Abstract
Background
Blockade of costimulatory molecules is a potent method of inducing long-term graft survival. We have previously addressed the issue of donor-reactive T cell precursor frequency on relative costimulation dependence, and found that the presence of a high precursor frequency of donor-reactive CD8+ T cells resulted in costimulation blockade-resistant graft rejection, whereas the presence of a low-frequency donor-reactive population did not. To address the mechanisms by which high frequency T cells obviated the requirement for costimulation, we asked whether a low frequency population responding concomitantly with a high frequency response also demonstrated costimulation independence.
Methods
A model system was established in which B6 mice containing a low frequency of anti-mOVA responders and a high frequency of anti-BALB/c responders received a skin graft from B6.mOVAxBALB/c F1 donors in the presence or absence of CTLA-4 Ig/anti-CD154 costimulatory blockade.
Results
Results revealed that in the presence of costimulation blockade, high frequency anti-BALB/c T cells augmented the effector activity of low frequency anti-mOVA T cells, but did not enhance the accumulation of anti-mOVA T cells capable of mediating graft rejection.
Conclusions
These results demonstrate that both antigen-specific and antigen-independent factors contribute to the relative costimulation-independence of high frequency T cell responses.
Keywords: transplantation, tolerance, T-lymphocyte, costimulation, precursor frequency
Introduction
Antigen-specific T cell precursor frequency is increasingly being appreciated as an important factor impacting the quality of antigen-specific T cell responses. For example, studies from several groups showed that the number of adoptively transferred TCR transgenic T cells affected the resulting kinetics of expansion and effector function (1, 2), altered the degree of memory cell survival (3, 4), and impacted the memory differentiation status of the transferred cells (5). While these studies were a cautionary tale regarding the relevance of using supra-physiologic numbers of transgenic T cells to study protective immune responses, the issue of responding T cell precursor frequency might be also relevant for the field of transplantation. Specifically, the number of T cells capable of responding to alloantigens is known to generally exceed that of T cells specific for typically encountered environmental antigens, with the precursor frequency of allospecific T cells reported to be 0.1–10% (6, 7), compared to 1 in 106 for nominal antigens (8). As such, allospecific T cells could be hypothesized to behave differently than lower frequency T cells responding to physiologic antigens.
Combined blockade of CD28 and CD40 pathways synergistically inhibits donor-reactive T cell responses and allograft rejection in rodents and non-human primates (9–12). Despite this potent synergistic effect, it is widely recognized and of considerable interest that combined CD28 and CD40 pathway blockade does not uniformly control rejection responses (13). Several groups have found a primary role for CD8+ T cells in costimulation blockade-resistant rejection (14–18). As CD28 blockers progress in clinical trials and the success of alternative approaches to target CD40 show promise in non-human primate models, the need to understand their effect on T cell responses and the mechanisms by which T cells can escape blockade of the these pathways warrants further study.
We recently showed that naive CD4+ and CD8+ T cell precursor frequency profoundly influenced the degree of proliferation and differentiation of responding donor-reactive T cell populations during transplantation, and their ability to mediate costimulation blockade-resistant rejection (19–21). Our investigations made use of a transgenic model system incorporating Act-mOVA mice as the donor strain. These mice constitutively express full-length mOVA protein under control of the β-actin promoter in all tissues, including skin (22). By analyzing primary anti-donor T cell responses in these mice, we demonstrated that graft-specific CD8+ T cells stimulated at high frequency proliferated and accumulated even in the presence of CTLA-4 Ig and anti-CD154, and resulted in more efficacious, multi-cytokine producing effector cells that more efficiently precipitated graft rejection (19). In contrast, graft-specific CD8+ T cells stimulated at lower frequency in the presence of costimulation blockade failed to accumulate, did not differentiate into high quality effectors, and were incapable of rejecting a skin graft (19).
Here, we sought to dissect the mechanisms underlying the resistance of high frequency donor-reactive CD8+ T cell populations to CD28 and CD40L-mediated costimulation blockade during graft rejection. Specifically, we asked whether a low frequency population specific for a defined minor antigen responding concomitantly with a high frequency alloreactive population also demonstrated costimulation independence. We established a model system in which B6 mice containing low frequency precursors specific for a surrogate minor antigen (mOVA) received a skin graft from B6.mOVAxBALB/c F1 donors. Following B6.mOVAxBALB/c skin transplantation, recipients were treated with CTLA-4 Ig and anti-CD154. Therefore, in this system, low frequency OVA-specific T cells were stimulated in the presence of high frequency anti-BALB/c responders in the presence or absence of costimulatory blockade. Herein, our results revealed that in the absence of treatment, high-frequency alloreactive T cells augmented the accumulation of low-frequency donor-reactive T cells of a different specificity, but failed to rescue their ability to mediate rejection following treatment with costimulation blockade. Our results therefore demonstrated that both antigen-specific and non-antigen-specific factors contributed to the relative costimulation-independence of high frequency T cell responses.
Materials and Methods
Mice
Adult male 6- to 8-week old C57BL/6, BALB.B and BALB/c were purchased from the Jackson Laboratory (Bar Harbor, ME). TCR transgenic OT-I and OT-II mice were purchased from Taconic, Inc. and were bred onto RAG−/− and Thy1.1+ backgrounds. Act-mOVA mice were provided by Dr. Marc Jenkins, Univ. of Minnesota (22). Act-mOVA mice (B6 background) were crossed with BALB/c animals to generate mOVAxBALB/c F1 mice (H-2bxd). Animals received humane care and treatment in accordance with Emory University Institutional Animal Care and Use Committee guidelines.
