Abstract
Central activation of somatostatin (sst) receptors by oligosomatostatin analogs inhibits growth hormone and stress-related rise in catecholamine plasma levels while stimulating grooming, feeding behaviors, gastric transit and acid secretion, which can be mimicked by selective sst2 receptor agonist. To evaluate the pattern of neuronal activation induced by peptide sst receptor agonists, we assessed Fos-expression in rat brain after intracerebroventricular (icv) injection of a newly developed selective sst2 agonist compared to the oligosomatostatin agonist, ODT8-SST, a pan-sst1–5 agonist. Ninety min after injection of vehicle (10µl) or previously established maximal orexigenic dose of peptides (1µg=1nmol/rat), brains were assessed for Fos-immunohistochemistry and doublelabeling. Food and water were removed after injection. The sst2 agonist and ODT8-SST induced a similar Fos distribution pattern except in the arcuate nucleus where only the sst2 agonist increased Fos. Compared to ODT8-SST, the sst2 agonist induced higher Fos-expression by 3.7-fold in the basolateral amygdaloid nucleus, 1.2-fold in the supraoptic nucleus (SON), 1.6-fold in the magnocellular paraventricular hypothalamic nucleus (mPVN), 4.1-fold in the external lateral parabrachial nucleus, and 2.6-fold in both the inferior olivary nucleus and superficial layer of the caudal spinal trigeminal nucleus. Doublelabeling in the hypothalamus showed that ODT8-SST activates 36% of oxytocin, 63% of vasopressin and 79% of sst2 immunoreactive neurons in the mPVN and 28%, 55% and 25% in the SON, respectively. Selective activation of sst2 receptor results in a more robust neuronal activation than the pan-sst1–5 agonist in various brain regions that may have relevance in sst2 mediated alterations of behavioral, autonomic and endocrine functions.
Keywords: somatostatin receptor, brain, oxytocin, vasopressin
1. Introduction
Somatostatin (SST) is a cyclic tetradecapeptide that was originally identified in the hypothalamus as a physiological inhibitor of growth hormone (GH) release (Brazeau et al., 1973). In addition to its hypophysiotropic function, SST acts in the brain to exert pleiotropic actions that are mediated by interactions with one or more of five G-protein coupled seven transmembrane domain receptors, designated sst1–5 on which SST interacts with similar nanomolar affinity (Leroux et al., 1997; Patel, 1999; Vanetti et al., 1992). Receptor subtype selective agonists and their availability are key tools to the understanding of receptor subtype(s) involved in the diverse effects induced by injection of SST in the brain (Erchegyi et al., 2008; Grace et al., 2006). Octreotide (also SMS 201–995) is a stable analog characterized to have exclusive binding affinity to sst2 > sst3 = sst5 receptors (Hoyer et al., 1995; Patel, 1999), and early on shown to influence several homeostatic functions including food intake, drinking behavior, and blood pressure when injected into the rat brain (Danguir, 1988; Hajdu et al., 2000). We also reported that the long-acting pan-sst1–5 oligosomatostatin agonist ODT8-SST (des-AA1,2,4,5,12,13-[DTrp8]-SST) (Barnes et al., 1981; Erchegyi et al., 2008) injected into the cerebrospinal fluid stimulates gastric function including acid secretion and emptying (Martinez et al., 2000; Yoneda et al., 1991) and light phase food intake in rats (Stengel, 2010a). ODT8-SST’s orexigenic effect is mediated primarily through the sst2 receptor since intracerebroventricular (icv) pretreatment with the sst2 antagonist, des-AA1,4–6,11–13-[pNO2-Phe2,DCys3,Tyr7,DAph(Cbm)8]-SRIF-2Nal-NH2 (Cescato et al., 2008) blocked this response (Stengel, 2010a). Moreover, the selective sst2 receptor agonist (sst2 agonist) des-AA1,4–6,11–13-[DPhe2,Aph7(Cbm),DTrp8]-Cbm-SRIF-Thr-NH2 (Grace et al., 2006) injected icv induces a similar increase in food consumption. In addition, both ODT8-SST and sst2 agonist injected icv increase body temperature and lead to a distinctive behavioral response characterized by excessive grooming including licking and scratching in rats (Stengel, 2010a; Stengel, 2010b). By contrast, sst2 agonists injected into the cerebrospinal fluid did not alter while selective or preferential sst5 agonists accelerated gastric transit (Martinez et al., 2000; Stengel, 2010b). These data are indicative of differential sst receptor subtypes involved in ODT8-SST behavioral and thermoregulatory effects compared with those involved in brain-gut interactions.
All somatostatin receptors are widely distributed throughout the central nervous system (Leroux et al., 1988; Moller et al., 2003; Schulz et al., 2000; Selmer et al., 2000; Stroh et al., 1999), especially in the cerebral cortex and hypothalamus where each receptor subtype has its specific pattern of expression with the sst2 receptor being the most abundant (Kumar, 2007). In the rodent brain, the sst2 receptor exists in two variant isoforms, sst2a and sst2b which differ in length and composition of their respective carboxyl-terminal domains as well as localization but exhibit the same binding properties (Sarret et al., 1998). In particular, anatomical support for the sst2a centrally mediated behavioral actions of somatostatin analogs is provided by the brain distribution of sst2a receptors in specific areas as determined by in situ hybridization and immunohistochemistry. Those encompass hypothalamic nuclei regulating endocrine and fluid homeostasis such as the supraoptic (SON) and paraventricular (PVN) nucleus, feeding centers such as the arcuate nucleus (Arc) (Csaba et al., 2003; Schindler et al., 1997; Stepanyan et al., 2003) as well as other areas governing motor behavior and visceral function such as the basolateral amygdaloid nucleus, hippocampus, locus coeruleus and parabrachial nucleus of the brainstem as well as spinal cord (Dournaud et al., 1996). However, still little is known about the pattern of brain activation induced by a selective sst2 receptor compared with a pan-sst receptor agonist when injected into the lateral brain ventricle at doses known to exert central actions to influence homeostatic function (Brown et al., 1984; Stengel, 2010b; Taché et al., 1981). Using Fos as a marker of neuronal activation (Dragunow and Faull, 1989; Sagar et al., 1988), one recent study showed that SST injected icv at a low dose significantly increased Fos expression in SON and PVN (Meddle et al., 2010) and a dose known to induce barrel rotation induces Fos mRNA in the cerebellum (Kamegai et al., 1993) in rats. Octreotide injected icv dampened Fos expression induced by corneal stimulation in the rat spinal trigeminal nucleus (Bereiter, 1997). Other in vivo or in vitro electrophysiological studies showed that activation of sst2 receptors largely produces an inhibitory effect on neurons in the rat locus coeruleus (Chessell et al., 1996), mouse hypothalamus (Lanneau et al., 1998), rat arcuate nucleus (Mori et al., 2010) and rat periaqueductal gray (Connor et al., 2004).
Therefore, the present study aimed to assess the pattern of neuronal activation in the brain in response to icv injection of the newly developed selective sst2 agonist, des-AA1,4–6,11–13-[DPhe2,Aph7(Cbm),DTrp8]-Cbm-SRIF-Thr-NH2 (Grace et al., 2006) using Fos immunohistochemistry and compared the expression to that induced by the pan-activator of sst receptors, ODT8-SST (Erchegyi et al., 2008). As both peptides have similar molecular weights (ODT8-SST: 1078.5 and sst2 agonist: 1132.5), the icv dose of 1 µg/rat was selected based on our previous dose response studies showing that both agonists under these conditions induced a maximal orexigenic effect in ad libitum fed rats during the light phase (Stengel, 2010a; Stengel, 2010b). Likewise, both compounds robustly influenced other behaviors such as grooming including scratching, washing and licking as well as thermoregulation when injected at 1µg/rat (Stengel, 2010a; Stengel, 2010b).
The brain mapping of Fos expression encompasses regions that have been described as sst2-immunoreactive (ir) namely the limbic system including the PVN, Arc and amygdala as well as brainstem nuclei (inferior olivary nucleus and spinal trigeminal nucleus) implicated in behavior and autonomic regulation. In addition, in view of the existing evidence that somatostatin and analogs injected icv elicit the release of oxytocin and vasopressin from the hypothalamus (Hajdu et al., 2000; Meddle et al., 2010; Rettig et al., 1989), we also performed double labeling to assess whether ODT8-SST injected icv activates oxytocin and vasopressin-containing cells in the SON and PVN. Lastly to get insight to the potential direct stimulatory action of the pan-sst agonist ODT8-SST on these populations of hypothalamic neuroendocrine neurons, we performed double immunolabeling of Fos and sst2a receptor using an antibody raised against the C-terminal 331–340 aa of the sst2a receptor and does not recognize the sst2b (Sternini et al., 1997).
2. Results
Rats injected icv with vehicle and food and water deprived thereafter displayed a low number of Fos-immunoreactive (ir) cells (<6 cells/nucleus) in all brain nuclei investigated as assessed 90 min after injection. Distributions of Fos-ir neurons induced by icv sst2 agonist and ODT8-SST are summarized in Table 1. In brief, sst2 agonist induces Fos expression mainly in the somatosensory and -motor cortex, striatum, basolateral amygdaloid nucleus (BLA), ventral premamillary nucleus, selective hypothalamic nuclei, parasubthalamic nucleus, cerebellum, external lateral parabrachial nucleus (LPBE), medullary reticular nucleus, inferior olivary nucleus (IO) and the superficial layer of the caudal spinal trigeminal nucleus (Sp5Cs). ODT8-SST induces a similar pattern although Fos expression was not as pronounced or absent in some nuclei such as the striatum, Arc or parasubthalamic nucleus (Table 1). In our study, neither the sst2 agonist nor ODT8-SST induced neuronal activation in the locus coeruleus which is consistent with previous electrophysiological studies showing that somatostatin exerts an inhibitory action on spontaneous firing of locus coeruleus neurons through activation of sst2 receptor (Chessell et al., 1996; Ikeda et al., 2009). Further quantitative analysis was performed targeting specific limbic (BLA), hypothalamic (SON, PVN, Arc) and brainstem nuclei (LPBE, IO, Sp5c). Two-way ANOVA showed a statistically significant interaction between treatment and region (F(12,98) = 38.2, p < 0.001). Results from the two different batches of rats that each contained all three treatment groups (vehicle, sst2 agonist or ODT8-SST) were not different.
Table 1.
