beta cell function in type 2 diabetes. Curr Opin Lipidol. 2010;21:178–185. doi: 10.1097/MOL.0b013e328339387b. [DOI] [PubMed] [Google Scholar]
- 6.Fryirs M, Barter PJ, Rye K-A. Cholesterol metabolism and pancreatic beta-cell function. Curr Opin Lipidol. 2009;20:159–164. doi: 10.1097/MOL.0b013e32832ac180. [DOI] [PubMed] [Google Scholar]
- 7.Xia F, Xie L, Mihic A, Chen Y, Gao X, Gaisano HY, Tsushima RG. Inhibition of cholesterol biosynthesis impairs insulin secretion and voltage gated calcium channel function in pancreatic beta cells. Endocrinology. 2008;149:5136–5145. doi: 10.1210/en.2008-0161. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald ML, Morris AL, Chroni A, Mendez AJ, Zannis VI, Freeman MW. ABCA1 and amphipathic apolipoproteins form high-affinity molecular complexes required for cholesterol efflux. J Lipid Res. 2004;45:287–294. doi: 10.1194/jlr.M300355-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Fryirs MA, Barter PJ, Appavaroo M, Tuch BE, Tabet F, Heather AK, Rye K-A. Effect of high density lipoproteins on pancreatic beta cell insulin secretion. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.207373. [DOI] [PubMed] [Google Scholar]
- 10.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rütti S, Ehses JA, Sibler RA, Prazak R, Rohrer L, Georgopoulos S, Meier DT, Niclauss N, Berney T, Donath MY, von Eckardstein A. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology. 2009;150:4521–4530. doi: 10.1210/en.2009-0252. [DOI] [PubMed] [Google Scholar]
- 12.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkamiu RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haidar B, Denis M, Marcil M, Krimbou L, Genest J., Jr. Apolipoprotein A-I activates cellular cAMP signaling through the ABCA1 transporter. J Biol Chem. 2004;279:9963–9969. doi: 10.1074/jbc.M313487200. [DOI] [PubMed] [Google Scholar]
- 14.Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW. Dysfunctional HDL: Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the abca1 pathway. J Biol Chem. doi: 10.1074/jbc.M110.118182. Epub Apr 8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passarelli M, Tang C, McDonald TO, O’Brien KD, Gerrity RG, Heinecke JW, Oram JF. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54:2198–205. doi: 10.2337/diabetes.54.7.2198. [DOI] [PubMed] [Google Scholar]