Skin Grafting and Costimulation Blockade
Full thickness skin grafts (~1 cm2) were transplanted onto the dorsal thorax of recipient mice and secured with a plastic adhesive bandage for 5 days. Graft survival was monitored by daily visual inspection. Rejection was defined as the complete loss of viable epidermal tissue. Where indicated, recipients of skin grafts received treatment with 500 μg each of hamster anti-mouse CD40L mAb (MR-1, BioXcell, West Lebanon, NH) and human CTLA-4 Ig (Bristol-Meyers Squibb) administered i.p. on the day of transplantation (day 0) as well as on post-transplant days 2, 4, and 6.
In Vivo CFSE Mixed Lymphocyte Reaction
2×107 CFSE-labeled (5 μM) B6 splenocytes were transferred into irradiated syngenic B6, mOVA, or BALB/c recipients on day 0. On day 4, recipient spleens were harvested and splenocytes were stained with CD4, CD8, and anti-H-2Kb to identify donor-derived cells. CFSE profiles shown are gated on H-2Kb+ CD8+ or CD4+ cells.
T Cell Adoptive Transfers
OT-I and OT-II TCR tg T cells were recovered from OT-IxThy1.1+xRAG−/− and OT- IIxThy1.1+xRAG−/− mice, respectively. The frequency of OT-I or OT-II T cells was determined by staining with anti-Vα2 (used by both TCRs) and anti-CD8 or anti-CD4, respectively (Pharmingen, San Diego, CA). Mice received a single i.v. injection of OT-I or OT-II T cells along with syngeneic B6 carrier splenocytes.
Flow Cytometric Analyses for Frequency and Absolute Number
Recipients of OT-I and/or OT-II T cells were sacrificed, and spleens and draining axillary lymph nodes were recovered. Cells were stained with Thy1.1-PerCP, CD8-PacOrange, and CD4-PacBlue (all BD Pharmingen) for flow cytometric analysis on a BD LSRII. The absolute number of antigen-specific T cells was determined by TruCount Bead Analysis (Pharmingen) according to manufacturer’s instuctions. Flow cytometric data were analyzed using FlowJo Software (Treestar, San Carlos, CA).
Intracellular Cytokine Staining
For measurement of IFN-γ and TNF secreting cells, suspensions of draining axillary LN cells were incubated in a 96 well plate (1×106 per well) with 10 nM OVA257-264 (SIINFEKL) (Emory University Microchemical Core Facility) and 10 μg/ml Brefeldin A (Pharmingen, San Diego, CA). After 6 hours in culture, cells were processed using an intracellular staining kit (Pharmingen, San Diego, CA) according to manufacturer’s instructions and stained with anti-TNF-PE, anti-IFN-γ-APC, anti-Thy1.1-PerCP, anti-CD8-Pacific Orange, and anti-CD4-Pacific Blue (Pharmingen).
Statistical Analyses
Survival times for skin graft experiments are presented on Kaplan-Meier survival curves and were compared by log-rank test. Numbers of donor-specific T cells and antibody responses were compared by Mann-Whitney non-parametric test. Statistical analyses were conducted using GraphPad Prism Software.
Results
mOVA x BALB/c model: a method to test the cooperation and competition between different populations of graft-specific T cells during graft rejection or acceptance
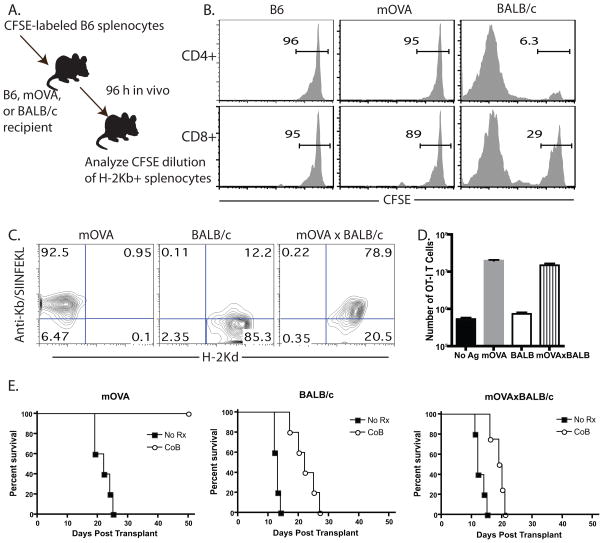
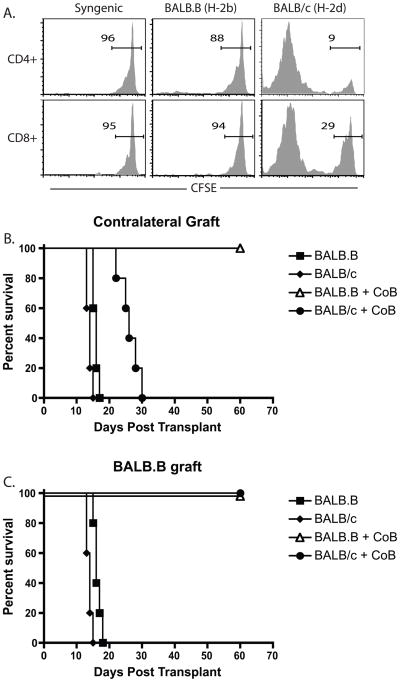
Given the fact that donor-specific T cell populations resisted CD28 and CD40L costimulation blockade during graft rejection when present at high but not low precursor frequency (19, 20), we sought to define the conditions under which a given T cell population responded as though the precursors were present at high frequency. In order to address this question we established a model system in which B6 mice containing a low-frequency population of precursors specific for a surrogate minor antigen, chicken ovalbumin (mOVA), and a high frequency of precursors specific for BALB/c alloantigens, received a skin graft from mOVAxBALB/c F1 donors. The low endogenous frequency of mOVA-specific cells and high frequency of BALB/c-specific cells was confirmed for both CD4+ and CD8+ B6 splenocytes in an in vivo mixed-lymphocyte assay (Figure 1A), where naive CFSE-labeled B6 splenocytes adoptively transferred into mOVA hosts exhibited minimal proliferation relative to that observed in B6 control hosts (Figure 1B). This is in contrast to the high degree of proliferation observed when B6 splenocytes were transferred into BALB/c hosts (Figure 1B).