Rat brain structures showing Fos immunoreactivity following icv injection of vehicle, sst2 agonist or ODT8-SST. Brain regions that do not express Fos immunoreactivity are omitted from the table.
| Brain structure | Density | |||
|---|---|---|---|---|
| vehicle | sst2 agonist | ODT8- SST |
||
| (n=5) | (n=6) | (n=6) | ||
| Forebrain | Piriform cortex | + | ++ | + |
| Insular cortex | − | ++ | − | |
| Entorhinal cortex | + | ++ | + | |
| Cingulate cortex | − | + | + | |
| Somatomotor cortex | + | ++ | + ~ ++ | |
| Lateral septum | − | + | − | |
| Striatum | − | ++ | − | |
| other cortical areas | + | ++ | + | |
| Anterior cortical amygdaloid nucleus | − | + | + | |
| Medial amygdaloid nucleus | + | + | + | |
| Central amygdaloid nucleus | − | − | − | |
| Basolateral amygdaloid nucleus | − | +++ | ++ | |
| Hypothalamus | Lateral preoptic area | − ~ + | + | − |
| Suprachiasmatic nucleus (Cap) | + | + | + | |
| Supraoptic nucleus | − | ++++ | +++ | |
| Accessory neurosecretory nuclei | − | + | + | |
| Paraventricular nucleus | ||||
| Parvicellular anterior | − | − | − | |
| Parvicellular medial | − | − ~ + | − | |
| Ventral | − | − | − | |
| Magnocellular medial | − | +++ | ++ | |
| Magnocellular lateral | − | +++ | ++ | |
| Posterior | − | − ~ + | − | |
| Medial hypothalamic area | + | + | − | |
| Dorsomedial hypothalamic nucleus | + | ++ | − | |
| Arcuate nucleus | − | ++ | − | |
| Ventral tuberomammillary nucleus | − | +++ | + | |
| Premamillary nucleus, ventral part | − | ++ | ++ | |
| Thalamus | Paraventricular thalamic nucleus, anterior part | + | + | − |
| Parasubthalamic nucleus | − | ++ | − | |
| Cerebellum | Cerebellar cortex | − | ++ | + |
| Midbrain | Periaqueductal Gray | + | + | − ~ + |
| Superior and inferior colliculi | ++ | ++ | + | |
| Pons | Pontine nuclei | ++ | ++ | + |
| External subnucleus of lateral parabrachial nucleus | − | ++ ~ +++ | + | |
| Medulla | Raphe pallidus | − | + | − ~ + |
| Area postrema | − | − | + | |
| Lateral reticular nucleus | + | − | − ~ + | |
| Medullary reticular nucleus, dorsal part | − | ++ | + | |
| Spinal trigeminal nucleus, superficial layer | − | +++ | ++ | |
| Inferior olivary nucleus | − | ++ | + | |
Density: “+” approximately 1–4 cells, “++” 5–10, “+++” 10–20 and “++++” >20 immunoreactive cells in a 100 µm×100 μm area of an ocular grid when the objective was 10×.
2.1. Sst2 agonist and ODT8-SST icv induce Fos expression in the basolateral amygdaloid nucleus
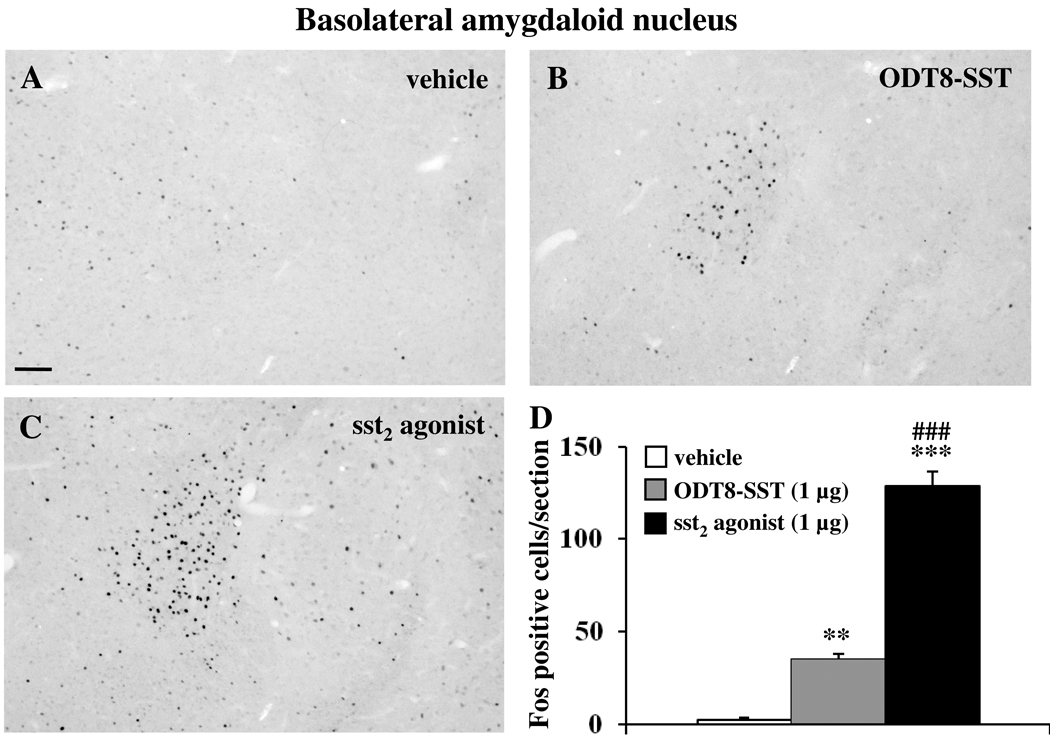
The icv injection of sst2 agonist and ODT8-SST (1 µg/rat) significantly increased the number of Fos-ir neurons in the BLA compared to control animals (vehicle: 2.5 ± 1.3, sst2 agonist: 128.8 ± 7.9, ODT8-SST: 35.0 ± 2.8; p < 0.01 vs. vehicle; Fig. 1). Injection of sst2 agonist resulted in a significant 3.6-fold higher number of Fos-expressing neurons compared with ODT8-SST (p<0.01).
Fig. 1.
Icv injection of sst2 agonist or ODT8-SST induces Fos expression in neurons of the basolateral amygdaloid (BLA) nucleus in conscious rats. Fos immunostaining in the BLA 90 min after icv injection of vehicle (A), ODT8-SST (B) and sst2 agonist (C). Unilateral cell count/section in the BLA (D) is expressed as mean ± SEM of 5–6 rats/group. ** p < 0.01; *** p < 0.001 compared with vehicle and ### p < 0.001 compared with ODT8-SST. The scale bar in (A) represents 100 µm.
2.2. Sst2 agonist and ODT8-SST icv induce Fos expression in distinct hypothalamic nuclei
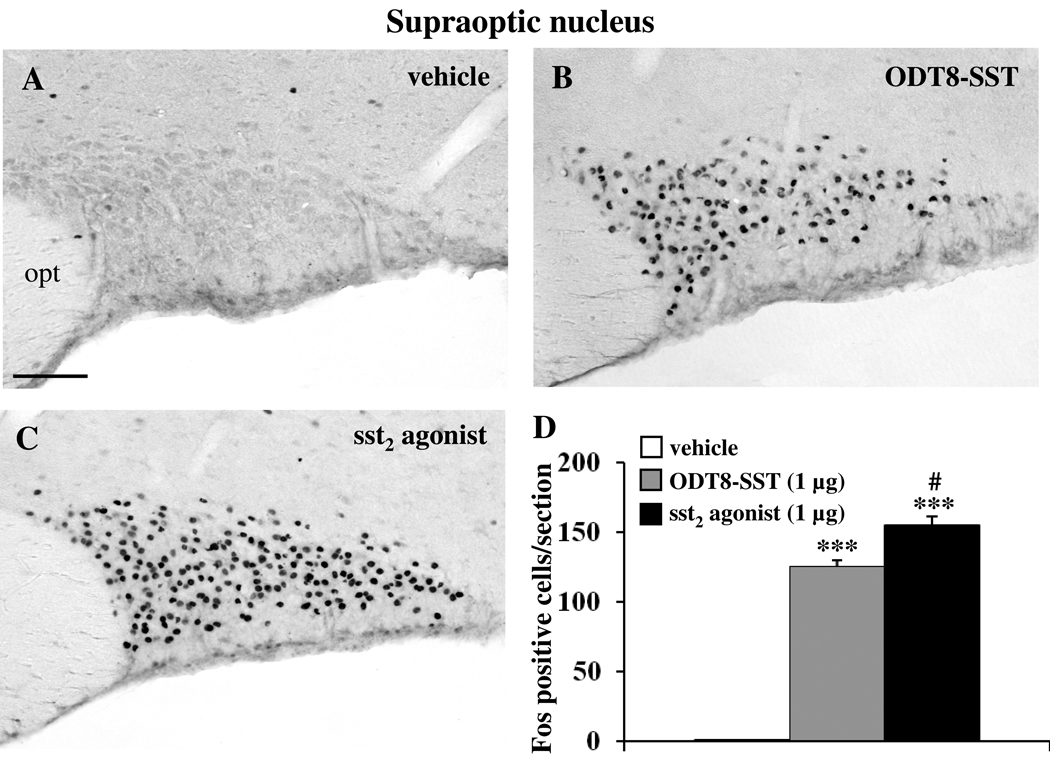
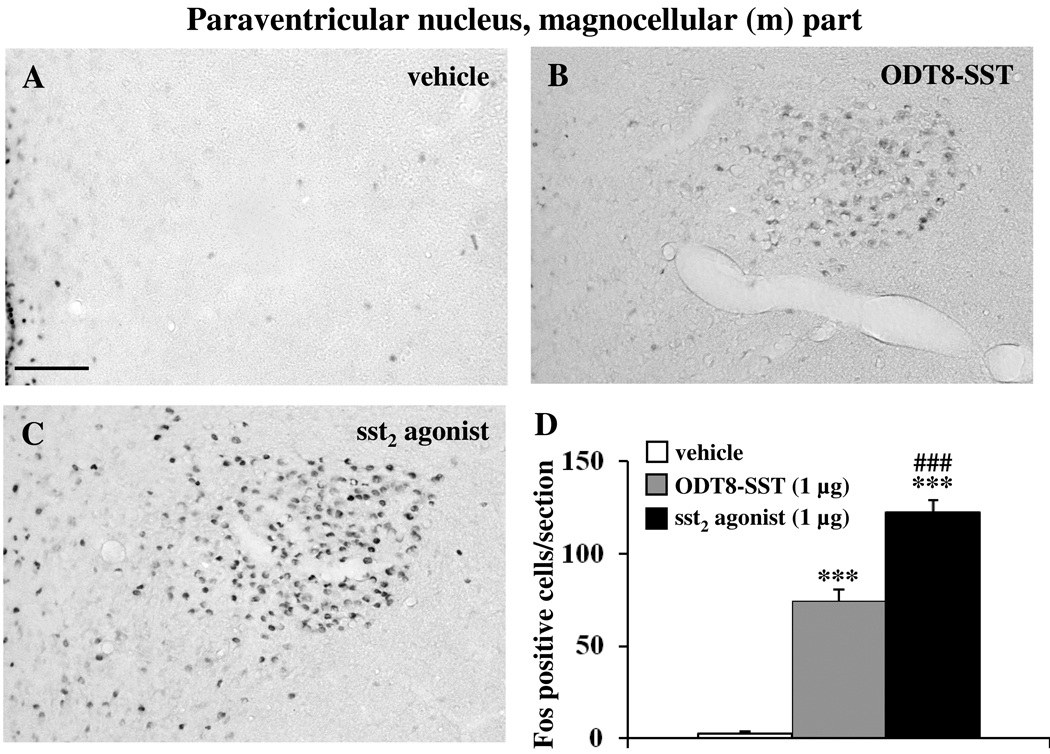
