Abstract
Background
Rituximab inhibited structural damage at 1 year in patients with rheumatoid arthritis (RA) who had had a previous inadequate response to tumour necrosis factor (TNF) inhibitors.
Objective
To assess structural damage progression through 2 years.
Methods
Intention-to-treat patients with one post-baseline radiograph (rituximab n=281; placebo n=187) received background methotrexate (MTX) and were randomised to rituximab (2×1000 mg infusions, 2 weeks apart) or placebo; patients were eligible for rituximab re-treatment every 6 months. By week 104, 82% of the placebo population had received ≥1 dose of rituximab. Radiographic end points included the change in total Sharp score (TSS), erosion and joint space narrowing scores at week 104.
Results
At week 104, significantly lower changes in TSS (1.14 vs 2.81; p<0.0001), erosion score (0.72 vs 1.80; p<0.0001) and joint space narrowing scores (0.42 vs 1.00; p<0.0009) were observed with rituximab plus MTX vs placebo plus MTX. Within the rituximab group, 87% who had no progression of joint damage at 1 year remained non-progressive at 2 years.
Conclusions
Rituximab plus MTX demonstrated significant and sustained effects on joint damage progression in patients with RA and a previously inadequate response to TNF inhibitors.
Introduction
Before the development of targeted biological treatments, irreversible joint damage and deformity leading to a progressive decline in functional status and increased work disability were common outcomes for patients with rheumatoid arthritis (RA).1 2 Biological treatments that inhibit tumour necrosis factor α (TNFα), T-cell costimulation or interleukin 6 have demonstrated the ability to inhibit radiographic progression in patients with either early or longstanding disease.3–8
Rituximab, a monoclonal antibody that selectively targets CD20-positive B cells, reduces the signs and symptoms of RA and has been proved to inhibit joint damage progression over 1 year in patients with RA for whom TNF inhibitors produced an inadequate response.9 10 Here we report the sustained effects of rituximab on the progression of joint damage over an extended period of 2 years.
Patients and methods
Patients
Patients in this post hoc analysis were participants in the phase III REFLEX study.9 Eligibility criteria for REFLEX have been described previously.9 Briefly, patients were included if they had active RA despite treatment with methotrexate (MTX) ≥10 mg/week and had experienced an inadequate response (lack of efficacy or intolerance) to at least one TNF inhibitor.
The study was performed in accordance with the Declaration of Helsinki. All participating sites received approval from their governing institutional review board (or equivalent) and all patients provided written informed consent.
Study protocol
REFLEX was a randomised, double-blind, placebo-controlled, phase III study with an option for further treatment courses under a separate extension study. Patients continued background MTX and were randomly assigned to placebo or rituximab (MabThera, Roche, Welwyn Garden City, UK; Rituxan, Genentech, South San Francisco, California, USA and Biogen Idec, San Diego, California, USA). Rituximab 1000 mg was administered by intravenous infusion on days 1 and 15. All patients received corticosteroid treatment, consisting of intravenous methylprednisolone 100 mg before each infusion and oral prednisone during the 2-week treatment period (60 mg on days 2−7, 30 mg on days 8−14).
From weeks 16 to 24, patients who failed to respond to treatment could receive rescue therapy. Patients randomised to placebo could receive rituximab and patients randomised to rituximab could receive standard care. Patients completing week 24 were eligible to receive further courses of rituximab within an open-label extension study. Further courses of rituximab were also available for placebo patients who had responded to rescue treatment.
Radiographs of hands, wrists (posterior/anterior) and feet (anterior/posterior) were performed at screening (baseline) and at weeks 24, 56 and 104, relative to randomisation. Radiographs were read at a central reading facility by two independent expert radiologists and scored using the Genant-modified Sharp scoring system.11 12 Radiologists were blinded to the treatment group assignment, chronological order of the radiographs and patients' clinical response.
