Abstract
T-cell based immunotherapies can be effective in the treatment of large vascularized tumors, but they rely on adoptive transfer of substantial numbers (~ 20 million) of tumor-specific T cells administered together with vaccination and high-dose IL-2. In this study, we report that ~10,000 T cells gene-engineered to express a single-chain IL-12 molecule can be therapeutically effective against established tumors in the absence of exogenous IL-2 and vaccine. Although IL-12 engineered cells did not persist long-term in hosts, they exhibited enhanced functionality and were detected in higher numbers intra-tumorally along with increased numbers of endogenous NK and CD8+ T cells just prior to regression. Importantly, transferred T cells isolated from tumors stably overproduced supra-physiological amounts of IL-12 and the therapeutic impact of IL-12 produced within the tumor microenvironment could not be mimicked with high doses of exogenously provided IL-12. Furthermore, anti-tumor effects could be recapitulated by engineering wild-type open-repertoire splenocytes to express both the single-chain IL-12 and a recombinant tumor-specific T-cell receptor (TCR), but only when individual cells expressed both the TCR and IL-12, indicating that arrested migration of T cells at the tumor site was required for their activities. Successful tumor eradication was dependent on a lymphodepleting pre-conditioning regimen that reduced the number of intra-tumoral CD4+ Foxp3+ T regulatory cells. Our findings reveal an approach to genetically modify T cells to reduce the cell number needed, eliminate the need for vaccines or systemic IL-2, and improve immunotherapy efficacy based on adoptive transfer of gene-engineered T cells.
Keywords: Adoptive transfer, IL-12, tumor microenvironment
Introduction
CD8+ T cells migrate through tumor with high instantaneous velocity but arrest upon contact with cognate antigen (1, 2). As a result, we reasoned that T cells might be able to overproduce an anti-tumor agent directly into a suppressive microenvironment that includes negative regulatory factors such as myeloid-derived suppressor cells and regulatory T cells (3-10). We chose to genetically engineer tumor antigen-specific CD8+ T cells to secrete IL-12, a potent pro-inflammatory and anti-angiogenic cytokine capable of activating multiple aspects of innate and adaptive anti-tumor immunity (11-20). The mechanisms of anti-tumor action are complex, as IL-12 can directly augment the functionality of multiple end effectors such as CD4+ (type 1), CD8+, and natural killer (NK) cells (12, 21-25).
In clinical trials, attempts to give IL-12 systemically have been restricted by toxicities, limiting the benefits seen in pre-clinical models (12, 20, 21, 24, 26, 27). Due to systemic dosing restrictions, the ability to reach therapeutic levels of IL-12 at the tumor site likely represents a major barrier for treatment of solid tumors. Several preclinical and clinical studies have demonstrated successful tumor regression by direct injection of IL-12 into lesions or genetic manipulations to induce tumor cells to produce IL-12 in situ (27-30). However, the majority of patients with metastatic cancer present with widespread disease not accessible to direct application at all sites and even if lesions are approachable, uniform distribution remains problematic. Several studies have engineered tumor cells, dendritic cells, and fibroblasts to overproduce a single chain functional IL-12, but achieving high local concentrations through a systemic approach remains elusive (26, 30-34).
In this study, we examined the effects of delivering IL-12 directly to the tumor site by adoptively transferring tumor-specific gene-engineered CD8+ T cells. We found that transferring small numbers of IL-12 engineered T cells into lymphodepleted hosts resulted in the destruction of large vascularized tumors without the need for vaccine or IL-2. We show that T cells engineered to produce IL-12 infiltrate tumors in higher numbers than non-transduced cells and continue to secrete supra-physiologic levels of IL-12 directly at the tumor site just prior to regression. T-cell receptor specificity was critical for tumor regression, suggesting that arrested migration of T cells at the tumor site was essential for successful treatments. These results describe a systemic approach to achieve high local concentrations of IL-12, leading to dramatic improvements in adoptive cell therapies.
Materials and Methods
Mice and Tumor Lines
Female thy1.1+ pmel-1 TCR transgenic (Tg) mice were generated in our laboratory (35) and made available at www.jax.org. Female C57BL/6 mice and C57BL/6 TCR-α-/- mice (obtained from Jackson Laboratories) bearing B16, an H-2b+/gp100+ murine melanoma, established and maintained as previously described (36), were used as recipients for adoptive immunotherapies. Experiments were conducted with the approval of the National Cancer Institute Animal Use and Care Committee.
Retroviral Production and Transduction
Single Chain IL-12 Vector
The cDNA for the p40 (Il12b) and p35 (Il12a) genes for IL-12 were linked by a sequence encoding a 15 amino acid (Gly4Ser)3 flexible linker and inserted into a MSGV-1 based retroviral vector with a woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) to help increase transgene expression. Pmel-1 Vector: The codon optimized pmel-1 TCR-α (Tcra) and TCR-β (Tcrb) cDNA genes were linked by a sequence encoding the foot and mouth disease picornavirus 2A “self-cleaving” linker and inserted into a MSGV-1 vector (37). Retroviral Production: Platinum Eco 293 based cells (Cell Bio Labs) were plated on poly-d-lysine coated 100mm plates (BD Biosciences) and transfected with 6 μg of pCL-Eco helper plasmid (Imgenex) and 9.3 μg of the MSGV-1IL-12 or the MSGV-1 pmel-1 TCR vector with lipofectamine 2000 (Invitrogen) overnight in antibiotic-free CM. Viral supernatants were harvested 36-48 hrs post transfection. Retroviral transductions: Pmel-1 splenocytes were cultured in the presence of 1 μM hgp10025-33 and CM containing 60 International Units (IU)/mL of recombinant human (rh) IL-2 (Chiron). For transduction of C57BL/6 splenocytes, 1 μg/mL of soluble anti-CD3 (BD Biosciences), and 1 μg/mL of soluble anti-CD28 (BD Biosciences) were used to stimulate bulk splenocytes. Two days later, splenocytes were collected and resuspended in retroviral supernatant with 60 IU/mL rhIL-2 and 10 μg/mL protamine sulfate (Abraxis Pharmaceutical Products), and spun at 1000g at 37° C for 90 minutes in 24 well plates. Cultured cells were adoptively transferred 3-5 days post transduction (> 90% CD8+ T cells).