Figure 1. B6 recipients of mOVAxBALB/c skin grafts experienced costimulation blockade-resistant rejection.
A, Diagram of experimental design in which B, Splenocytes from B6 mice were CFSE-labeled and adoptively transferred into B6, mOVA, or BALB/c hosts. Recipients were sacrificed five days later and splenocytes were stained with anti-H-2Kb to identify responder B6 cells, anti-CD4 and anti-CD8, and analyzed for CFSE division. Data shown are gated on CD4+ or CD8+ T cells. C, mOVA, BALB/c, or mOVAxBALB/c splenocytes were analyzed by flow cytometry for expression of H-2Kd and the presence of Kb/SIINFEKL peptide:MHC complexes. B, OT-I T cells were stimulated in vitro with mOVA, BALB/c or mOVAxBALB/c stimulators, and the number of OT-I T cells were quantified by TruCount analysis five days later. C, Skin from mOVA, BALB/c or mOVA x BALB/c donors was placed onto B6 recipients, which were left untreated or were treated with CTLA-4 Ig and anti-CD154 on days 0, 2, 4, 6. Results indicated that while the mOVA graft was protected by CoB (MST >60 d), both BALB/c and mOVA x BALB/c grafts showed only modest prolongation under costimulation blockade. Data shown are representative of three independent experiments with 4–5 mice per group.
In this model, as demonstrated by staining with a monoclonal antibody recognizing Kb/SIINFEKL complexes, mOVAxBALB/c F1 tissue expressed both H-2d and H-2b (MHC antigens) and mOVA, which was processed and presented on H-2Kb molecules (Figure 1C). We verified that OVA-specific OT-I T cells proliferated equivalently in response to mOVA and mOVAxBALB/c stimulators in an in vitro MLR (Figure 1D). To confirm that mOVAxBALB/c grafts underwent costimulation blockade-resistant rejection, skin from mOVA, BALB/c or mOVAxBALB/c donors was placed onto B6 recipients in the presence or absence of costimulation blockade. Results indicated that while the mOVA graft was protected by costimulation blockade (MST >60 d, Figure 3C, left panel), both BALB/c and mOVAxBALB/c grafts showed only modest prolongation following treatment with costimulation blockade (MSTs, BALB/c: 13 vs 22 d and mOVAxBALB/c 12 vs 20d, untreated and treated, respectively, Figure 1E).
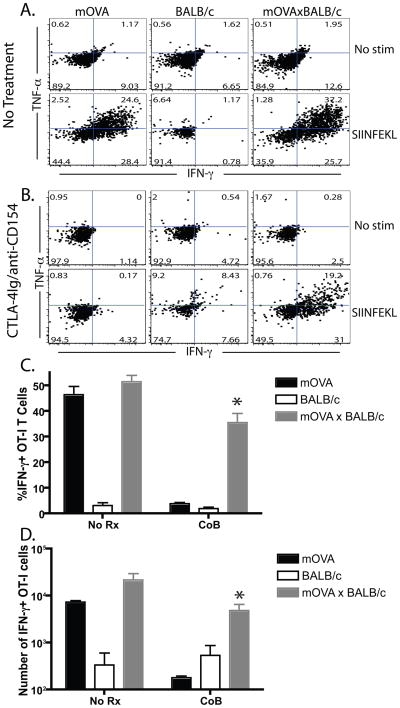
Figure 3. Stimulation by an mOVAxBALB/c skin graft increased cytokine production in OT-I T cells arising from low frequency precursors.
Recipients of 106 Thy1.1+ OT- I and OT-II T cells were transplanted and treated as indicated. Draining LN cells were restimulated in vitro with for 6 hours. Results indicated that frequencies (A,B) and absolute numbers (C) of IFN-γ and TNF -producing CD8+ Thy1.1+ anti-mOVA T cells were augmented the mOVAxBALB/c graft recipients relative to the mOVA recipients both in the presence and absence of costimulation blockade. Data shown are representative of three independent experiments with 3–5 mice per group.