Radiographic outcome measures
Radiographic assessments included the mean change in total Genant-modified Sharp score (mTSS), the erosion score, the joint space narrowing score and the proportion of patients with no further joint damage progression (defined as a change in mTSS ≤0). All assessments compared baseline and week 104. Radiographic changes were also determined during discrete time intervals of baseline to 24 weeks, 24–56 weeks and 56–104 weeks. The annualised progression rate (APR) was calculated to provide a measure of the rate of change in progression standardised to a common time interval. The APR for each patient was calculated as follows:
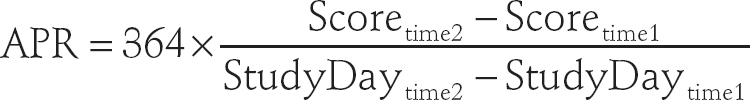
|
Statistical analysis
The primary population for the radiographic analysis was defined as all patients (including those withdrawing or receiving rescue) included in the REFLEX intention-to-treat (ITT) population who had at least one post-baseline radiograph (either 24, 56 or 104 weeks). All missing data were imputed using linear extrapolation of the progression observed from baseline to the week 24/week 56 radiographs. Analyses were conducted using a non-parametric analysis (Van Elteren test), stratified by region (USA vs non-USA) and baseline rheumatoid factor (positive vs negative). In addition, sensitivity analyses were conducted using observed data only.
Results
Patient characteristics and disposition
A total of 517 patients were randomised: 308 to rituximab plus MTX and 209 to placebo plus MTX. Of these, 468 patients (281 rituximab patients and 187 placebo patients) were included in the REFLEX ITT population, and had a baseline film at screening and at least one post-baseline radiograph. A total of 197 rituximab and 135 placebo patients had radiographs at both baseline and week 104. The baseline characteristics and measures of disease activity were similar in both treatment groups and were similar to those of the original ITT population (table 1).
Table 1.
Baseline demographic characteristics of the patients*
| Characteristics | Placebo plus MTX (n=187) | Rituximab plus MTX (n=281) |
|---|---|---|
| Female/male (n (%)) | 150 (80)/37 (20) | 228 (81)/53 (19) |
| Age (years) | 52.9 (12.1) | 52.5 (12.2) |
| Disease duration (years) | 11.7 (7.7) | 11.9 (8.2) |
| Swollen joint count | 23.1 (12.8) | 23.2 (11.9) |
| Tender joint count | 33.2 (15.7) | 33.2 (15.1) |
| CRP (mg/dl) | 3.7 (3.8) | 3.7 (3.9) |
| Anti-CCP positive (n (%)) | 82 (44) | 130 (46) |
| ESR (mm/h) | 48.7 (26.5) | 47.8 (25.6) |
| HAQ-DI score | 1.9 (0.54) | 1.8 (0.57) |
| Total Genant–modified Sharp score | 32.5 (31.5) | 30.6 (26.7) |
Except where indicated otherwise values are the mean (SD).
CCP, cyclic citrullinated peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate.
By week 104, 165/281 patients (59%) in the rituximab group had received two or more courses of rituximab. Of the 187 patients randomised to placebo, 154 (82%) had received at least one dose of rituximab before their last observed radiograph, with 94 (50%) having received two or more courses. Only 33 patients (18%) initially randomised to placebo did not receive rituximab treatment.
Radiographic efficacy
The mean change in the mTSS from baseline to 104 weeks was significantly lower in the rituximab group than in the placebo group (1.14 vs 2.81, respectively; p<0.0001). Significant differences in the mean change in erosion and joint space narrowing scores were also observed (figure 1).
Figure 1.
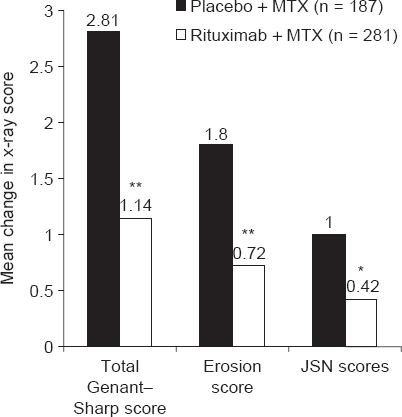
Changes from baseline to 2 years in total Genant–modified Sharp, erosion and joint space narrowing (JSN) scores in patients treated with rituximab (2 × 1000 mg) plus methotrexate (MTX) or placebo plus MTX *p<0.005; **p<0.0001 versus placebo plus MTX.