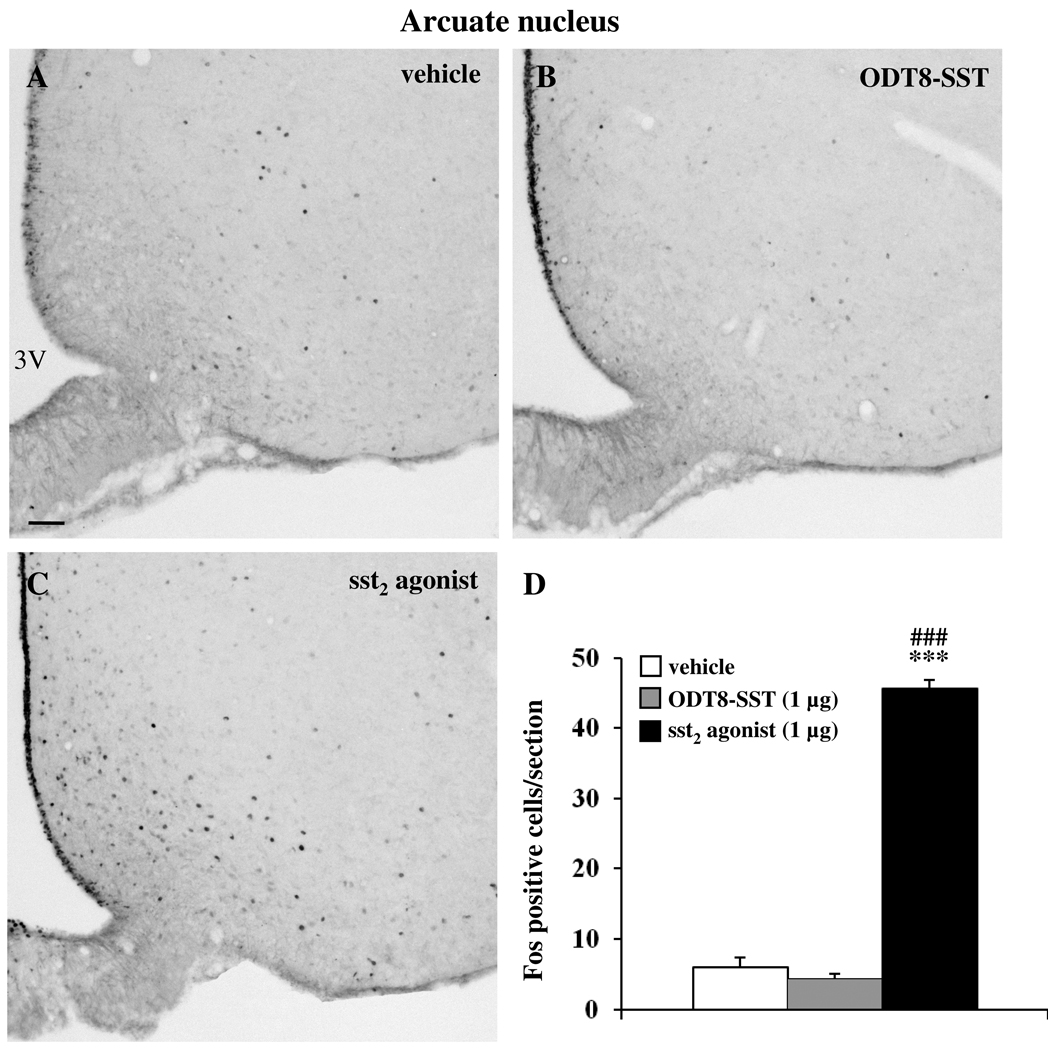
Icv injection of sst2 agonist and ODT8-SST increased the number of Fos-ir neurons compared to control animals in the SON (vehicle: 0.5 ± 0.3, sst2 agonist: 154.6 ± 7.1, ODT8-SST: 125.3 ± 4.3; p < 0.01 vs. vehicle; Fig. 2) and the Fos response induced by sst2 agonist was significantly 1.2-fold higher compared to ODT8-SST (p < 0.01). In the magnocellular paraventricular nucleus (mPVN), icv injection of sst2 agonist and ODT8-SST increased the number of Fos-ir neurons compared to control animals (vehicle: 2.5 ± 1.4, sst2 agonist: 122.0 ± 6.9, ODT8-SST: 74.4 ± 6.3; p < 0.001 vs. vehicle; Fig 3). The sst2 agonist induced a significantly 1.6-fold higher response compared with ODT8-SST injected animals (p < 0.001). In the Arc, only the sst2 agonist induced Fos expression while after ODT8-SST neuronal activation resembled that of vehicle injected animals (vehicle: 6.0 ± 1.3, sst2 agonist: 45.6 ± 1.3, ODT8-SST: 4.4 ± 0.6; p < 0.001 sst2 agonist vs. vehicle or ODT8-SST; Fig. 4).
Fig. 2.
Fos immunostaining in the supraoptic nucleus (SON) 90 min after icv injection of either vehicle (A), ODT8-SST (B) or sst2 agonist (C). Unilateral cell count/section in the SON (D) is expressed as mean ± SEM of 5–6 rats/group. *** p < 0.001 compared with vehicle and ## p < 0.01 compared with ODT8-SST. The scale bar in (A) represents 100 µm. Other abbreviation: opt: optic tract.
Fig. 3.
Injection of sst2 agonist or ODT8-SST induces Fos expression in magnocellular neurons of the hypothalamic paraventricular nucleus (mPVN). Chronically icv cannulated conscious rats were injected with vehicle (A), ODT8-SST (B) or sst2 agonist (C) and 90 min later transcardially perfused for immunohistochemical detection of Fos protein. Unilateral cell count/section in the mPVN (D) is expressed as mean ± SEM of 5–6 rats/group. *** p < 0.001 compared with vehicle and ### p < 0.001 compared with ODT8-SST. The scale bar in (A) represents 100 µm.
Fig. 4.
Icv injection of sst2 agonist (C) but not ODT8-SST (B) induces Fos expression in neurons of the arcuate nucleus (Arc) compared with vehicle treated animals (A). The scale bar in (A) represents 100 µm. Unilateral cell count/section in the Arc (D) is expressed as mean ± SEM of 5–6 rats/group. *** p < 0.001 compared to vehicle and ### p < 0.001 compared to ODT8-SST. Other abbreviations: 3V: third ventricle.
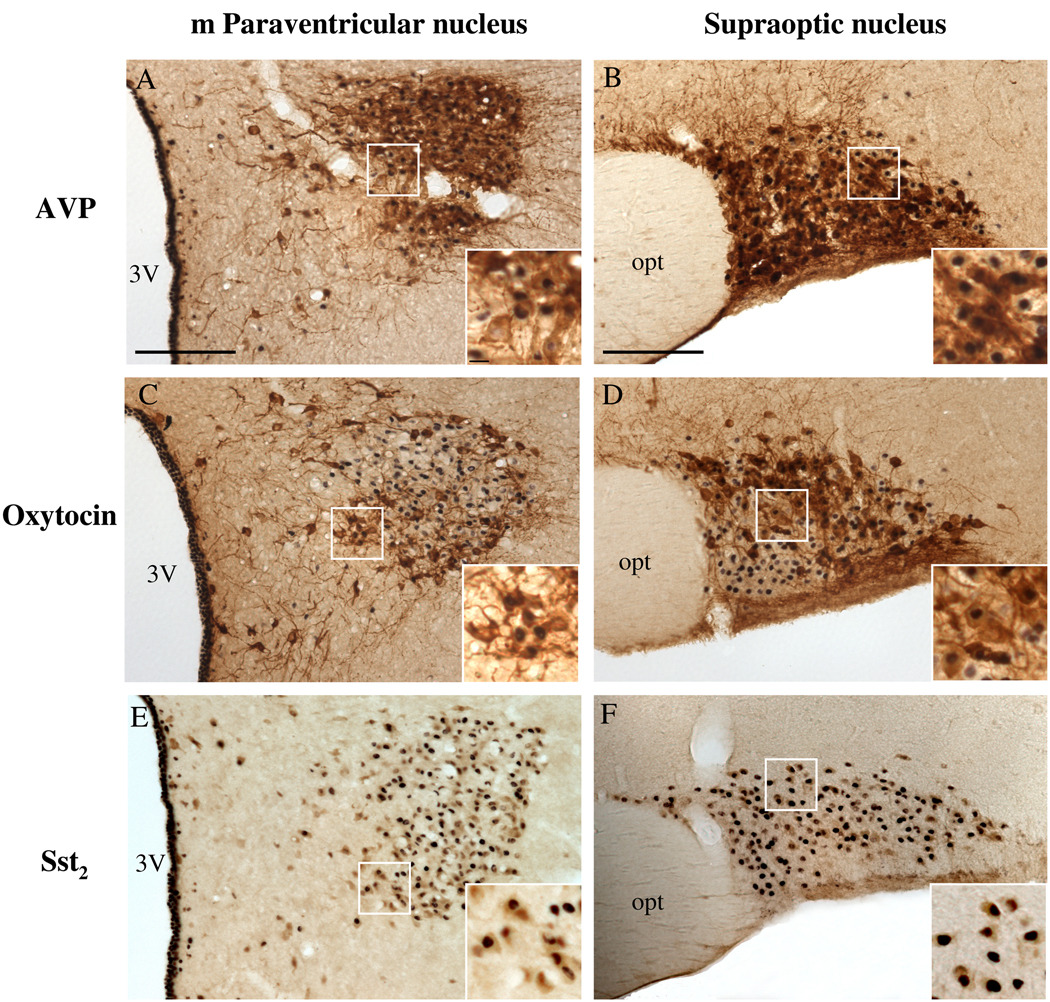
2.3. ODT8-SST icv activates oxytocin and arginine-vasopressin (AVP) neurons as well as neurons bearing the sst2a receptor in the PVN and SON
The distribution of oxytocin and AVP-ir neurons in the magnocellular PVN and SON was consistent with that previously described (Rhodes et al., 1981; Wang et al., 2009). Briefly, in the mPVN, the medial part consists primarily of oxytocin-producing cells, while the lateral mPVN is formed by a core of vasopressin-producing cells rimmed by oxytocin cells. In the principal part of the SON, oxytocin-producing cells were located rostrally and dorsally, while the AVP-ir cells were more caudally and ventrally located.
In the mPVN after icv injection of ODT8-SST, 36.3% of the Fos-ir neurons were oxytocin-positive (mean ± SEM of double labeled neurons, ODT8-SST vs. vehicle: 25.7 ± 11.7 vs. 0.2 ± 0.0, Fig. 5A) and 62.8% of Fos-ir neurons were also AVP-ir (42.1 ± 10.7 vs. 0.3 ± 0.2; p < 0.05, Fig. 5C). In the SON, 27.9% of the Fos-ir neurons were oxytocin-positive (40.3 ± 6.9 vs. 0.0 ± 0.0; p < 0.01, Fig. 5B) and 55.4% were AVP-ir (70.5 ± 4.0 vs. 0.2 ± 0.2; p < 0.001, Fig. 5D).