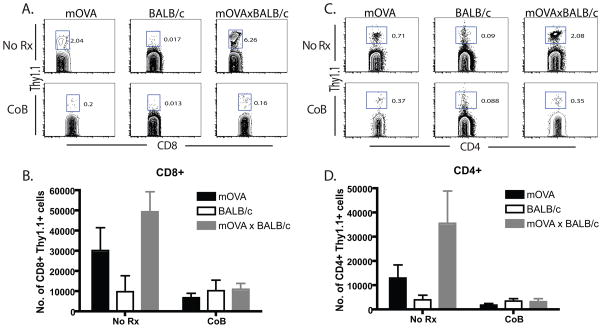
Evidence for cooperation: high frequency T cells of different antigen specificity augment the expansion of low frequency populations and rescue their effector function following costimulation blockade
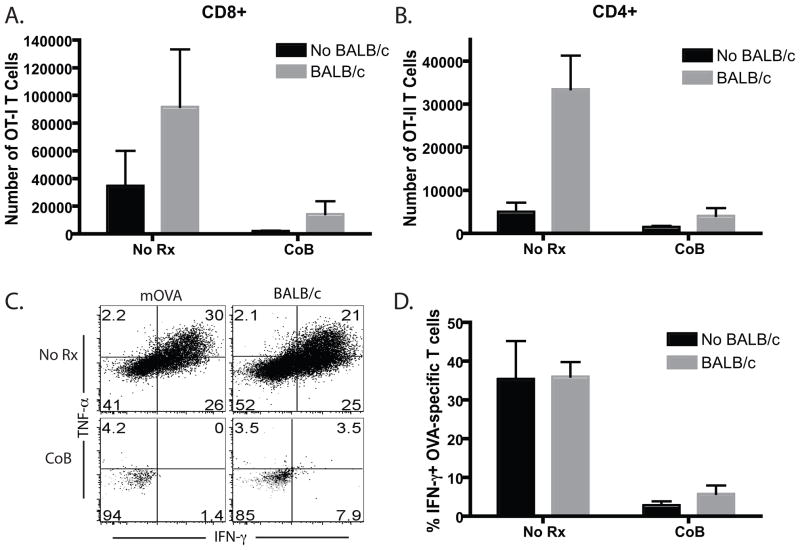
We analyzed the response of the low frequency mOVA-specific responders in the presence or absence of a high frequency anti-BALB/c response. Briefly, recipients of 106 Thy1.1+ OT- I and OT-II T cells were transplanted with skin from mOVA, BALB/c or mOVAxBALB/c donors and left untreated or were treated with CTLA-4 Ig and anti-CD154. We have previously shown that the transfer of 106 OT-I and OT-II T cells results in a precursor frequency of ~0.4% of the CD8+ and CD4+ T cell compartments, respectively, and at this low precursor frequency mice do not exhibit costimulation blockade-resistant rejection of mOVA skin grafts (19). Recipients were sacrificed at day 10 and draining lymph nodes (LN) were harvested. In the absence of treatment, both the frequency (Figure 2A) and absolute number (Figure 2B) of mOVA-specific Thy1.1+ CD8+ T cells were enhanced in mice that received an mOVAxBALB/c graft relative to mice that received an mOVA control graft (p<0.05). A similar increase was observed in the CD4+ OVA-specific population in mice that had received an mOVAxBALB/c skin graft relative to those that received an mOVA graft (Figures 2C, 2D). Mice that received BALB/c allografts served as negative controls. Thus, the proliferative response of low-frequency T cells was increased when high-frequency donor-reactive T cells with different antigen specificity responded in the same LN. Interestingly, however, this “bystander” augmentation failed to rescue the low frequency proliferative response from attenuation with costimulation blockade (p=0.05), as demonstrated by a reduction in the expansion and accumulation of donor-reactive Thy1.1+ CD4+ and CD8+ T cells in recipients of both mOVA and mOVAxBALB/c following treatment with costimulation blockade (Figure 2).
Figure 2. Stimulation by an mOVAxBALB/c skin graft did not augment the accumulation of OT-I T cells arising from low frequency precursors in the presence of costimulation blockade.
Recipients of 106 Thy1.1+ OT- I and OT-II T cells were transplanted with skin from mOVA, BALB/c or mOVA x BALB/c donors and left untreated or were treated with CTLA-4 Ig and anti-CD154 on days 0, 2, 4, 6. Mice were sacrificed at day 10 and draining LN were harvested. Results indicated that while frequencies and absolute numbers of CD8+ (A,B) and CD4+ (C,D) Thy1.1+ anti-mOVA T cells were augmented the mOVAxBALB/c graft recipients relative to the mOVA recipients, these populations were not augmented in the presence of costimulation blockade. Mice receiving a BALB/c allograft served as negative controls for frequency of OT-I T cells in the absence of antigenic stimulation. Data shown are representative of four independent experiments with 3–5 mice per group.