Adoptive Cell Transfer
Six to twelve week old mice (n=5 for all groups) were injected subcutaneously with 5 × 105 B16 melanoma cells. Ten to fourteen days later, they were irradiated with 5 Gy total body irradiation (TBI) and given IL-12 transduced pmel-1 CD8+ T cells or pmel-1 TCR and IL-12 double transduced C57BL/6 by tail vein. Tumors were measured using digital calipers and the tumor area was calculated as the product of perpendicular diameter by investigators in a blinded manner. All experiments were performed independently at least twice with similar results and all tumor curve data is shown as mean +/− standard error of the mean.
Analysis of Adoptively Transferred Cells: Flow Cytometry, Cell Enumeration, Cytokine Release, Histology, and Real Time PCR
Prior to transfer, cells were characterized by flow cytometry for CD8α, CD62L, CD44, IL-7Rα, IL-2Rα and Sca-1 (BD Biosciences). IL-12, IFN-γ, TNF-α and IL-2 were analyzed using intracellular staining kits (BD Biosciences) with or without a 4 hour stimulation with phorbolmyristate acetate, 50 ng/mL (PMA) and ionomycin, 1 μg/mL (Sigma). Following transfer, tumor samples were harvested and lymphocytes were isolated using lympholyte cell separation media (Cedarlane Laboratories) and enumerated by flow cytometry. Transferred pmel-thy1.1+ cells were analyzed by flow cytometry for thy 1.1 (CD90.1), NK1.1, CD4, CD8α and IL-12 expression. Hematoxylin and Eosin staining was performed on paraffin fixed tumor samples and analyzed at 100X magnification by an Olympus IX-FLA microscope. Real-time reverse transcription PCR were performed as previously described (38).
Statistical Analysis
Tumor growth slopes were compared using Wilcoxon rank sum test. One-way ANOVA and student t-tests were used to test for significant differences in enumeration assays. P < 0.05 was considered significant.
Results
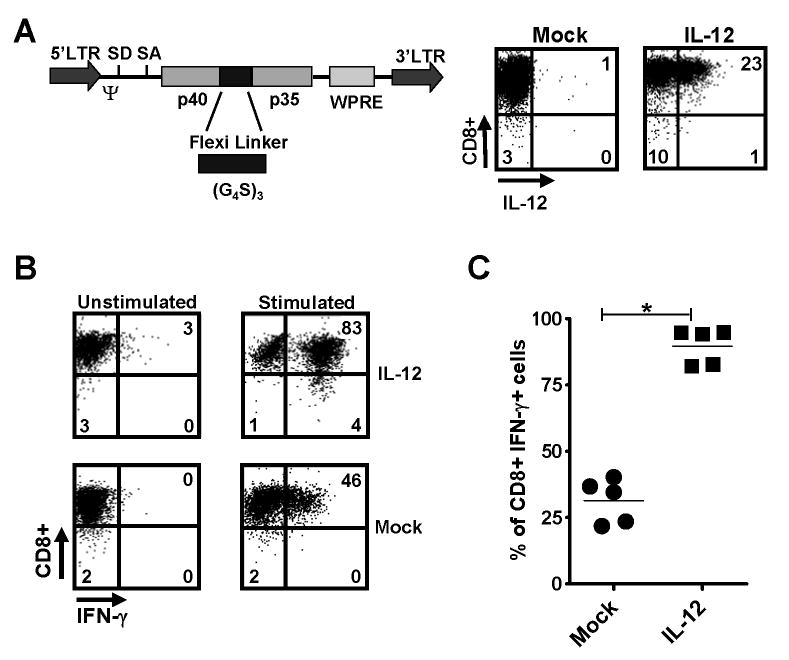
To assess the ability of tumor-specific T cells to over-produce IL-12, we constructed a retrovirus based on a derivative of the murine stem cell virus, MSGV-1 (39), encoding a single-chain, bioactive IL-12 obtained by fusing the p35 and p40 subunits with a flexible (Gly4Ser)3 linker (40-42) and designated the construct as MSGV-1IL-12 (Fig. 1A left panel). We transduced pmel-1 CD8+ T cells, which express a transgenic T-cell receptor specific for the melanoma-associated antigen, gp100, with this vector to express high levels of IL-12 (designated as pmel-1IL-12-TD; Fig. 1A right panel). We next examined the phenotype of pmel-1IL-12-TD T cells used for adoptive transfer experiments and showed several distinct characteristics compared to mock-transduced cells (Supplementary Fig. 1). IL-12 engineered cells also expressed higher levels of IFN-γ (Fig. 1B, C) and TNF-α (Supplementary Fig. 2A) upon secondary stimulation, but expressed less IL-2 (Supplementary Fig. 2B). Two critical T-box transcription factors for CD8+ T cells were altered with increased relative T-bet expression and down-regulation of eomesodermin (Supplementary Fig. 3) (43). On the other hand, IL-12 engineered cells underwent apoptosis when prolonged in culture beyond 7 days, failed to proliferate upon secondary stimulation (Supplementary Fig. 4) and exhibited a small decrease in cytotoxic ability (Supplementary Fig. 5).
Figure 1.
Pmel-1 CD8+ T cells engineered to overproduce IL-12 possess a distinct phenotype and enhanced functionality. A, The MSGV-1IL-12 retroviral vector (left panel), which encodes the p40 and p35 subunits of IL-12 linked by (Gly4Ser)3 flexible linker. SD, splice done; SA, splice accceptor; LTR, long terminal repeat; Ψ, packaging sequence. Pmel splenocytes were stimulated with 1 μM hgp10025–33 for 2 days, transduced with the MSGV-1IL-12 retrovirus, and analyzed by flow cytometry 3 days after for IL-12 expression (right panel). B, IL-12 transduced pmel-1 CD8+ cells from A were analyzed by intracellular staining without or with a 4hr secondary stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. C, The percentage of CD8+ IFN-γ+ cells following secondary stimulation in mock versus IL-12 transduced pmel cells was quantified (n=5; * p < 0.05). Experiments in A, B, and C are representative of at least 2 independent experiments.