Fig. 5.
Immunohistochemical double staining of Fos- and AVP-immunoreactivity (A, B) or oxytocin-immunoreactivity (C, D) or sst2a-immunoreactivity (E, F) in the magnocellular PVN (A, C, E) and SON (B, D, F) 90 min after icv injection of 1 µg ODT8-SST or vehicle (n=3 per group). The inserts show higher magnification of Fos-expressing neurons that co-label with AVP (A, B), oxytocin (C, D) or sst2a-immunoreactivity (E, F). The scale bar in A and B represents 100 µm and 10 µm in the insert A. Other abbreviations: 3V: third ventricle, opt: optic tract.
Icv ODT8-SST induced robust Fos expression in the mPVN and SON. Therefore, double labeling analysis of sst2a and Fos immunoreactivity was focused on these two nuclei. Sst2a immunoreactivity in nerve cell bodies and some terminals was distributed throughout the mPVN and SON. Of these sst2a-bearing neurons, 56.3 % in the mPVN (64.7 ± 6.3 vs. 0.0 ± 0.0; p < 0.001) and 69.7 % in the SON (34.5 ± 4.4 vs. 0.1 ± 0.1; p < 0.01) showed Fos immunoreactivity after injection of ODT8-SST (Fig. 5E, F). On the other hand, 25.5% of the Fos-ir neurons in the SON and 78.9% of the Fos-ir neurons in the mPVN were co-labeled for the sst2a receptor. Furthermore, cell count of sst2a expressing neurons was significantly higher in the mPVN in icv ODT8-SST injected animals compared to vehicle (114.9 ± 8.3 vs. 85.5 ± 1.4; p < 0.05).
2.4. Sst2 agonist and ODT8-SST icv induce Fos expression in the lateral parabrachial nucleus
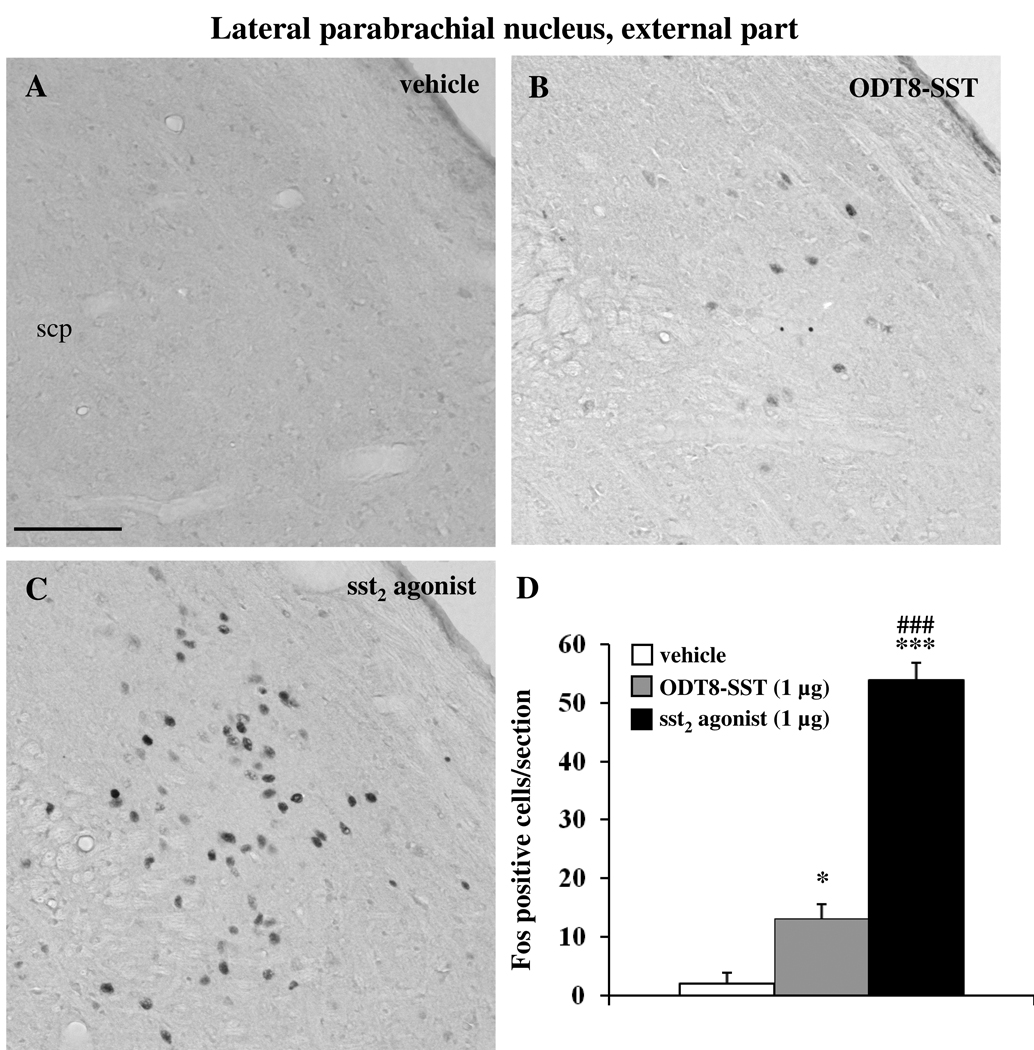
Icv injection of sst2 agonist and ODT8-SST increased the number of Fos-ir neurons compared to control animals in the external part of the lateral parabrachial nucleus (LPBE, vehicle: 2.0 ± 1.9, sst2 agonist: 54.0 ± 2.9, ODT8-SST: 13.1 ± 2.5; p < 0.05 vs. vehicle; Fig. 6). Sst2 agonist induced a significantly 4.1-fold higher number of Fos-positive neurons compared to ODT8-SST (p < 0.001).
Fig. 6.
Immunohistochemical staining for Fos in the external part of the lateral parabrachial nucleus (LPBE). Rats were icv injected with either vehicle (A), ODT8-SST (B) or sst2 agonist (C), 90 min later brains were harvested and processed for Fos immunohistochemistry. The scale bar in (A) represents 100 µm. Unilateral cell count/section in the LPBE (D) is expressed as mean ± SEM of 5–6 rats/group. * p < 0.05 and *** p < 0.001 vs. vehicle; ### p < 0.001 compared with ODT8-SST. Other abbreviations: scp: superior cerebellar peduncle.
2.5. Sst2 agonist and ODT8-SST icv induce Fos expression in brainstem neurons
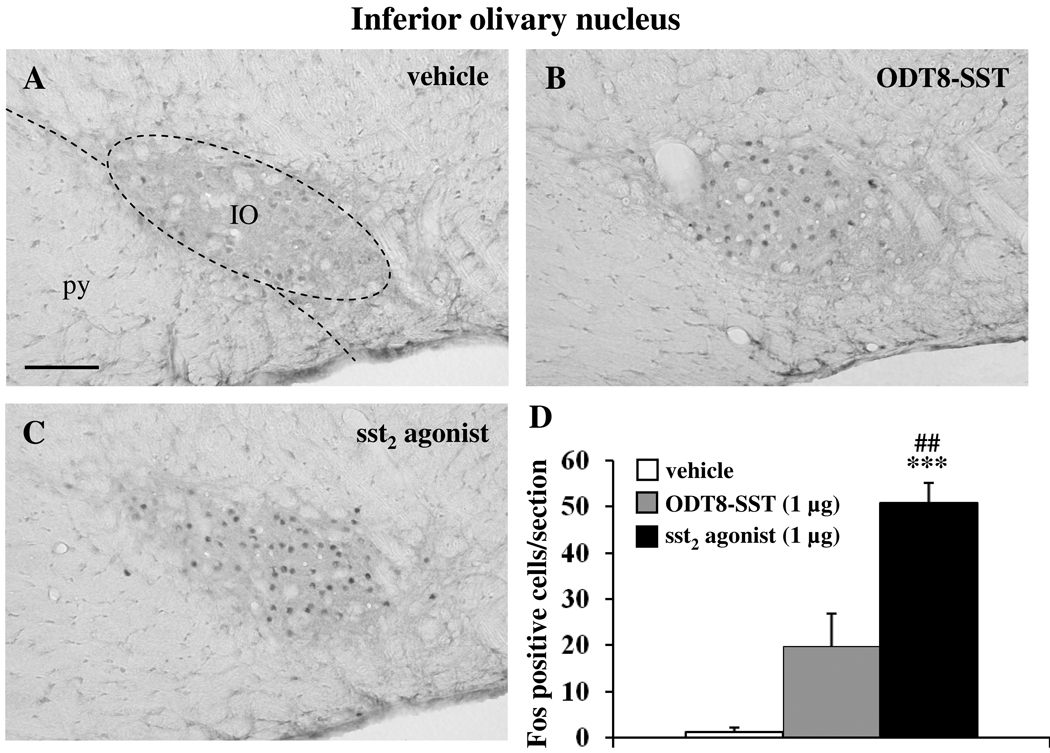
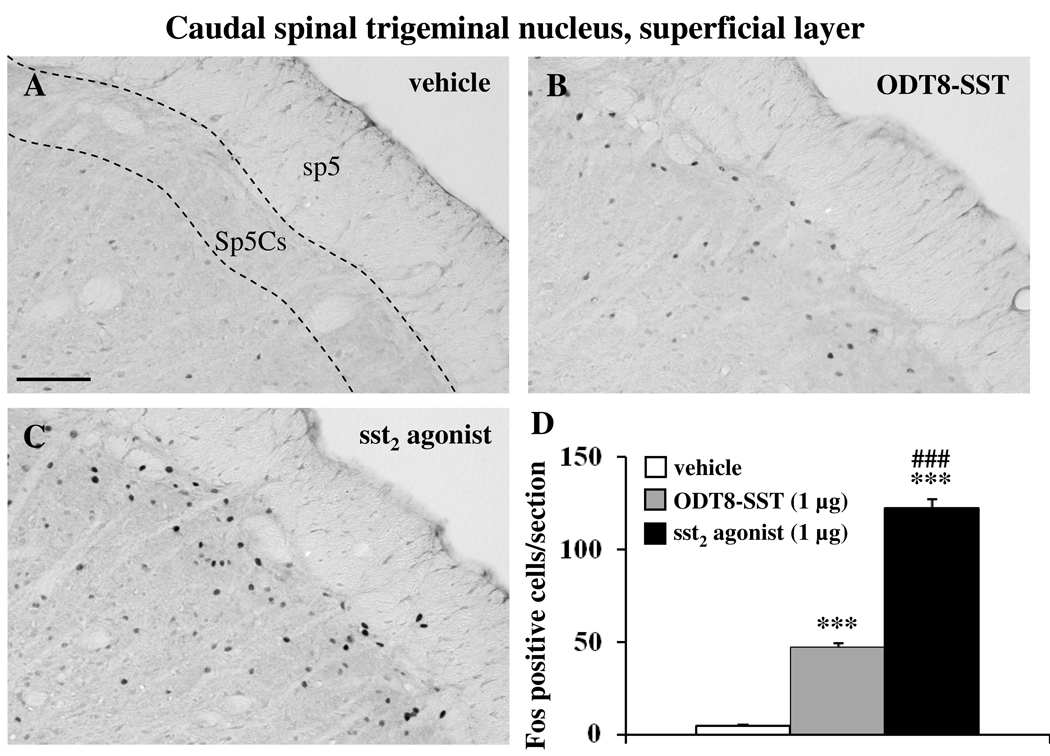
Icv injection of sst2 agonist and ODT8-SST increased the number of Fos-positive neurons compared to control animals in the inferior olivary nucleus (IO, vehicle: 1.3 ± 0.9, sst2 agonist: 50.9 ± 4.4, ODT8-SST: 19.8 ± 7.1; p < 0.001 vs. vehicle; Fig 7) and superficial layer of the caudal spinal trigeminal nucleus (Sp5Cs, vehicle: 4.3 ± 0.8, sst2 agonist: 122.5 ± 5.0, ODT8-SST: 47.1 ± 2.5; p < 0.001 vs. vehicle; Fig 8). In both nuclei, sst2 injection resulted in a significantly 2.6-fold higher number of Fos neurons compared with ODT8-SST icv injection (p < 0.01).