Stimulation by an mOVAxBALB/c skin graft increases cytokine production of OT-I T cells arising from low frequency precursors
In order to assess the effect of a high frequency response on the cytokine effector function of low frequency donor-specific T cell populations, recipients of 106 Thy1.1+ OT- I and OT-II T cells were transplanted with skin from mOVA, BALB/c or mOVAxBALB/c donors and left untreated or were treated with CTLA-4 Ig and anti-CD154. Mice were sacrificed at day 10 and draining LN cells were restimulated in vitro with SIINFEKL peptide. Frequencies of IFN-γ and TNF-producing CD8+ Thy1.1+ anti-mOVA T cells were unchanged in untreated mOVAxBALB/c graft recipients relative to untreated mOVA recipients (Figure 3A). However, IFN-γ and TNF production by OVA- specific cells isolated from costimulation blockade-treated recipients of an mOVAxBALB/c graft was enhanced as compared to cytokine production by recipients of an mOVA graft (Figure 3B). This finding is reflected in both the frequency and absolute number of IFN-γ-producing effectors (Figures 3C and 3D, respectively). These data therefore demonstrated that high frequency populations specific for a non-shared antigen could augment the cytokine production of a low-frequency donor-reactive T cell population in the presence of costimulation blockade.
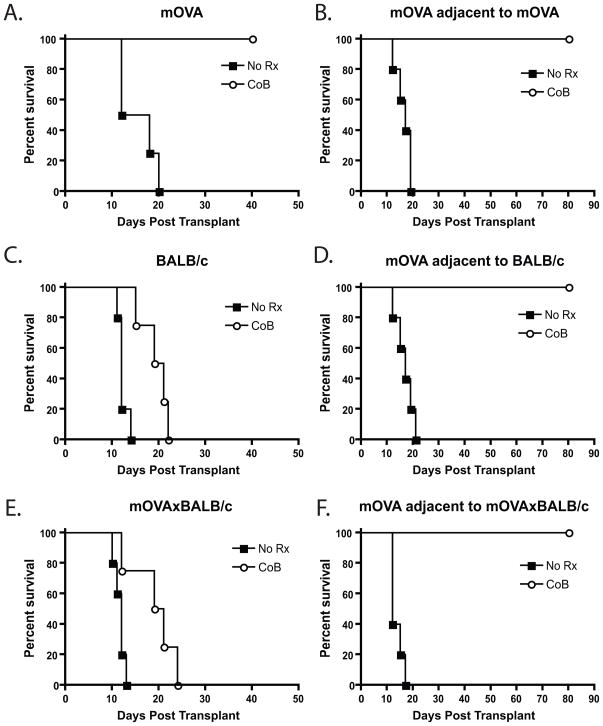
High frequency T cells of different antigen specificity did not rescue the ability of low frequency donor-reactive cells to mediate costimulation blockade-resistant rejection
The above results demonstrated that high frequency T cells of a different specificity responding in the same LN could improve the effector function of a low frequency response following costimulation blockade. However, results from Figure 4 showed that the high frequency anti-BALB/c response did not augment the accumulation of absolute numbers of OT-I T cells arising from low frequency naive precursors in the presence of costimulation blockade. Therefore, we next tested the ability of a contralateral B6.mOVA skin graft to be rejected in the presence of costimulation blockade. Mice that received 106 OT-I and OT-II T cells were transplanted with either an mOVA, BALB/c, or mOVAxBALB/c skin graft on the left dorsal thorax, and all groups received a B6.mOVA skin graft on the right dorsal thorax. Recipients were left untreated or were treated with costimulation blockade. Untreated recipients that had received either an mOVA or BALB/c skin graft on the left thorax (Figure 4A, 4C) rejected their right thorax mOVA skin grafts with an MST of 17 d (Figure 4B, 4D). Untreated recipients of mOVAxBALB/c grafts on the left side (Figure 4E) rejected their right-side mOVA graft with an MST of 12 d (Figure 4F, p<0.05), consistent with findings of enhanced proliferation and accumulation of OT-I T cells in untreated recipients of mOVAxBALB/c grafts. Strikingly, however, the enhancing effects of the anti-BALB/c response on the effector cytokine response of the low frequency T cells was not enough to allow them to mediate rejection in the presence of costimulation blockade, as was evidenced by their inability to reject a contralateral mOVA skin graft (MST > 80 d in all groups, Figure 4B, 4D, 4F), despite the fact that the mOVAxBALB/c graft on the left side had undergone costimulation blockade-mediated rejection (Figure 4E). From these results we concluded that the presence of a high frequency donor-reactive population of a distinct antigen specificity was insufficient to “rescue” the ability of a low frequency donor reactive population to mediate rejection in the presence of costimulatory blockade.
Figure 4. Stimulation of OT-I T cells by an mOVAxBALB/c skin graft did not result in costimulation blockade-resistant rejection of a second mOVA skin graft.
Recipients of 106 Thy1.1+ OT- I and OT-II T cells were transplanted with skin from mOVA (A), BALB/c (B) or mOVA x BALB/c (C) donors with or without costimulation blockade on the left dorsal thorax. These recipients were then challenged with a simultaneous mOVA skin graft on the right dorsal thorax (D, E, and F) and monitored for graft rejection. Results indicated that even if primed in the presence of an mOVAxBALB/c skin graft (Figure 4C), OT-I effectors arising from low frequency precursors failed to mediate costimulation blockade resistant rejection of an mOVA skin graft (Figure 4E). Data shown are representative of three independent experiments with 5 mice per group.