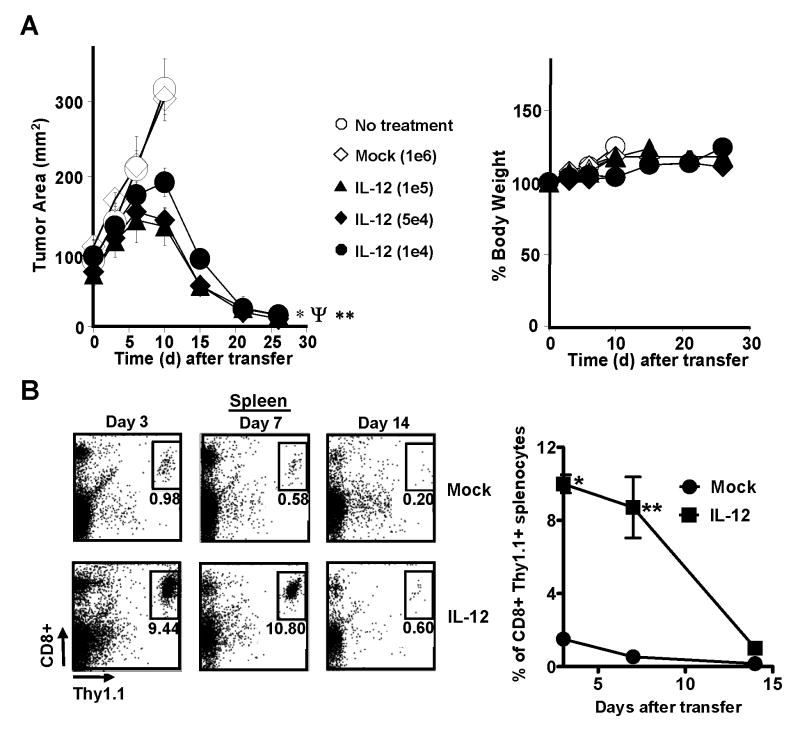
To assess the in vivo efficacy and toxicity of pmel-1IL-12-TD cells, we adoptively transferred decreasing doses of the gene-modified T cells cultured in vitro 5-7 days into sublethally-irradiated mice (5 Gy total body irradiation; TBI) bearing large vascularized B16 tumors established for 14 days. Surprisingly, we found that as few as 10,000 pmel-1 IL-12-TD cells mediated significant tumor destruction (Fig. 2A; left panel). This potent anti-tumor response was observed in the absence of high-dose recombinant IL-2 (rIL-2) and a gp100 vaccine, which we previously found were required for effective anti-tumor immunity (35). It is noteworthy that we observed tumor destruction without significant weight loss (Fig. 2A; right panel) or pathological end-organ damage (Supplementary Fig. 6). Although all the experiments in this study were completed without observable toxicities, administration of doses greater than 500,000 pmel-1IL-12-TD cells given alone or in concert with IL-2 and vaccination produced weight loss and decreased survival (Supplementary Fig. 7). These toxicities were clearly related to the increased number of cells transferred and hence, likely correlated to the amount of IL-12 produced systemically, a well described clinical phenomenon (44). Experiments in mice treated with less than 500,000 cells did not show any clear signs of systemic side effects. Thus, small numbers of pmel-1 CD8+ T cells engineered to secrete IL-12 safely enabled the destruction of large established tumors.
Figure 2.
Adoptive transfer of IL-12 engineered pmel-1 CD8+ T cells induces the regression of large, established tumors without exogenous IL-2 and vaccine. A, Anti-tumor activity following the adoptive transfer of 1×105*, 5×104Ψ, or 1×104** pmel-1IL-12-TD cells into sublethaly-irradiated (5 Gy) mice bearing B16 tumors (n=5) established for 14 days (*, Ψ, **, p <0.05 compared no treatment) with no evidence of weight loss (right panel). B, 2.5×105 mock versus IL-12 transduced pmel-1 thy1.1+ CD8+ T cells were transferred into sublethaly-irradiated (5 Gy) mice (n=3) and spleens were harvested on days 3, 7, and 14 after transfer and analyzed by flow cytometry for the percentage of transferred CD8+ thy1.1+ cells (left panel). B (right panel), Quantification of the percentage of CD8+ thy1.1+ cells in spleens (*,** p < 0.05 compared to mock). Experiments of A, and B are representative of at least 2 independent experiments.
Due to pmel-1IL-12-TD cells undergoing apoptosis after one week in culture, we were surprised by the potency of these cells and next assessed the in vivo persistence of transferred cells, a characteristic classically associated with improved tumor regression (38). We adoptively transferred thy1.1 gene-marked, pmel-1IL-12-TD cells into sublethally-irradiated (5 Gy) tumor-bearing mice and detected increased engraftment of pmel-1IL-12-TD cells compared to non-transduced cells on day 3 and 7, but found that the persistence of IL-12 engineered cells dramatically dropped on day 14 (Fig. 2B).