Fig. 7.
Fos immunohistochemical staining in the inferior olivary nucleus (IO) assessed 90 min after icv injection of vehicle (A), ODT8-SST (B) or sst2 agonist (C). The scale bar in (A) represents 100 µm. Cell counts per section (unilateral) in the IO (D) are expressed as mean ± SEM of 5–6 rats/group. *** p < 0.001 compared to vehicle and ## p < 0.01 compared to ODT8-SST. Other abbreviation: py: pyramidal tract.
Fig. 8.
Immunohistochemical staining for Fos in the superficial layer of the caudal part of the spinal trigeminal nucleus (Sp5Cs) in icv vehicle rats (A) and after icv ODT8-SST (B) or sst2 agonist (C). The scale bar in (A) represents 100 µm. Cell counts per section (unilateral) in the Sp5Cs (D) are expressed as mean ± SEM of 5–6 rats/group. *** p < 0.001 compared to vehicle and ### p < 0.001 compared to ODT8-SST. Other abbreviation: sp5: spinal trigeminal tract.
3. Discussion
The newly developed selective peptide sst2 agonist elicited neuronal activation in distinct brain nuclei such as the BLA, SON, mPVN, Arc, LPBE, IO and Sp5Cs. Likewise, the pan-activator of somatostatin receptors, ODT8-SST, induced a similar pattern of Fos activation, however the magnitude of the Fos response in all nuclei was 1.2 to 4.1-fold lower compared to the sst2 agonist and, in addition, the Arc was not activated. Such differences may be related to several factors: (1) ODT8-SST displays lower affinity to the sst2 receptor than the selective sst2 agonist [IC50 binding affinity of sst2 agonist to sst2 receptor: 7.5–20 nM (Grace et al., 2006) compared to 41.0 ± 8.7 nM for ODT8-SST (Erchegyi et al., 2008)]; (2) ODT8-SST also binds to the other four receptor subtypes that may exert an inhibitory effect on sst2-activated neurons; and (3) lower amounts of the ODT8-SST may reach sst2 receptors as the peptide also targets the other four sst receptors which may not lead to Fos induction. These later possibilities are supported by a recent report where intracellular voltage clamp recordings showed that somatostatin depressed excitatory but not inhibitory transmission in hippocampus slices (Tallent and Siggins, 1997). Another in vitro study showed that somatostatin reduced inhibitory presynaptic currents mediated mostly by the sst2 subtype while the somatostatin-induced inhibition of excitatory transmission was mainly mediated by sst1 or sst4 (Momiyama and Zaborszky, 2006). These data are indicative that different sst subtypes are involved in somatostatin-induced effects on inhibitory and excitatory currents.
In the BLA, icv injection of sst2 agonist and ODT8-SST induced Fos immunoreactivity with a response 3.6-fold higher in sst2 compared with ODT8-SST-injected rats. The highest concentrations of sst receptors are found in the basal and basolateral amygdaloid nuclei and in the ventral and dorsal subiculum (Leroux et al., 1993) and high levels of sst2a immunostaining have been reported in nerve terminals of the BLA (Dournaud et al., 1996; Schindler et al., 1997). The BLA has strong implications in anxiety and fear-related behaviors and anxiolytic as well as antidepressant effects have been reported after icv injection of somatostatin (Engin et al., 2008; Engin and Treit, 2009). In particular, icv injection of somatostatin, sst2 or sst3 agonists, unlike other selective agonists, abolished immobility of rats, often observed in the forced swim test, also called ‘behavioral despair’ as a measure for depressive behavior (Engin et al., 2008; Engin and Treit, 2009). Likewise, in the plus-maze test, icv somatostatin and sst2 agonist resulted in increased exploration of the open arms with a magnitude comparable to the anxiolytic effect of 10 µg diazepam while other selective sst1, sst3, sst4 and sst5 agonists had no effect in rats (Engin et al., 2008; Engin and Treit, 2009). The mechanism of the anxiolytic-like effects of somatostatin is most likely GABAergic since pretreatment with bicuculline, a GABAA receptor antagonist, reversed this effect (Engin et al., 2008; Engin and Treit, 2009). Most of the non-pyramidal neurons in the BLA are GABAergic (McDonald and Pearson, 1989) and somatostatin and neuropeptide Y (NPY) are co-localized in this neuronal population (McDonald, 1989; Moore and Black, 1991). It is possible that somatostatin and sst2 agonist produce anxiolytic effects through an interaction with NPY, which also exerts anxiolytic effects upon microinjection into the BLA (Sajdyk et al., 2008) and reduces excitability of neurons projecting from the amygdala (Sosulina et al., 2008). Thus, it may be speculated that the activation of GABAergic and/or NPYergic amygdaloid neurons in the BLA by sst2 agonist or ODT8-SST may underlie the anxiolytic and antidepressive effects observed after icv injection of the peptides (Engin et al., 2008; Engin and Treit, 2009). The effects of microinjection of sst2 agonist directly into the BLA on the open maze and forced swim tests, thereby directly targeting the sst2 receptor in the BLA should be evaluated along with the blockade of NPY or GABAergic neurons by specific antagonists to further corroborate this assumption.
After icv injection of sst2 agonist we found Fos-ir hypothalamic neurons in the Arc and magnocellular system comprised of SON and mPVN, known to play a role in drinking and feeding behaviors (Arora and Anubhuti, 2006; Olszewski et al., 2007; Wang et al., 2002). Activation of Arc neurons has been described under fasting conditions in mice (Coppola et al., 2007; Stengel et al., 2009a; Wang et al., 1998) but also after the first nocturnal eating surge in rats (Comoli et al., 2005). We also found Fos expression in the ventral premammillary nucleus that has ascending projections to the somatostatin-containing neurons in the periventricular nucleus and the lateral area of the Arc (Canteras et al., 1992). Somatostatin derived from the periventricular nucleus (Olias et al., 2004) excites sst2 receptor-bearing NPY neurons in the Arc (Lanneau et al., 2000). This pathway might represent a circuit by which icv sst2 agonist activates Arc neurons in the hypothalamus. In contrast to the sst2 agonist, ODT8-SST failed to activate Arc neurons. The differential activation of Arc neurons by sst2 agonist and ODT8-SST while both peptides injected icv at such a dose increased food intake (Stengel, 2010a; Stengel, 2010b), suggests that their orexigenic effects may be mediated by distinct pathways. Consistent with a lacking Fos expression by ODT8-SST, previous electrophysiological studies showed that the pan-sst receptor agonist, somatostatin, applied to Arc neurons induced a direct postsynaptic inhibition of firing activity in 80% of Arc neurons and none were excited (Mori et al., 2010). Those neurons were mainly neuroendocrine growth hormone releasing hormone-containing cells projecting to the median eminence (Mori 2010) and shown also to express the sst1 receptor (Stroh et al., 2009; Tannenbaum et al., 1998). They were distinct from those in the ventromedial Arc projecting to the PVN and more likely to express the sst2 which is found in the medial segment of the Arc along the wall of the 3rd ventricle (Tannenbaum et al., 1998).
Double labeling focusing on the magnocellular hypothalamic system showed sst2a receptor immunostaining in cell bodies of the mPVN and SON and of these, 56% in the mPVN and 70% in the SON also expressed Fos immunoreactivity induced by ODT8-SST. This supports a potential direct sst2a receptor-mediated action of ODT8-SST on these hypothalamic neurons to induce Fos expression. The other hypothalamic Fos-positive neurons not bearing sst2a receptors may be activated indirectly through projections from sst2a-expressing activated neurons in the mPVN and SON or reflect a combined activation of other sst receptors. The selectivity of the sst2a antibody has previously been established (Sternini et al., 1997). Detailed analysis of the immunostaining in our study showed that a higher number of sst2a-ir cell bodies were present after injection of ODT8-SST in the PVN. This is in line with previous data establishing that the cellular distribution of the sst2a receptor can be stimulated in vivo by icv injection of octreotide, an sst2a agonist, in the hypothalamus (Csaba et al., 2003).
Of interest was the finding that 28–36% of Fos neurons were oxytocin-ir and 55–63% of activated neurons were AVP-ir in the SON and mPVN, respectively, after icv ODT8-SST. These results are in keeping with a recent report showing that icv injection of somatostatin in female rats also increased Fos expression in magnocellular hypothalamic neurons, the firing rate of both oxytocin and vasopressin neurons in the SON as well as plasma oxytocin levels (Meddle et al., 2010). Previous studies also established that icv injection of somatostatin released AVP into the circulation in conscious rats and the augmented release of this peptide was responsible for the major part of the pressor response to icv somatostatin (Brown, 1988; Rettig et al., 1989). Our observation that more than half of the ODT8-SST-activated neurons contain vasopressin provides anatomical support for this endocrine response. It is also possible that the icv ODT8-SST-activated AVP neurons are involved in drinking behavior. Our recent study showed that icv ODT8-SST increased drinking behavior in rats (Stengel, 2010a; Stengel, 2010b). Similarly, another sst agonist, octreotide, injected icv increased water intake in rats together with vasopressin release (Hajdu et al., 2000). Oxytocin is known to inhibit food intake (Douglas et al., 2007; Sabatier et al., 2007; Verbalis et al., 1995). However, this pathway is unlikely to play a role in ODT8-SST-induced food intake alterations. Firstly, icv ODT8-SST increases and not decreases food intake (Stengel, 2010a; Stengel, 2010b). Secondly, the oxytocin inhibitory effect on food intake involves oxytocin neurons in the parvicellular PVN (Douglas et al., 2007) where ODT8-SST does not increase Fos expression. Thirdly, the Fos induction in the SON and mPVN elicited by food intake is associated with ingestion of food and consecutive stomach distension and meal termination while there is no or little increase in Fos expression in the SON and mPVN before a meal (Comoli et al., 2005; Johnstone et al., 2006). In our study, food and water were removed to avoid interfering effects of Fos induction resulting from increased food ingestion and water intake. It could be possible that ODT8-SST-activated oxytocin neurons play a role to counteract some stressful behavior, such as scratching since oxytocin has anxiolytic effect (Blume et al., 2008). However, it cannot be excluded that oxytocin and vasopressin are involved in other central actions of somatostatin (Legros, 2001) including analgesia (Dahaba et al., 2009) as recent evidence showed that oxytocin-induced analgesia is mediated by the vasopressin-1A receptor (Schorscher-Petcu et al., 2010).