High frequency anti-BALB/c T cells did not augment the ability of low frequency BALB.B-reactive T cells to mediate costimulation blockade-resistant rejection
In order to confirm the above results in a non-transgenic model, we assessed the ability of high frequency anti-BALB/c responses to augment the function of low-frequency anti-BALB.B responses (Figure 5A) and precipitate costimulation blockade-resistant rejection of a contralateral BALB.B skin graft. Briefly, B6 mice were grafted with either BALB/c (H-2d) or BALB.B (H-2b) skin grafts on the left side in the presence or absence of costimulation blockade. All mice then received a BALB.B skin graft on their right dorsal thorax. While untreated recipients of BALB/c and BALB.B grafts rejected their skin grafts with MSTs of 14 and 16 days respectively, costimulation blockade-treated recipients of BALB/c grafts experienced prolonged graft survival with an MST of 26 days (Figure 5B). As expected, recipients of BALB.B grafts enjoyed long-term graft survival of > 70 days (Figure 5B). Despite the costimulation blockade-resistant rejection observed in the recipients of BALB/c grafts, a simultaneous BALB.B skin graft on the same recipient was not rejected (Figure 5C). These results confirm our findings from the mOVAxBALB/c system, and indicate that the presence of a high-frequency anti-BALB/c T cell response responding in the same milieu as a low-frequency anti-BALB.B graft was not sufficient to “rescue” the ability of these cells to precipitate rejection of a contralateral BALB.B graft in the presence of costimulation blockade.
Figure 5. High frequency anti-BALB/c T cells did not augment the ability of low frequency BALB.B-reactive T cells to mediate costimulation blockade-resistant rejection.
A, Splenocytes from B6 mice were CFSE-labeled and adoptively transferred into B6 or BALB.B hosts. Recipients were sacrificed four days later and splenocytes were stained with anti-H-2Kb to identify responder B6 cells, anti-CD4 and anti-CD8, and analyzed for CFSE division. Data shown are gated on CD4+ or CD8+ T cells. B) B6 mice were grafted with either BALB/c (H-2d) or BALB.B (H-2b) skin grafts on the left side in the presence or absence of costimulation blockade. C) Mice also received a BALB.B skin graft on their right dorsal thorax. Despite the costimulation blockade-resistant rejection observed in the recipients of BALB/c grafts (MST 26d), a simultaneous BALB.B skin graft on the same recipient was not rejected (MST > 70 days).
Given these findings, we went on to ascertain whether the observed enhancement in the low-frequency donor-reactive T cell response in the presence of a high-frequency population of distinct specificity shown earlier (Figures 2 and 3) functioned in cis or in trans. To this end, we analyzed the OVA-reactive CD4+ and CD8+ T cell responses to an mOVA skin graft in the presence or absence of an adjacent BALB/c skin graft. Results indicated that the absolute number of both CD4+ and CD8+ donor-reactive OVA-specific T cells were enhanced in the presence of adjacent BALB/c skin graft (Figure 6A, 6B), indicating that this phenomenon functions in trans. However, in contrast, the observed augmentation in the frequency of cytokine-producing cells in costimulation blockade-treated recipients of an mOVAxBALB/c as compared to an mOVA skin graft (as shown in Figure 3) was not observed in the presence of an adjacent BALB/c skin graft (Figure 6C, D), indicating that this effect functions in cis.
Figure 6. Impact of adjacent BALB/c skin graft on CD4+ and CD8+ anti-OVA T cell responses.
Recipients of 106 Thy1.1+ OT- I and OT-II T cells were transplanted with skin from an mOVA donor on the right dorsal thorax in the presence or absence of costimulation blockade Groups of mice also received a simultaneous adjacent BALB/c skin graft on the left dorsal thorax. Results indicated that the absolute number of both CD4+ and CD8+ donor-reactive OVA-specific T cells were enhanced in the presence of adjacent BALB/c skin graft (Figure 6A, 6B). In contrast, however, there was no augmentation in the frequency of cytokine-producing cells in costimulation blockade-treated recipients in the presence of an adjacent BALB/c (Figure 6C, D). Data shown are representative of two independent experiments with 3 mice/group.
Discussion
The results from this study demonstrated that high frequency alloreactive T cells of a different specificity responding in the same LN in untreated recipients enhanced the accumulation of effectors generated from a low frequency response, and rescued the cytokine production in low-frequency donor-reactive T cells in costimulation blockade-treated recipients. This allogeneic “adjuvant” effect has been previously documented, particularly in the tumor immunology field. For example, studies have shown that allogeneic DC expressing tumor antigens enhanced activation of antigen-specific T cells relative to stimulation with autologous DC expressing the same antigens (23). Furthermore, the presence of alloreactive T cells secreting large amounts of type I interferons and expressing high levels of CD154 have been shown to increase anti-tumor responses in vivo (24, 25). Our results are consistent with these findings in that increased CD154-mediated signals might promote increased DC activation in unblocked recipients, leading to enhanced activation of T cell populations specific for a distinct antigen.