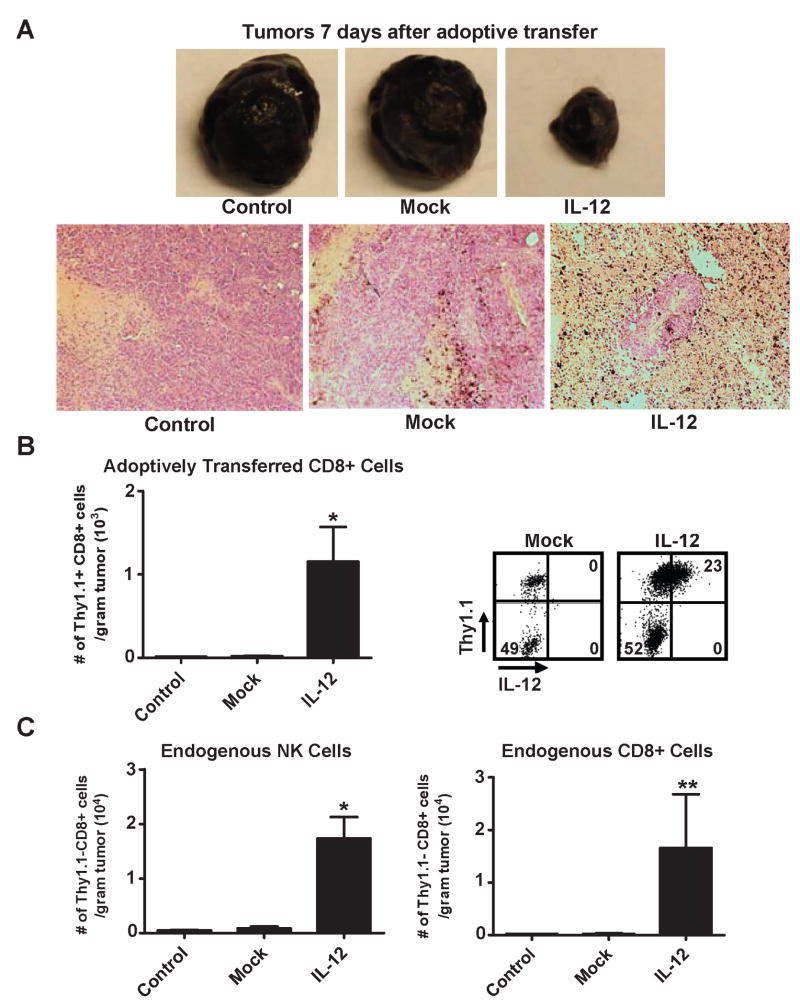
We were surprised by the degree of tumor destruction despite the lack of persistence and therefore hypothesized that there maybe differences at the tumor site contributing to the marked enhancement in response. Similar to previous experiments, we adoptively transferred thy1.1 gene-marked, pmel-1IL-12-TD cells into sublethally-irradiated tumor-bearing mice, harvested tumors one week after transfer and witnessed an increase in cellular infiltratation in conjunction with higher amounts of necrosis within tumors of mice treated with pmel-1IL-12-TD cells (Fig. 3A). We next quantified the characteristics and numbers of tumor infiltrating cells and found that mice treated with pmel-1IL-12-TD cells had a significant increase in the number of CD8+ thy 1.1+ adoptively transferred T cells (Fig. 3B; left panel). Importantly, the transgene expression of IL-12 in transferred cells remained robust (Fig. 3B; right panel), indicating the ability of these cells to secrete supraphysiologic amounts of IL-12 directly into the tumor microenvironment just prior to regression. We next assessed infiltration of tumors by endogenous cells (thy1.1-) and observed a significant increase in NK cell infiltration and an increased trend in endogenous CD8+ T cell infiltration (Fig. 3C). On the other hand, we did not observe any differences in the number of endogenous CD4+ T cells infiltrating the tumors (data not shown). It is important to note that all mice received a 5 Gy TBI lymphodepleting regimen just prior to adoptive transfer, indicating that tumors were infiltrated by reconstituting endogenous cells. Thus, pmel-1IL-12-TD cells were capable of delivering IL-12 at therapeutic levels directly into the tumor microenvironment, leading to an increase in infiltration of transferred cells along with endogenous NK and CD8+ T cells.
Figure 3.
Treatment with IL-12 engineered CD8+ T cells leads to increased tumor infiltration of adoptively transferred cells that stably expressing IL-12, and increased tumor infiltration by endogenous NK and CD8+ T cells. A, Representative subcutaneous tumor samples (top panel) excised 7 days (n=5) following the transfer of 5×105 mock or IL-12 transduced pmel-1 CD8+ T cells into sublethaly-irradiated (5 Gy) mice bearing B16 tumors established for 14 days. Corresponding H and E stains (bottom panel; 100X magnification) for tumors in the top panel. B, 5×105 mock versus IL-12 transduced pmel-1 thy1.1+ CD8+ T cells were transferred into sublethaly-irradiated (5Gy) mice (n=3), and tumors were excised 7 days after transfer, mechanically disrupted, and enumerated by flow cytometry for infiltration of adoptively transferred thy1.1+ CD8+ cells per gram of tumor (left panel; * p < 0.05 compared to control). B (right panel), IL-12 expression in tumor infiltrating thy1.1+ CD8+ cells (gated on the CD8+ population). C, Tumor samples from B, were also analyzed by flow cytometry for the number of endogenous thy1.1-, NK1.1+ cells (left panel; * p < 0.05 compared to control) and thy1.1-, CD8+ cells (right panel; ** p < 0.10 compared to control) per gram of tumor. All flow cytometry plots gated on live propidium iodide (PI)- populations. Experiments in A, B, and C representative of at least 2 independent experiments.