We showed Fos expression in the parasubthalamic nucleus (PSTN) that, due to its extensive brain network, is thought to influence and gate viscerosensory and gustatory information related to food processing occurring in both the cephalic and the consummatory phases of feeding behavior (Goto and Swanson, 2004). This is supported by anterograde tracing studies showing that the PSTN sends densely packed fibers to salivatory nuclei innervating head mucous along with salivary glands (Contreras et al., 1980; Hiura, 1977; Nicholson and Severin, 1981) as well as components of the parabrachial nucleus (Goto and Swanson, 2004; Mascaro et al., 2009) that process gustatory (Hamilton and Norgren, 1984) and vagal gastrointestinal (Leslie et al., 1982; Shapiro and Miselis, 1985) information. Thus, the PSTN and the lateral parabrachial nucleus are important relay stations to connect the brainstem with forebrain areas (Cechetto et al., 1985; Feil and Herbert, 1995; Herbert et al., 1990). Both nuclei may be part of the circuitry by which sst agonists exert the strong induction of feeding behavior in rats. The lateral parabrachial nucleus also integrates visceral and somatic signals as a part of nociceptive (Hylden et al., 1989), thermoregulatory (Kobayashi and Osaka, 2003; Morrison et al., 2008) and feeding circuits (Becskei et al., 2007; Wilson et al., 2003). In this study, icv injection of ODT8-SST significantly induced Fos-ir neurons in the LPBE and administration of the sst2 agonist led to a 4-fold stronger increase in activated neurons compared to ODT8-SST. Such an activation might be part of the neuronal network involved in increased body temperature (Brown, 1982; Fisher et al., 1985) and feeding that are induced by icv injection of sst2 agonist or ODT8-SST at such a dose (Stengel, 2010a; Stengel, 2010b).
The IO was activated as shown by Fos induction after icv injection of sst2 agonist and ODT8-SST, to a lesser extent. This may represent the underlying substrata for related hyperthermia observed within 1 hour of ODT8-SST and sst2 agonist injection (Brown, 1982; Fisher et al., 1985; Stengel, 2010a; Stengel, 2010b). Indeed, there is evidence that procaine microinjected into the midbrain elevates body temperature by removing the inhibitory tone on non-shivering thermogenesis and increased Fos expression in IO neurons of rats, while IO lesions completely blocked the observed increase in body temperature (Uno and Shibata, 2001). Other studies established that the IO represents an essential part of the central efferent pathway to thermogenic effectors such as the brown adipose tissue (Uno and Shibata, 2001).
The Sp5Cs expressed 3-times more Fos neurons after injection of sst2 agonist compared to ODT8-SST. Electrical stimulation of trigeminal afferents in humans and animals (Jenkins et al., 2004; Messlinger et al., 1995) causes release of calcitonin-gene related peptide (CGRP), indicating a major role of CGRP in the trigeminal system (Goadsby and Edvinsson, 1993; Zagami et al., 1990). Somatostatin-ir fibers and cells (Alvarez and Priestley, 1990) and somatostatin mRNA (Yin, 1995) are abundant in the superficial laminae of Sp5C, and a moderate density of sst receptors can be detected throughout the caudal portions of Sp5 (Senaris et al., 1994). Furthermore, 80% of sst2a-ir trigeminal ganglionic neurons that innervate Sp5C co-expressed CGRP (Ichikawa et al., 2003). Co-injection of CGRP and somatostatin intrathecally, but not CGRP alone, elicited a biting/scratching response lasting about 20 min (Wiesenfeld-Hallin et al., 1984; Wiesenfeld-Hallin, 1986). Both, icv sst2 agonist and to a lesser extent ODT8-SST induce pronounced grooming behavior including scratching that could be mediated by the release of CGRP in the spinal trigeminal system and is accompanied by robust Fos expression in this area. Treatment with a CGRP antagonist prior to sst2 agonist injection will clarify whether the scratching response could be abolished.
In summary, this study provides evidence of a robust Fos expression in the BLA, SON, mPVN, Arc, LPBE, IO and Sp5c induced by the selective sst2 agonist and the pan sst1–5 agonist ODT8-SST although with a lower magnitude when peptides are injected icv at a similar nanomolar dose. In addition, ODT8-SST activates 28–36% of oxytocin and 55–63% of AVP neurons as well as 56–70% of sst2a-bearing neurons in the magnocellular parts of the hypothalamus. These findings provide neuronal substrata for recently published functional data showing that activation of sst2 receptors at such icv dose enhances feeding, grooming behavior and body temperature in rats and that somatostatin increases circulating levels of oxytocin and AVP and thus give new insight into the central pathways that may underlie these effects.
4. Experimental procedures
4.1. Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA, body weight: 280–350g) were group housed (four animals/cage) under conditions of controlled illumination (12:12 h light/dark cycle, lights on/off: 06.00 h/18.00 h), humidity, and temperature (22 ± 2 °C). Animals were fed with a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water ad libitum. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committees at Veterans Affairs Greater Los Angeles Healthcare System (animal protocol number 99127-07).
4.2. Peptides
ODT8-SST [des-AA1,2,4,5,12,13-[DTrp8]-somatostatin, MW 1078.5, compound #1 in (Erchegyi et al., 2008) and the sst2 agonist, des-AA1,4–6,11–13-[DPhe2,Aph7(Cbm),DTrp8]-Cbm-SRIF-Thr-NH2, MW: 1132.5, 1 µg/rat, compound #3 in (Grace et al., 2006) (Clayton Foundation Laboratories, Salk Institute, La Jolla, CA) were synthesized and purity assessed as previously described (Erchegyi et al., 2008; Grace et al., 2006). Peptides were kept in powder form at −80 °C and dissolved in pyrogen-free distilled water immediately before the experiments.
4.3. Intracerebroventricular cannulation and injection
Intracerebroventricular cannulation was performed as described before (Martinez and Taché, 2001; Stengel et al., 2009b). Rats were anesthetized with an intraperitoneally applied mixture of ketamine hydrochloride (75 mg/kg bw, Ketanest, Fort Dodge Laboratories Inc., Fort Dodge, IA) and xylazine (5 mg/kg, Rompun, Mobay Corporation, Shawnee, KS), placed in a stereotaxic apparatus, and implanted with a chronic guide cannula (22-gauge, Plastic One Products, Roanoke, VA) into the right lateral brain ventricle. Stereotaxic coordinates obtained from the Paxinos and Watson brain atlas (Paxinos, 2007) were 0.8 mm posterior to the bregma, 1.5 mm right lateral, and 3.5 mm ventral. The guide cannula was secured by dental cement and anchored by four stainless steel screws fixed to the skull and occluded. After surgery animals were allowed to recover for 7 days and housed individually. During that time rats were handled for 5 days to become accustomed to icv injection through the guide cannula. For icv injections, a 28-gauge cannula (1 mm longer than the guide cannula) was connected to a 25 µl Hamilton syringe by a PE-50 catheter (Intramedic Polyethylene Tubing, Clay Adams, NJ) filled with freshly prepared injection solution consisting of vehicle (pyrogen-free distilled water), ODT8-SST (1 µg/rat in 10 µl) or the sst2 agonist (1 µg/rat in 10 µl). The volume of 10 µl was delivered by pressure injection over 1 min in lightly hand restrained conscious rats which were afterwards returned to their home cages without access to food and water. Correct icv injection of peptides was ascertained by monitoring the distinct scratching and grooming behaviors that rats display within 15 min (Stengel, 2010a; Stengel, 2010b). Only animals that displayed this behavior were included for transcardial perfusion and subsequent Fos immunohistochemistry. The 1 µg doses selected for the peptide sst agonists were based on our previous dose response studies showing a maximal orexigenic response in rats (Stengel, 2010a; Stengel, 2010b).
4.4. Tissue processing and Fos immunohistochemistry
Ninety min after icv injection rats were deeply anesthetized with sodium pentobarbital (70 mg/kg, intraperitoneally, Nembutal, Abbott Lab.) and transcardially perfused as described before (Goebel et al., 2009; Wang et al., 2000). Briefly, the thoracic cavity was opened and a cannula inserted into the ascending aorta via the left heart ventricle. Perfusion consisted of a quick flush with sodium chloride (0.9% NaCl) and 500 ml of 4% paraformaldehyde, 14% saturated picric acid in 0.1 M phosphate buffer pH 7.2). Brains were removed and post-fixed overnight in the same fixative, rinsed and cryoprotected in 10% sucrose for 24 h and snap-frozen in dry ice-cooled 2-methylbutane. Three sets of coronal sections (25 µm) of the whole brain were cut using a cryostat (Microm International GmbH, Walldorf, Germany). Free floating brain sections were incubated in rabbit anti-c-Fos serum (1:10,000, Oncogene, Cambridge, MA) as primary antibody followed by avidin-biotin-peroxidase complex (ABC, Vector, Vermont, CA) method and visualization by 3,3’-diaminobenzidine tetrachloride (DAB) as previously described (Goebel et al., 2009; Stengel et al., 2010).
The correct location of the cannula into the right lateral ventricle was verified by light microscopy in stained brain sections at the end of the experiment. No animal had to be excluded from data analysis. The experiments were performed in two different batches of rats. Each batch contained all three treatment groups and immunohistochemical processing was performed in parallel for all three treatment groups.
4.5. Double immunohistochemistry for Fos and oxytocin, AVP or sst2a receptor in the PVN and SON in icv ODT8-SST-treated rats
Hypothalamic brain sections of icv vehicle and ODT8-SST (1 µg/rat) were incubated in rabbit anti-c-Fos serum (1:10,000) as described above and visualized by DAB and nickel ammonium sulfate. Thereafter, sections were incubated overnight at 4 °C in either rabbit anti-oxytocin (1:1,000, T-4084, Peninsula Laboratories, San Carlos, CA), rabbit anti-AVP (1:2,000, T-4563, Peninsula Laboratories) (Wang et al., 2009) or rabbit anti-sst2a (1:10,000, CURE # 9452) (Sternini et al., 1997) and visualized by DAB. There is neither cross-reactivity of the anti-Goebel et al: Fos after somatostatin analogs 21 oxytocin antiserum and AVP nor anti-AVP antiserum and oxytocin (technical note of the company). The selectivity of the sst2a antibody raised against the C-terminal 331–340 aa of the sst2a receptor has previously been established (Sternini et al., 1997). This antibody did not label sst2b receptor transfected cells while it gave an immunosignal in sst2a receptor-transfected cells which was confined to the cell surface. In addition, specificity was ascertained by preabsorption with the immunogenic peptide which abolished immunostaining (Sternini et al., 1997). Moreover, the antibody stained the same gastrointestinal tissue structures as another sst2a antibody although with less intensity (Sternini et al., 1997).