Interestingly, however, this “companion” augmentation did not rescue the low frequency proliferative response from attenuation following treatment with costimulation blockade, as demonstrated by a reduction in the expansion and accumulation of donor-reactive Thy1.1+ CD4+ and CD8+ T cells. In addition, the enhancing effects of the anti-BALB/c response on the effector cytokine response of the low frequency T cells was not enough to allow them to mediate costimulation blockade-resistant rejection, as was evidenced by the inability of cells in an adjacent lymph node to exhibit this enhancement in trans and to reject a contralateral mOVA skin graft (Figures 4 and 6). From these results and our previous findings showing that high-frequency populations bearing the same antigen-specificity could mediate costimulation blockade-resistant rejection (19), we infer that donor-reactive populations recognizing the same antigen need to be present at high frequency in order to mediate costimulation blockade-resistant rejection of donor tissue. Therefore, we hypothesize that competition for antigen is a key factor playing a role in resistance to costimulation blockade, in that increased competition for antigen may limit the amount of TCR signal received by any one cell. In support of this, results from previous studies have demonstrated decreased cell division in cells stimulated at high frequency, suggesting that competition for antigen exists within populations stimulated at high frequency (1, 2, 19). The idea that attenuated TCR-mediated signals are critical for high precursor frequency populations to obviate the need for costimulation during graft rejection might suggest that therapeutic intervention designed to augment TCR signaling could facilitate attenuation of donor-reactive T cell responses and promote graft survival under these conditions.
The finding that a high frequency anti-BALB/c response could not “rescue” the low frequency anti-OVA T cell response in this model raises questions about the nature of the high frequency alloreactive population in a fully mismatched setting. Specifically, it implies that the high frequency anti-BALB/c response itself does not function as many low frequency populations that each recognize distinct alloepitopes and that do not compete for antigen. This idea is supported by early work on the nature of alloreactivity and the underlying basis for high precursor frequency alloreactive populations, which suggested that the binding energy for T cell activation derived almost entirely from TCR interaction with the MHC complexes (26). In a related theory, Bevan hypothesized that the high frequency of allospecific T cell responses could be due to the recognition of a spectrum of related peptide:MHC ligands by a pool of different T cell clones (26). However, more recent work has clearly demonstrated the critical role of the peptide in allorecognition, and suggests that alloreactive T cells are highly peptide-specific (27–32). Based on this knowledge, presumably each low frequency anti-BALB/c population would function independently, and would be expected to exhibit a dependence on costimulation for activation and graft rejection. Rather, our data implied that at least a subset of the anti-BALB/c response was functioning as a high-frequency population, it that it did not require costimulation for graft rejection. These apparently conflicting results may be reconciled by a recent finding in both the MHC class I and II systems showing that alloreactive T cells were polyspecific, in that they could recognize several unrelated peptides presented by the same MHC molecule (28). This polyspecificity, purported to be the basis for the high frequency of allorecognition (33), may provide an explanation for how a pool of peptide-specific alloreactive T cells can compete for related antigens, thereby undergoing limited cell division and avoiding the deleterious effects of extensive division in the absence of costimulation (34).
The findings presented here raise the question of which cell type (i.e. alloreactive CD4+ vs CD8+ T cells) might be responsible for augmenting the response of the low-frequency anti-OVA CD8+ T cells in this model. While experiments in CD4- or CD8-deficient animals may have allowed us to address this issue, these experiments would ultimately be confounded by the potential of homeostatic proliferation in these recipients. Therefore, although we have previously shown that high-frequency populations of either CD4+ or CD8+ T cells of the same antigen specificity resulted in an augmentation of the anti-OVA response and precipitated costimulation blockade-resistant rejection (19, 20), whether both CD4+ and CD8+ T cells can do the same for cells of different antigen specificity remains an open question.
Our results also raise the issue of whether indirect or direct alloreactivity plays a more prominent role in the augmentation of a low-frequency anti-donor T cell response. Because the OVA epitopes can be presented by both donor and recipient antigen-presenting cells in this model, anti-BALB/c T cell populations responding through either the direct or indirect pathway could be engaging the same APCs as the anti-OVA T cells. Therefore, additional studies are required in order to dissect the relative roles of direct vs. indirect alloimmunity in this model.
While high frequency antigen-non-specific populations did not rescue low frequency populations from costimulation blockade-induced apoptosis, we demonstrated that high frequency antigen-non-specific population could augment the cytokine-producing ability of a low-frequency donor-reactive T cell population. This finding suggests that there are factors that can be shared between distinct populations of donor-reactive T cells that may be important for the generation of cytokine-producing effector cells. We speculate that paracrine cytokines secreted from, or alternate costimulatory molecules expressed on, high frequency T cells may impact the surrounding T cells directly or may act indirectly to augment the low frequency response via differential or enhanced activation of an APC intermediate. Characterization of the pathways most critical for mediating this “companion” augmentation of cytokine production could provide new targets for therapeutic intervention in order to attenuate high-frequency allo-reactive T cell responses.
One potential caveat of these experiments is that they assessed the effects of a polyclonal high-frequency population on a single monoclonal low-frequency population. The OT-I T cell receptor is considered to be a high-affinity receptor, and the possibility remains that lower-affinity low frequency clones would not be affected in a similar manner. In addition, the bioavailability of costimulation blockade may be altered in the high-frequency pool due to limiting reagent per cell. However, this can be ruled out for CTLA-4 Ig, since this blocking reagent binds to the APC, the frequency of which would be unchanged in the presence of high or low frequency T cell populations. Furthermore, if limited bioavailability of blocking reagent were playing a role, one would predict that a proportion of the low frequency population would be inefficiently blocked as well.