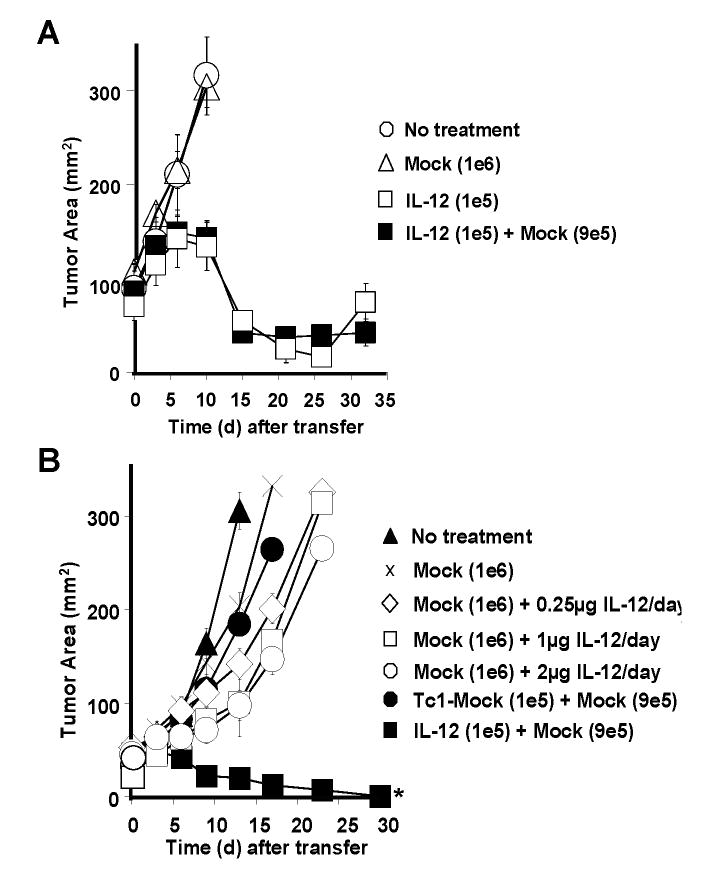
Based on the results from cells infiltrating the tumor, we next assessed the ability of non-transduced pmel-1 CD8+ T cells (mock) to assist in tumor eradication. We hypothesized that co-transferring non-transduced pmel-1 CD8+ T cells in combination with pmel-1IL-12-TD cells may impact anti-tumor responses. We adoptively transferred pmel-1IL-12-TD cells alone or in combination with mock transduced pmel-1 CD8+ T cells into sublethally-irradiated tumor-bearing mice. We did not observe any significant differences in anti-tumor responses by adding non-transduced pmel-1 CD8+ T cells, most likely due to the potency of pmel-1IL-12-TD cells alone (Fig. 4A). To further understand the nature of these anti-tumor responses, we tested the importance of local versus systemic IL-12. We sought to ascertain whether we could replicate the successful treatments using exogenously provided IL-12. We compared pmel-1IL-12-TD cells to mock-transduced pmel-1 CD8+ T cells adoptively transferred into sublethally-irradiated tumor-bearing mice in combination with increasing doses of exogenous recombinant murine IL-12 (rIL-12) given at doses up to 2 μg/day. Unlike pmel-1IL-12-TD, exogenously provided rIL-12 given together with pmel-1 CD8+ T cells alone did not induce major tumor regression (Fig. 4B). We also compared pmel-1 CD8+ T cells exposed to rIL-12 ex vivo (Tc1) to pmel-1 CD8+ T cells engineered to secrete IL-12 and found that pmel-1 Tc1 cells failed to exhibit anti-tumor responses similar to pmel-1IL-12-TD cells when transferred in similar low numbers (Fig. 4B). Thus, IL-12 produced by the transferred T cells appeared to be more effective than that provided exogenously.
Figure 4.
IL-12 engineered pmel-1 CD8+ T cells display enhanced anti-tumor responses compared to rIL-12 administered exogenously. A, Anti-tumor activity following the adoptive transfer of 1×105 pmel-1IL-12-TD cells alone or in combination with 9×105 mock transduced pmel-1 CD8+ T cells into sublethaly-irradiated (5Gy) mice bearing B16 tumors (n=5) established for 14 days. B, Treatment responses in sublethally-irradiated (5 Gy) tumor bearing mice (n=5) following the transfer of 1×106 mock-transduced pmel-1 cells with exogenous rIL-12 daily administered intraperitoneally for 3 days (0.25 μg, 1 μg, or 2 μg), 1×105 mock-transduced Tc1 cells (polarized with 3.33 ng ml-1 IL-12 ex vivo) or 1×105 pmel-1IL-12-TD cells co-transferred with 9×105 mock-transduced cells (* p<0.05 compared to all other treatment groups). Experiments in A and B are representative of at least 2 independent experiments.
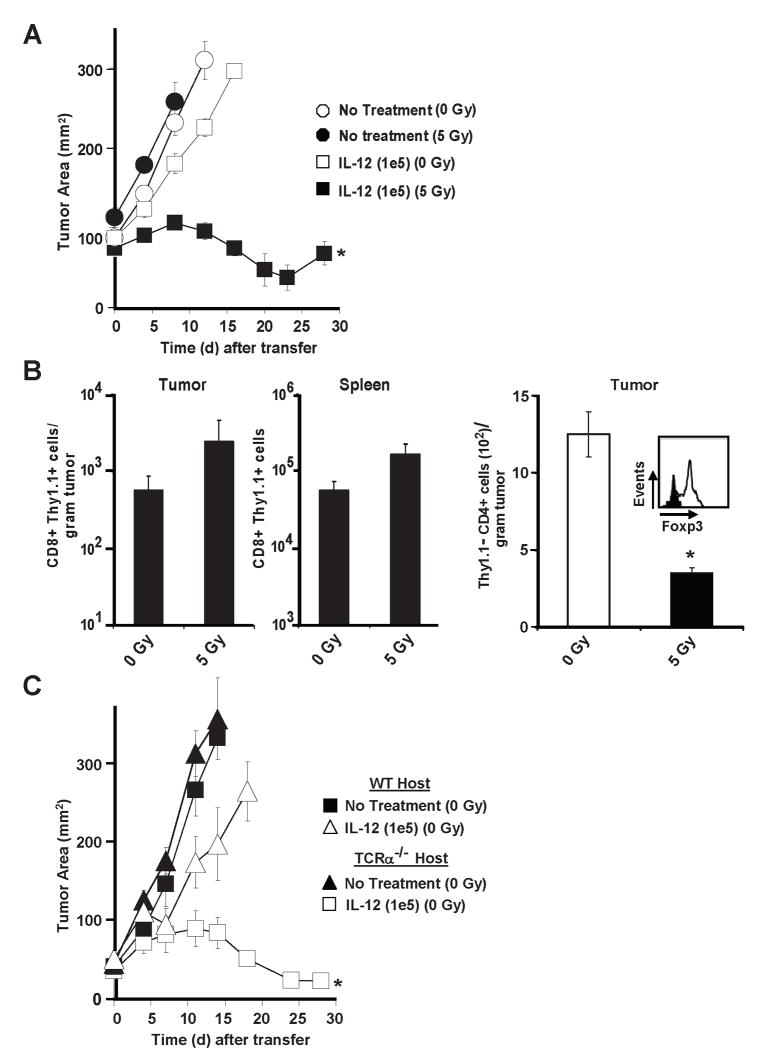
The reason for the selective retention and efficacy of IL-12 producing tumor-specific T cells was unclear, but the requirement of host immunodepletion was unambiguous in this model (Fig. 5A). Therapy failed when endogenous cells were not depleted by a preparative dose of 5 Gy TBI. We hypothesized that host immunodepletion functioned to create a “niche” that facilitated engraftment of adoptively transferred cells, but we did not measure significantly greater numbers of pmel-1IL-12-TD cells in the tumors or the spleens of mice receiving 5 Gy TBI (Fig. 5B; left panel). Instead, we observed that lymphodepleted mice had significantly lower numbers of intra-tumoral CD4+ T cells (Fig. 5B; right panel) and that these remaining T cells from mice receiving 5 Gy TBI had lower expression of Foxp3, the transcription factor marking regulatory T cells (Fig. 5B; right panel). To further test the negative functional impact of endogenous T cells, we transferred pmelIL-12-TD cells into non-ablated wild type versus TCRα-/- (no endogenous T cells) tumor bearing mice (Fig. 5C). We observed a potent anti-tumor response in non-ablated TCRα-/- mice, indicating that endogenous T cells were indeed imparting a negative or regulatory impact on the adoptively transferred pmelIL-12-TD cells. Several studies by our laboratory and others have described the importance of a preparative ablative regimen for improving adoptive T cell transfer experiments and once again we show a similar requirement in this model (36, 45-47).
Figure 5.
Host irradiation (5 Gy) is required for anti-tumor immunity of adoptively transferred pmel-1IL-12-TD. A, Tumor treatment of sublethally-irradiated (5 Gy) or non-irradiated (0 Gy) WT B16 tumor bearing mice (n=5) treated with 1×105 pmel-1IL-12-TD cells. B, Enumeration for thy1.1+ CD8+ T cells from isolated tumor samples and spleens (n=4) 6 days after the adoptive transfer of 5×105 thy1.1+-marked pmel-1IL-12-TD into 0 Gy or 5 Gy treated hosts (left panel). Enumeration (n=5) for thy1.1- CD4+ T cells in tumor samples (right panel; * p<0.05 compared to 0 Gy treatment). Flow-cytometry of isolated thy1.1- CD4+ T cells for Foxp3 expression (right panel; gated on thy1.1-, CD4+ population). All flow cytometry samples gated on live propidium iodide (PI-)populations. C. Anti-tumor responses following the treatment of 1×105 pmel-1IL-12-TD cells into non-ablated (0 Gy) WT or TCRα-/- B16 tumor bearing mice (n=5). Experiments in A and B are representative of at least 2 independent expermiments.
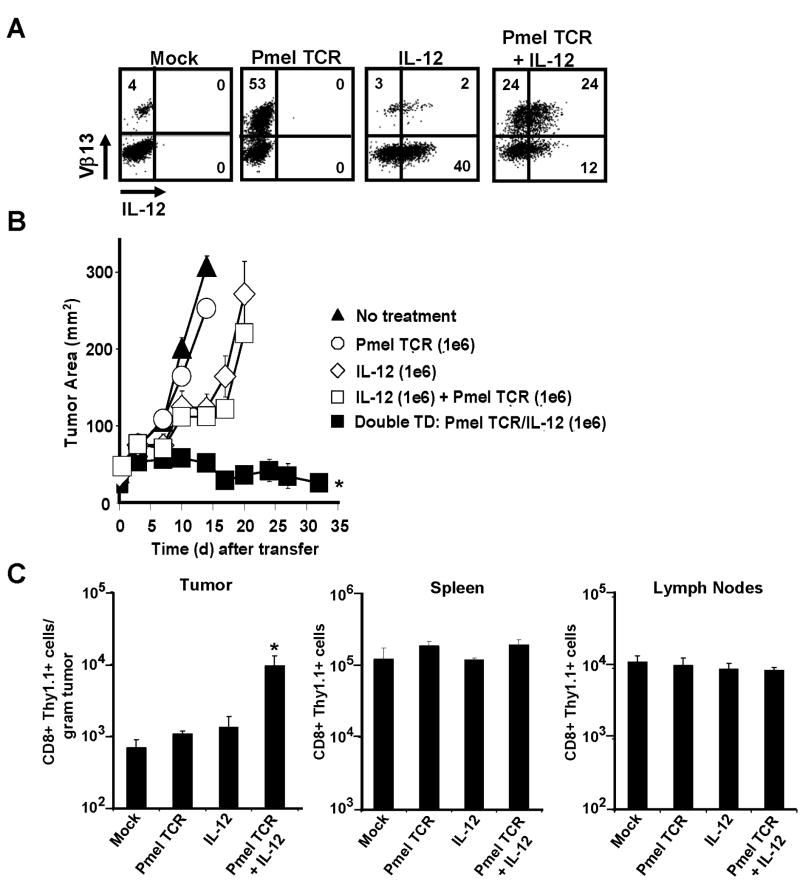
The one aspect of the therapeutic response that still remained unclear was the importance for a tumor antigen specific TCR. To address the question of specificity using “open-repertoire” CD8+ T cells, we developed a retrovirus that encoded the α/β pmel-1 TCR. We created three distinct cell types from a common precursor pool of CD8+ splenocytes expressing: a) the pmel-1 TCR alone; b) IL-12 alone; or c) both the pmel-1 TCR and IL-12 (Fig. 6A). We found that the amount of IL-12 produced by single- and double-transduced cells was similar (Fig. 6A). Interestingly, open-repertoire CD8+ T cells expressing either the single-chain IL-12 or the pmel-1 TCR failed to induce tumor regression in sublethally-irradiated tumor-bearing mice (Fig. 6B). The treatment was also ineffective when mice were given a 1:1 mixture of CD8+ T cells expressing the pmel-1 TCR mixed with cells expressing the single chain IL-12. Only double-transduced CD8+ T cells expressing both the pmel-1 TCR and IL-12 on the same cell were able to induce potent anti-tumor responses (Fig. 6B). We also found that CD8+ T cells possessing both the pmel-1 TCR and IL-12, but not either component alone, accumulated at the tumor site in significantly higher numbers (Fig. 6C). These results indicated that IL-12 must be produced by T cells with specificity for the tumor in order to increase local infiltration and induce anti-tumor responses. Furthermore, our experiments revealed that enhanced engraftment of the gene-engineered T cells possessing both the pmel-1 TCR and IL-12 occurred significantly at the tumor site but not within the spleens or lymph nodes of the same recipient mice (Fig. 6C). These results were consistent with the observations seen previously with transgenic pmel-1 CD8+ T cells transduced with IL-12.