4.6. Cell counting
For quantitative assessment, the number of Fos-immunoreactive cells was counted unilaterally (right side). Unilateral cell count was performed based on our previous studies in which we demonstrated no hemispheric differences (Goebel et al., 2009). The density of Fos-ir cells in each nucleus or area was determined in a field of 100 µm × 100 µm using a 10× objective with a grid in the ocular of the microscope in at least five sections, and assigned according to a scale of + to ++++ in which “+” corresponds to approximately 1–4 cells, “++” 5–10, “+++” 10–20 and “++++” more than 20 immunoreactive cells (Goebel, 2009). Coordinates of selected brain nuclei were identified with the Paxinos and Watson's atlas (Paxinos, 2007) and are given in mm from bregma: SON, 12 sections: −0.6 to −1.56; mPVN, 4 sections: −1.44 to −1.8; arcuate nucleus, 10 sections: −1.92 to −2.76; basolateral amygdaloid nucleus, 6 sections: −1.8 to −2.28; lateral parabrachial nucleus, external part, 5 sections: −8.88 to −9.24; superficial layer of the caudal part of the spinal trigeminal nucleus, 10 sections: −14.64 to −13.92; inferior olivary nucleus, 5 sections: −14.28 to −13.92 using light microscopy (Axioscop II, Carl Zeiss, Germany). The average number of single labeled Fos-ir cells/unilateral section for each animal was calculated. Since no consecutive sections were used for the detection, no corrections for double counting were applied. Double immunohistochemical staining for Fos and oxytocin, AVP or sst2a was assessed in the mPVN and SON. Cells with dark blue nuclear staining were Fos-ir and cells with strong brown cytoplasmic staining were oxytocin, AVP or sst2a-ir. The investigator was blinded to the treatment. Images were acquired by a digital camera (Hamamatsu, Bridgewater, NJ) using the image acquisition system SimplePCI (Hamamatsu Corporation, Sewickley, PA).
4.7. Statistical analysis
Data are expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. Differences between groups were considered significant when p < 0.05.
Acknowledgements
This work was supported by German Research Foundation fellowship grants GO 1718/1-1 (M.G.) and STE 1765/1-1 (A.S.), the VA Research Career Scientist Award, NIHDK 33061 (Y.T.) and Center grant DK-41301 (Animal Core, Y.T.). We are grateful to Mrs. Honghui Liang for her excellent technical support and Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez FJ, Priestley JV. Anatomy of somatostatin-immunoreactive fibres and cell bodies in the rat trigeminal subnucleus caudalis. Neuroscience. 1990;38:343–357. doi: 10.1016/0306-4522(90)90033-z. [DOI] [PubMed] [Google Scholar]
- Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity--a review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Barnes AJ, Long RG, Adrian TE, Vale W, Brown MR, Rivier JE, Hanley J, Ghatei MA, Sarson DL, Bloom SR. Effect of a long-acting octapeptide analogue of somatostatin on growth hormone and pancreatic and gastrointestinal hormones in man. Clin Sci (Lond) 1981;61:653–656. doi: 10.1042/cs0610653. [DOI] [PubMed] [Google Scholar]
- Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA. Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Res. 2007;1162:76–84. doi: 10.1016/j.brainres.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Bereiter DA. Morphine and somatostatin analogue reduce c-fos expression in trigeminal subnucleus caudalis produced by corneal stimulation in the rat. Neuroscience. 1997;77:863–874. doi: 10.1016/s0306-4522(96)00541-6. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brown MR. Bombesin and somatostatin related peptides: effects on oxygen consumption. Brain Res. 1982;242:243–246. doi: 10.1016/0006-8993(82)90306-7. [DOI] [PubMed] [Google Scholar]
- Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: role of somatostatins. Endocrinology. 1984;114:1546–1549. doi: 10.1210/endo-114-5-1546. [DOI] [PubMed] [Google Scholar]
- Brown MR. Somatostatin-28 effects on central nervous system regulation of vasopressin secretion and blood pressure. Neuroendocrinology. 1988;47:556–562. doi: 10.1159/000124975. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- Cescato R, Erchegyi J, Waser B, Piccand V, Maecke HR, Rivier JE, Reubi JC. Design and in vitro characterization of highly sst2-selective somatostatin antagonists suitable for radiotargeting. J Med Chem. 2008;51:4030–4037. doi: 10.1021/jm701618q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Black MD, Feniuk W, Humphrey PP. Operational characteristics of somatostatin receptors mediating inhibitory actions on rat locus coeruleus neurones. Br J Pharmacol. 1996;117:1673–1678. doi: 10.1111/j.1476-5381.1996.tb15338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Negrao N, Goto M, Canteras NS. Functional mapping of the prosencephalic systems involved in organizing predatory behavior in rats. Neuroscience. 2005;130:1055–1067. doi: 10.1016/j.neuroscience.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Connor M, Bagley EE, Mitchell VA, Ingram SL, Christie MJ, Humphrey PP, Vaughan CW. Cellular actions of somatostatin on rat periaqueductal grey neurons in vitro. Br J Pharmacol. 2004;142:1273–1280. doi: 10.1038/sj.bjp.0705894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras RJ, Gomez MM, Norgren R. Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol. 1980;190:373–394. doi: 10.1002/cne.901900211. [DOI] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaba Z, Simon A, Helboe L, Epelbaum J, Dournaud P. Targeting sst2A receptor-expressing cells in the rat hypothalamus through in vivo agonist stimulation: neuroanatomical evidence for a major role of this subtype in mediating somatostatin functions. Endocrinology. 2003;144:1564–1573. doi: 10.1210/en.2002-221090. [DOI] [PubMed] [Google Scholar]
- Dahaba AA, Mueller G, Mattiassich G, Rumpold-Seitlinger G, Bornemann H, Rehak PH, Linck G, Mischinger HJ, Metzler H. Effect of somatostatin analogue octreotide on pain relief after major abdominal surgery. Eur J Pain. 2009;13:861–864. doi: 10.1016/j.ejpain.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Danguir J. Food intake in rats is increased by intracerebroventricular infusion of the somatostatin analogue SMS 201–995 and is decreased by somatostatin antiserum. Peptides. 1988;9:211–213. doi: 10.1016/0196-9781(88)90030-7. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–365. doi: 10.1016/j.physbeh.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Dournaud P, Gu YZ, Schonbrunn A, Mazella J, Tannenbaum GS, Beaudet A. Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody. J Neurosci. 1996;16:4468–4478. doi: 10.1523/JNEUROSCI.16-14-04468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Engin E, Stellbrink J, Treit D, Dickson CT. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience. 2008;157:666–676. doi: 10.1016/j.neuroscience.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology (Berl) 2009;206:281–289. doi: 10.1007/s00213-009-1605-5. [DOI] [PubMed] [Google Scholar]
- Erchegyi J, Grace CR, Samant M, Cescato R, Piccand V, Riek R, Reubi JC, Rivier JE. Ring size of somatostatin analogues (ODT-8) modulates receptor selectivity and binding affinity. J Med Chem. 2008;51:2668–2675. doi: 10.1021/jm701444y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker-Fuse nuclei. J Comp Neurol. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- Fisher LA, Cave CR, Brown MR. Central nervous system effects of bombesin on the cardiovascular response to cold exposure. Brain Res. 1985;341:261–268. doi: 10.1016/0006-8993(85)91065-0. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Taché Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–124. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Lambrecht NWG, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–246. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Swanson LW. Axonal projections from the parasubthalamic nucleus. J Comp Neurol. 2004;469:581–607. doi: 10.1002/cne.11036. [DOI] [PubMed] [Google Scholar]
- Grace CR, Erchegyi J, Koerber SC, Reubi JC, Rivier J, Riek R. Novel sst2-selective somatostatin agonists. Three-dimensional consensus structure by NMR. J Med Chem. 2006;49:4487–4496. doi: 10.1021/jm060363v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu I, Obal F, Jr, Gardi J, Laczi F, Krueger JM. Octreotide-induced drinking, vasopressin, and pressure responses: role of central angiotensin and ACh. Am J Physiol Regul Integr Comp Physiol. 2000;279:R271–R277. doi: 10.1152/ajpregu.2000.279.1.R271. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hiura T. Salivatory neurons innervate the submandibular and sublingual glands in the rat: horseradish peroxidase study. Brain Res. 1977;137:145–149. doi: 10.1016/0006-8993(77)91017-4. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE, Reisine T. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Anton F, Nahin RL. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience. 1989;28:27–37. doi: 10.1016/0306-4522(89)90229-7. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Schulz S, Hollt V, Sugimoto T. The somatostatin sst2A receptor in the rat trigeminal ganglion. Neuroscience. 2003;120:807–813. doi: 10.1016/s0306-4522(03)00364-6. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Kotani A, Koshikawa N, Cools AR. Somatostatin receptors in the nucleus accumbens modulate dopamine-dependent but not acetylcholine-dependent turning behaviour of rats. Neuroscience. 2009;159:974–981. doi: 10.1016/j.neuroscience.2009.01.053. [DOI] [PubMed] [Google Scholar]
- Jenkins DW, Langmead CJ, Parsons AA, Strijbos PJ. Regulation of calcitonin gene-related peptide release from rat trigeminal nucleus caudalis slices in vitro. Neurosci Lett. 2004;366:241–244. doi: 10.1016/j.neulet.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–321. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Minami S, Sugihara H, Wakabayashi I. Barrel rotation evoked by intracerebroventricular injection of somatostatin and arginine-vasopressin is accompanied by the induction of c-fos gene expression in the granular cells of rat cerebellum. Brain Res Mol Brain Res. 1993;18:115–120. doi: 10.1016/0169-328x(93)90179-s. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T. Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflugers Arch. 2003;446:760–765. doi: 10.1007/s00424-003-1119-7. [DOI] [PubMed] [Google Scholar]
- Kumar U. Colocalization of somatostatin receptor subtypes (SSTR1-5) with somatostatin, NADPH-diaphorase (NADPH-d), and tyrosine hydroxylase in the rat hypothalamus. J Comp Neurol. 2007;504:185–205. doi: 10.1002/cne.21444. [DOI] [PubMed] [Google Scholar]
- Lanneau C, Viollet C, Faivre-Bauman A, Loudes C, Kordon C, Epelbaum J, Gardette R. Somatostatin receptor subtypes sst1 and sst2 elicit opposite effects on the response to glutamate of mouse hypothalamic neurones: an electrophysiological and single cell RT-PCR study. Eur J Neurosci. 1998;10:204–212. doi: 10.1046/j.1460-9568.1998.00041.x. [DOI] [PubMed] [Google Scholar]