In conclusion, our results demonstrate that both antigen-specific and non-antigen-specific factors can contribute to the outcome of a high frequency T cell response. High frequency bystander populations augmented the effector cytokine production of a low-frequency donor-reactive T cell population, but were insufficient to inhibit cell death in the presence of costimulation blockade and allow breakthrough graft rejection. Future studies to determine the precise nature of the signals that mediate these effects are warranted.
Acknowledgments
This work was supported by NIH K22 AI079409 to MLF and NIH R37 AI40519 to CPL.
Abbreviations
- mOVA
membrane bound chicken ovalbumin
- CoB
costimulation blockade
References
- 1.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26 (6):827. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirth T, Harty JT. Initial TCR transgenic precursor frequency alters functional behaviour of CD8 T cells responding to acute infection. Adv Exp Med Biol. 2009;633:71. doi: 10.1007/978-0-387-79311-5_7. [DOI] [PubMed] [Google Scholar]
- 3.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312 (5770):114. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 4.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104 (38):15045. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6 (8):793. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166 (2):973. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 7.Lindahl KF, Wilson DB. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. Journal of Experimental Medicine. 1977;145 (3):508. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blattman JN, Antia R, Sourdive DJ, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195 (5):657. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 10.Adams AB, Shirasugi N, Jones TR, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174 (1):542. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 11.Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74 (7):933. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Larsen CP, Alexander DZ, Hollenbaugh D, et al. CD40-gp39 interactions play a critical role during allograft rejection. Transplantation. 1996;61 (1):4. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Williams MA, Trambley J, Ha J, et al. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165 (12):6849. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 14.Jones ND, Van Maurik A, Hara M, et al. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165 (2):1111. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 15.Trambley J, Bingaman AW, Lin A, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104 (12):1715. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Z, Meng L, Kim O, et al. CD8-T cell-mediated rejection of intestinal allografts is resistant to inhibition of the CD40/CD154 costimulatory pathway. Transplantation. 2001;71 (9):1351. doi: 10.1097/00007890-200105150-00033. [DOI] [PubMed] [Google Scholar]
- 17.Newell KA, He G, Guo Z, et al. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol. 1999;163 (5):2358. [PubMed] [Google Scholar]
- 18.Markees TG, Phillips NE, Gordon EJ, et al. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J Clin Invest. 1998;101 (11):2446. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ML, Koehn BH, Wagener ME, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007 doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J Immunol. 2008;180 (11):7203. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koehn BH, Ford ML, Ferrer IR, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008;181 (8):5313. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3 (11):1355. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 23.Merrick A, Diaz RM, O’Donnell D, Selby P, Vile R, Melcher A. Autologous versus allogeneic peptide-pulsed dendritic cells for anti-tumour vaccination: expression of allogeneic MHC supports activation of antigen specific T cells, but impairs early naive cytotoxic priming and anti-tumour therapy. Cancer Immunol Immunother. 2008;57 (6):897. doi: 10.1007/s00262-007-0426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Har-Noy M, Zeira M, Weiss L, Fingerut E, Or R, Slavin S. Allogeneic CD3/CD28 cross-linked Th1 memory cells provide potent adjuvant effects for active immunotherapy of leukemia/lymphoma. Leuk Res. 2009;33 (4):525. doi: 10.1016/j.leukres.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Har-Noy M, Zeira M, Weiss L, Slavin S. Completely mismatched allogeneic CD3/CD28 cross-linked Th1 memory cells elicit anti-leukemia effects in unconditioned hosts without GVHD toxicity. Leuk Res. 2008;32 (12):1903. doi: 10.1016/j.leukres.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Bevan MJ. High determinant density may explain the phenomenon of alloreactivity. Immunology Today. 1984;5 (3):128. doi: 10.1016/0167-5699(84)90233-0. [DOI] [PubMed] [Google Scholar]
- 27.Udaka K, Tsomides TJ, Eisen HN. A naturally occurring peptide recognized by alloreactive CD8+ cytotoxic T lymphocytes in association with a class I MHC protein. Cell. 1992;69 (6):989. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- 28.Felix NJ, Donermeyer DL, Horvath S, et al. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8 (4):388. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 29.Heath WR, Kane KP, Mescher MF, Sherman LA. Alloreactive T cells discriminate among a diverse set of endogenous peptides. Proc Natl Acad Sci U S A. 1991;88 (12):5101. doi: 10.1073/pnas.88.12.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heath WR, Sherman LA. Cell-type-specific recognition of allogeneic cells by alloreactive cytotoxic T cells: a consequence of peptide-dependent allorecognition. Eur J Immunol. 1991;21 (1):153. doi: 10.1002/eji.1830210123. [DOI] [PubMed] [Google Scholar]
- 31.Obst R, Munz C, Stevanovic S, Rammensee HG. Allo- and self-restricted cytotoxic T lymphocytes against a peptide library: evidence for a functionally diverse allorestricted T cell repertoire. Eur J Immunol. 1998;28 (8):2432. doi: 10.1002/(SICI)1521-4141(199808)28:08<2432::AID-IMMU2432>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Panina-Bordignon P, Corradin G, Roosnek E, Sette A, Lanzavecchia A. Recognition by class II alloreactive T cells of processed determinants from human serum proteins. Science. 1991;252 (5012):1548. doi: 10.1126/science.1710827. [DOI] [PubMed] [Google Scholar]
- 33.Wucherpfennig KW, Allen PM, Celada F, et al. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol. 2007;19 (4):216. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7 (12):942. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]