Figure 6.
Tumor antigen-specific T-cell receptors are critical for the therapeutic responses of IL-12 engineered T cells. A, Cytofluorometric analysis of open-repertoire (WT) CD8+ T cells expressing IL-12 or pmel-1 TCR (Vβ-13+ staining) individually or on the same cell following retroviral transduction. All plots gated on CD8+ cells. B, Anti-tumor responses in sublethally-irradiated (5 Gy) tumor-bearing WT mice (n=5) treated with 1×106 single-transduced cells (expressing either the pmel-1 TCR or IL-12), a combination of both single transduced populations (2×106 cells), or 1×106 CD8+ T cells co-expressing both the pmel-1 TCR and IL-12 (* p <0.05 compared to all other treatments). C, Enumeration of tumor, spleen and draining lymph nodes (n=5) for adoptively transferred open-repertoire CD8+ thy1.1+ T cells engineered to express IL-12 and/or the pmel-1 TCR as in panel (A). * p <0.05, compared to mock. All flow cytometry samples gated on live propidium iodide (PI-)populations. Experiments in A, B, and C are representative of at least 2 independent experiments.
Discussion
The adoptive transfer of autologous tumor-infiltrating lymphocytes (TIL) or TCR redirected peripheral blood lymphocytes (PBL) is a promising treatment for patients with metastatic cancer (48, 49). The experiments described in this report demonstrate that small numbers of tumor-antigen-specific CD8+ T cells engineered to produce high levels of IL-12 can lead to the regression of large vascularized tumors without the need for exogenous IL-2 or vaccine in lymphodepleted hosts. The marked improvement in treatment was associated with an increase in tumor infiltration by adoptively transferred T cells, along with an increase in endogenous NK cells and endogenous CD8+ T cells from the reconstituting host. Based on the delayed kinetics of tumor destruction, modulation of endogenous host immune cells likely plays an important role in facilitating tumor destruction, but the impact of host NK cells and CD8+ T cells remains unclear.
Nevertheless, one of our key findings in this study included isolating gene engineered T cells from tumors one week following adoptive transfer and observing the continued stable expression of high levels of IL-12. At the same time point, we also observed an increase in the degree of necrosis within tumors treated with pmel-1IL-12-TD T cells, an observation not surprising given the overall decrease in tumor size. We found that the expression of a tumor-specific TCR was critical for the ability of T cells to deliver IL-12 to the tumor microenvironment, suggesting that the arrested migration of T cells upon recognizing cognate antigen played a mechanistic role in the local delivery of IL-12.
Interestingly, a preparative lymphodepleting regimen, known to deplete suppressive factors such as regulatory T cells was necessary for successful tumor treatments. We were surprised by the requirement for lymphodepletion, largely due to the observation that the transfer of IL-12 engineered T cells into a lymphodepleted host resulted in an increase in endogenous NK and CD8+ cells within the tumor. Our laboratory and others have described the ability of host cells to function as cytokine sinks, limiting the ability for homeostatic activation of adoptively transferred cells (36). Lymphodepletion likely removes endogenous regulatory T cells, such as CD4+ Foxp3+ cells, along with other T cells that function as suppressors and ‘sinks’, allowing for transferred IL-12 engineered T cells to arrest within the tumor upon recognizing cognate antigen. Once the host immune system reconstitutes, the changes within the tumor microenvironment induced by IL-12 likely trigger multiple downstream factors contributing to the profound changes observed. Thus, the reconstituting endogenous immune response to IL-12 secretion within the tumor may play a significant role in the anti-tumor effects described in our experiments.
In regard to treatment related toxicities, none of the mice in this study had observable toxicities attributed to the transferred T cells. However, during initial pilot experiments, we observed decreased survival when we transferred greater than 500,000 pmelIL-12-TD cells. This observation likely correlates directly to the level of systemic IL-12 produced by the constitutive expression of IL-12 in retrovirally transduced T cells. When we transferred 500,000 cells or less, thus decreasing the amount of systemic IL-12, we did not observe the previously witnessed toxicities. While TCR mispairing leading to off-target recognition has been raised as a theoretical concern, we did not observe any graft-versus-host-like toxicities in experiments following double transduction of open repertoire CD8+ T cells with the pmel TCR and IL-12 (50). The inability of IL-12 engineered cells to persist long-term likely contributed to mitigating any toxicities related to TCR redirection. In regard to efforts to translate our findings into human clinical trials, a careful phase I dose escalation trial will likely be necessary to obtain a safe therapeutic treatment cell number. Our group has taken additional measures to reduce the level of systemic IL-12 toxicity by developing retroviral vectors driven by an NFAT (nuclear-factor of activated T cells) promoter, capable of producing the single chain functional IL-12 only upon TCR stimulation (manuscript in preparation).