- Lanneau C, Peineau S, Petit F, Epelbaum J, Gardette R. Somatostatin modulation of excitatory synaptic transmission between periventricular and arcuate hypothalamic nuclei in vitro. J Neurophysiol. 2000;84:1464–1474. doi: 10.1152/jn.2000.84.3.1464. [DOI] [PubMed] [Google Scholar]
- Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Leroux P, Gonzalez BJ, Laquerriere A, Bodenant C, Vaudry H. Autoradiographic study of somatostatin receptors in the rat hypothalamus: validation of a GTP-induced desaturation procedure. Neuroendocrinology. 1988;47:533–544. doi: 10.1159/000124966. [DOI] [PubMed] [Google Scholar]
- Leroux P, Weissmann D, Pujol JF, Vaudry H. Quantitative autoradiography of somatostatin receptors in the rat limbic system. J Comp Neurol. 1993;331:389–401. doi: 10.1002/cne.903310308. [DOI] [PubMed] [Google Scholar]
- Leroux P, Bucharles C, Bologna E, Vaudry H. des-AA-1,2,5[D-Trp8, IAmp9]somatostatin-14 allows the identification of native rat somatostatin sst1 receptor subtype. Eur J Pharmacol. 1997;337:333–336. doi: 10.1016/s0014-2999(97)01282-x. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Gwyn DG, Hopkins DA. The central distribution of the cervical vagus nerve and gastric afferent and efferent projections in the rat. Brain Res Bull. 1982;8:37–43. doi: 10.1016/0361-9230(82)90025-9. [DOI] [PubMed] [Google Scholar]
- Martinez V, Rivier J, Coy D, Taché Y. Intracisternal injection of somatostatin receptor 5-preferring agonists induces a vagal cholinergic stimulation of gastric emptying in rats. J Pharmacol Exp Ther. 2000;293:1099–1105. [PubMed] [Google Scholar]
- Martinez V, Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29–35. doi: 10.1016/s0006-8993(00)03277-7. [DOI] [PubMed] [Google Scholar]
- Mascaro MB, Prosdocimi FC, Bittencourt JC, Elias CF. Forebrain projections to brainstem nuclei involved in the control of mandibular movements in rats. Eur J Oral Sci. 2009;117:676–684. doi: 10.1111/j.1600-0722.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bull PM, Leng G, Russell JA, Ludwig M. Somatostatin actions on rat supraoptic nucleus oxytocin and vasopressin neurones. J Neuroendocrinol. 2010;22:438–445. doi: 10.1111/j.1365-2826.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Hanesch U, Kurosawa M, Pawlak M, Schmidt RF. Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can J Physiol Pharmacol. 1995;73:1020–1024. doi: 10.1139/y95-143. [DOI] [PubMed] [Google Scholar]
- Moller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta. 2003;1616:1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Momiyama T, Zaborszky L. Somatostatin presynaptically inhibits both GABA and glutamate release onto rat basal forebrain cholinergic neurons. J Neurophysiol. 2006;96:686–694. doi: 10.1152/jn.00507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MR, Black PM. Neuropeptides. Neurosurg Rev. 1991;14:97–110. doi: 10.1007/BF00313031. [DOI] [PubMed] [Google Scholar]
- Mori K, Kim J, Sasaki K. Electrophysiological effect of ghrelin and somatostatin on rat hypothalamic arcuate neurons in vitro. Peptides. 2010;31:1139–1145. doi: 10.1016/j.peptides.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JE, Severin CM. The superior and inferior salivatory nuclei in the rat. Neurosci Lett. 1981;21:149–154. doi: 10.1016/0304-3940(81)90373-6. [DOI] [PubMed] [Google Scholar]
- Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Bomberg EM, Martell A, Grace MK, Levine AS. Intraventricular ghrelin activates oxytocin neurons: implications in feeding behavior. Neuroreport. 2007;18:499–503. doi: 10.1097/WNR.0b013e328058684e. [DOI] [PubMed] [Google Scholar]
- Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Vol. San Diego: Academic Press; 2007. [Google Scholar]
- Rettig R, Geist R, Sauer U, Rohmeiss P, Unger T. Central effects of somatostatin: pressor response, AVP release, and sympathoinhibition. Am J Physiol. 1989;257:R588–R594. doi: 10.1152/ajpregu.1989.257.3.R588. [DOI] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Rowe I, Leng G. Central release of oxytocin and the ventromedial hypothalamus. Biochem Soc Trans. 2007;35:1247–1251. doi: 10.1042/BST0351247. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Botto JM, Vincent JP, Mazella J, Beaudet A. Preferential expression of sst2A over sst2B somatostatin receptor splice variant in rat brain and pituitary. Neuroendocrinology. 1998;68:37–43. doi: 10.1159/000054348. [DOI] [PubMed] [Google Scholar]
- Schindler M, Sellers LA, Humphrey PP, Emson PC. Immunohistochemical localization of the somatostatin SST2(A) receptor in the rat brain and spinal cord. Neuroscience. 1997;76:225–240. doi: 10.1016/s0306-4522(96)00388-0. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-Induced Analgesia and Scratching Are Mediated by the Vasopressin-1A Receptor in the Mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Handel M, Schreff M, Schmidt H, Hollt V. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J Physiol Paris. 2000;94:259–264. doi: 10.1016/s0928-4257(00)00212-6. [DOI] [PubMed] [Google Scholar]
- Selmer I, Schindler M, Allen JP, Humphrey PP, Emson PC. Advances in understanding neuronal somatostatin receptors. Regul Pept. 2000;90:1–18. doi: 10.1016/s0167-0115(00)00108-7. [DOI] [PubMed] [Google Scholar]
- Senaris RM, Humphrey PP, Emson PC. Distribution of somatostatin receptors 1, 2 and 3 mRNA in rat brain and pituitary. Eur J Neurosci. 1994;6:1883–1896. doi: 10.1111/j.1460-9568.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Schwesig G, Seifert G, Pape HC. Neuropeptide Y activates a G-protein-coupled inwardly rectifying potassium current and dampens excitability in the lateral amygdala. Mol Cell Neurosci. 2008;39:491–498. doi: 10.1016/j.mcn.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Million M, Stenzel-Poore MP, Kobelt P, Mönnikes H, Taché Y, Wang L. CRF over-expressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology. 2009a;150:153–160. doi: 10.1210/en.2008-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NW, Taché Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009b;150:4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides. 2010;31:263–270. doi: 10.1016/j.peptides.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog, ODT8-SST induces a somatostatin2 receptor mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010a doi: 10.1210/en.2010-0195. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Selective central activation of somatostatin2 receptor increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol. 2010b in press. [PMC free article] [PubMed] [Google Scholar]
- Stepanyan Z, Kocharyan A, Pyrski M, Hubschle T, Watson AM, Schulz S, Meyerhof W. Leptin-target neurones of the rat hypothalamus express somatostatin receptors. J Neuroendocrinol. 2003;15:822–830. doi: 10.1046/j.1365-2826.2003.01077.x. [DOI] [PubMed] [Google Scholar]
- Sternini C, Wong H, Wu SV, de Giorgio R, Yang M, Reeve J, Jr, Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol. 1997;386:396–408. [PubMed] [Google Scholar]
- Stroh T, Kreienkamp HJ, Beaudet A. Immunohistochemical distribution of the somatostatin receptor subtype 5 in the adult rat brain: predominant expression in the basal forebrain. J Comp Neurol. 1999;412:69–82. doi: 10.1002/(sici)1096-9861(19990913)412:1<69::aid-cne5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Stroh T, van Schouwenburg MR, Beaudet A, Tannenbaum GS. Subcellular dynamics of somatostatin receptor subtype 1 in the rat arcuate nucleus: receptor localization and synaptic connectivity vary in parallel with the ultradian rhythm of growth hormone secretion. J Neurosci. 2009;29:8198–8205. doi: 10.1523/JNEUROSCI.0336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y, Rivier J, Vale W, Brown M. Is somatostatin or a somatostatin-like peptide involved in central nervous system control of gastric secretion? Regul Pept. 1981;1:307–315. doi: 10.1016/0167-0115(81)90054-9. [DOI] [PubMed] [Google Scholar]
- Tallent MK, Siggins GR. Somatostatin depresses excitatory but not inhibitory neurotransmission in rat CA1 hippocampus. J Neurophysiol. 1997;78:3008–3018. doi: 10.1152/jn.1997.78.6.3008. [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Zhang WH, Lapointe M, Zeitler P, Beaudet A. Growth hormone-releasing hormone neurons in the arcuate nucleus express both Sst1 and Sst2 somatostatin receptor genes. Endocrinology. 1998;139:1450–1453. doi: 10.1210/endo.139.3.5977. [DOI] [PubMed] [Google Scholar]
- Uno T, Shibata M. Role of inferior olive and thoracic IML neurons in nonshivering thermogenesis in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R536–R546. doi: 10.1152/ajpregu.2001.280.2.R536. [DOI] [PubMed] [Google Scholar]
- Vanetti M, Kouba M, Wang X, Vogt G, Hollt V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B) FEBS Lett. 1992;311:290–294. doi: 10.1016/0014-5793(92)81122-3. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM. Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv Exp Med Biol. 1995;395:209–225. [PubMed] [Google Scholar]
- Wang L, Martinez V, Barrachina MD, Taché Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 1998;791:157–166. doi: 10.1016/s0006-8993(98)00091-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Vale W, Taché Y. Fos induction in selective hypothalamic neuroendocrine and medullary nuclei by intravenous injection of urocortin and corticotropin-releasing factor in rats. Brain Res. 2000;855:47–57. doi: 10.1016/s0006-8993(99)02200-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Larauche M, Taché Y. Proximal colon distension induces Fos expression in oxytocin-, vasopressin-, CRF- and catecholamines-containing neurons in rat brain. Brain Res. 2009;1247:79–91. doi: 10.1016/j.brainres.2008.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Hokfelt T, Lundberg JM, Forssmann WG, Reinecke M, Tschopp FA, Fischer JA. Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett. 1984;52:199–204. doi: 10.1016/0304-3940(84)90374-4. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Somatostatin and calcitonin gene-related peptide synergistically modulate spinal sensory and reflex mechanisms in the rat: behavioral and electrophysiological studies. Neurosci Lett. 1986;67:319–323. doi: 10.1016/0304-3940(86)90329-0. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for muopioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–R1065. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- Yin KJ. Distribution of somatostatin mRNA containing neurons in the primary pain relaying nuclei of the rat. Anat Rec. 1995;241:579–584. doi: 10.1002/ar.1092410415. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Raybould H, Taché Y. Central action of somatostatin analog, SMS 201–995, to stimulate gastric acid secretion in rats. Peptides. 1991;12:401–406. doi: 10.1016/0196-9781(91)90076-2. [DOI] [PubMed] [Google Scholar]
- Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990;16:69–75. doi: 10.1016/0143-4179(90)90114-e. [DOI] [PubMed] [Google Scholar]