In conclusion, engineering tumor infiltrating lymphocytes (TIL) or antigen-specific peripheral blood lymphocytes (PBL) with IL-12 may dramatically improve cellular therapies against metastatic cancer. Furthermore, decreasing the number of cells required for adoptive transfer and eliminating the need for systemic IL-2 may help facilitate the widespread acceptance of a promising treatment modality. We recently completed the construction and amplification of a gammaretrovirus encoding a single-chain human IL-12 using “good manufacturing process” (GMP) conditions. This retrovirus will be used for the transduction of naturally-occurring and receptor-engineered T cells in the treatment of patients with advanced cancers. In the future, we may be able to engineer tumor-specific lymphocytes to locally deliver a wide array of immunomodulating payloads.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The authors would like to thank Richard Lee, Department of Pathology, National Cancer Institute, for blinded pathologic scores, Dorina Frasheri for excellent technical assistance.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118(4):1390–7. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204(2):345–56. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 4.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164(7):3596–9. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 70(1):68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronte V, Wang M, Overwijk WW, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161(10):5313–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204(1):49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 10.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–68. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 13.Gerosa F, Paganin C, Peritt D, et al. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183(6):2559–69. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 15.Manetti R, Gerosa F, Giudizi MG, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994;179(4):1273–83. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitola S, Strasly M, Prato M, Ghia P, Bussolino F. IL-12 regulates an endothelial cell-lymphocyte network: effect on metalloproteinase-9 production. J Immunol. 2003;171(7):3725–33. doi: 10.4049/jimmunol.171.7.3725. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa M, Yu WG, Umehara K, et al. Multiple roles of interferon-gamma in the mediation of interleukin 12-induced tumor regression. Cancer Res. 1998;58(11):2426–32. [PubMed] [Google Scholar]
- 18.Strasly M, Cavallo F, Geuna M, et al. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J Immunol. 2001;166(6):3890–9. doi: 10.4049/jimmunol.166.6.3890. [DOI] [PubMed] [Google Scholar]
- 19.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295(5553):338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 20.Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87(8):581–6. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 21.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markiewicz MA, Wise EL, Buchwald ZS, et al. IL-12 enhances CTL synapse formation and induces self-reactivity. J Immunol. 2009;182(3):1351–61. doi: 10.4049/jimmunol.182.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165(5):2665–70. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 24.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7(11):1705–21. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wigginton JM, Wiltrout RH. IL-12/IL-2 combination cytokine therapy for solid tumours: translation from bench to bedside. Expert Opin Biol Ther. 2002;2(5):513–24. doi: 10.1517/14712598.2.5.513. [DOI] [PubMed] [Google Scholar]
- 26.Zitvogel L, Tahara H, Robbins PD, et al. Cancer immunotherapy of established tumors with IL-12 Effective delivery by genetically engineered fibroblasts. J Immunol. 1995;155(3):1393–403. [PubMed] [Google Scholar]
- 27.Curti A, Parenza M, Colombo MP. Autologous and MHC class I-negative allogeneic tumor cells secreting IL-12 together cure disseminated A20 lymphoma. Blood. 2003;101(2):568–75. doi: 10.1182/blood-2002-03-0991. [DOI] [PubMed] [Google Scholar]
- 28.Kang WK, Park C, Yoon HL, et al. Interleukin 12 gene therapy of cancer by peritumoral injection of transduced autologous fibroblasts: outcome of a phase I study. Hum Gene Ther. 2001;12(6):671–84. doi: 10.1089/104303401300057388. [DOI] [PubMed] [Google Scholar]
- 29.van Herpen CM, van der Laak JA, de Vries IJ, et al. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin Cancer Res. 2005;11(5):1899–909. doi: 10.1158/1078-0432.CCR-04-1524. [DOI] [PubMed] [Google Scholar]
- 30.Rook AH, Wood GS, Yoo EK, et al. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood. 1999;94(3):902–8. [PubMed] [Google Scholar]
- 31.Tahara H, Zitvogel L, Storkus WJ, et al. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol. 1995;154(12):6466–74. [PubMed] [Google Scholar]
- 32.Tatsumi T, Huang J, Gooding WE, et al. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer Res. 2003;63(19):6378–86. [PubMed] [Google Scholar]
- 33.Mazzolini G, Alfaro C, Sangro B, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23(5):999–1010. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 34.Rodolfo M, Colombo MP. Interleukin-12 as an adjuvant for cancer immunotherapy. Methods. 1999;19(1):114–20. doi: 10.1006/meth.1999.0836. [DOI] [PubMed] [Google Scholar]
- 35.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106(41):17469–74. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–13. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16(4):457–72. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson R, Macdonald I, Corbett T, Hacking G, Lowdell MW, Prentice HG. Construction and biological characterization of an interleukin-12 fusion protein (Flexi-12): delivery to acute myeloid leukemic blasts using adeno-associated virus. Hum Gene Ther. 1997;8(9):1125–35. doi: 10.1089/hum.1997.8.9-1125. [DOI] [PubMed] [Google Scholar]
- 41.Lieschke GJ, Rao PK, Gately MK, Mulligan RC. Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo. Nat Biotechnol. 1997;15(1):35–40. doi: 10.1038/nbt0197-35. [DOI] [PubMed] [Google Scholar]
- 42.Wagner HJ, Bollard CM, Vigouroux S, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin’s disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004;11(2):81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 43.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177(11):7515–9. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 44.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–8. [PubMed] [Google Scholar]
- 45.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117(8):2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174(5):2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wrzesinski C, Paulos CM, Kaiser A, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 33(1):1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10(1):5–18. doi: 10.1016/j.ymthe.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 16(5):565–70. doi: 10.1038/nm.2128. 1p following 70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.