The proportion of patients with no progression in joint damage over 2 years was significantly higher in the rituximab group than in the placebo group (57% vs 39%, respectively; p<0.0001; figure 2A). Similarly, a higher proportion of patients randomised to rituximab had no change in erosion scores over the 2 years compared with patients randomised to placebo (60% vs 44%, respectively; p=0.0003; figure 2B). Sensitivity analyses using observed data were consistent with the primary analyses.
Figure 2.
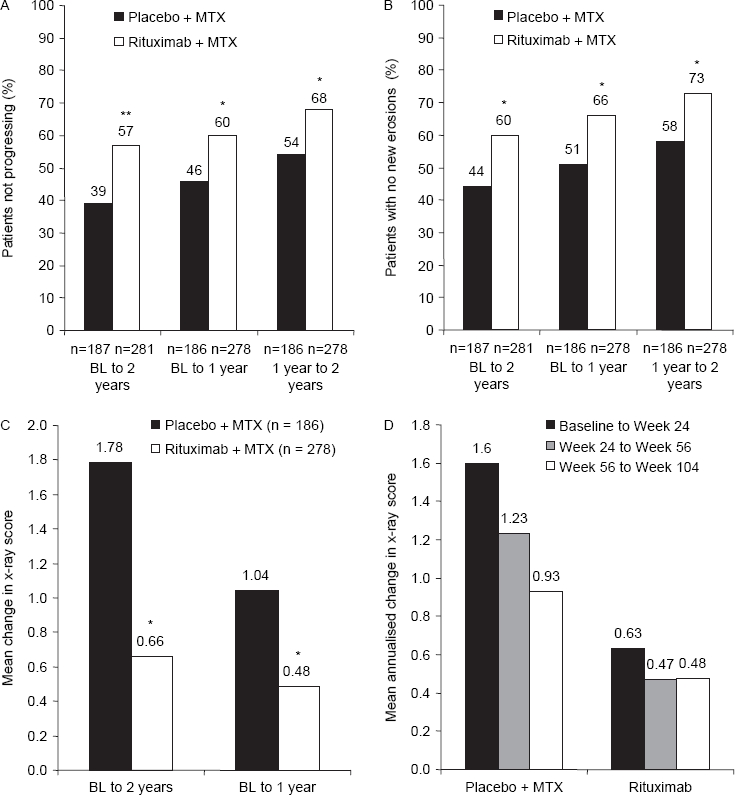
(A) Proportion of patients not progressing over the duration of the study. (B) Proportion of patients with no new erosions over the duration of the study. (C) Treatment effect of placebo plus MTX and rituximab (2 × 1000 mg) plus MTX on total Genant–modified Sharp score. (D) Annualised rate of progression in total Genant–modified Sharp score. BL, baseline; MTX, methotrexate *p<0.005; **p<0.0001.
Over discrete time intervals, the rate and extent of progression of joint damage in patients randomised to rituximab remained consistent. The rituximab group exhibited consistent mean changes in the mTSS during the first and second years, while the placebo group—most of whom had by then received rituximab—showed slower rates of change during their second year (figure 2C). Similarly, whereas in the rituximab group the APR remained consistent, in the placebo group it gradually declined from 1.60 points/year during the initial 24-week period to 0.93 points/year in the second year (figure 2D).
The proportion of patients with no change in their mTSS (figure 2A) and with no new erosions increased during each time period in each treatment group (figure 2B). Of those patients randomised to rituximab who did not progress during the first year, 87% did not progress during the second year either.
Discussion
Inhibition of structural joint damage by rituximab in patients with with RA and a previous inadequate response to TNF inhibitors was first described over a 1-year period.10 Here we have demonstrated that the initial effects of rituximab are maintained over an extended interval of 2 years, with all measures of joint damage significantly improved compared with placebo plus MTX.
Treatment with rituximab was associated with a significantly higher proportion of patients with no progression of joint damage over the 2 years compared with placebo plus MTX. The proportion of patients with no progression (57%) achieved with rituximab treatment compares well with that seen with other treatments with biological agents, albeit in less treatment-refractory patient populations.6 13 14 Importantly, of those rituximab patients with no progression in the first year, 87% maintained a non-progressive status during the second year.
Although patients were initially randomised to either rituximab or placebo, 82% of placebo patients had switched to rituximab by 2 years. The impact of this switch on the progression of joint damage in this placebo–rituximab group is evident by the reduced changes in scores between time periods and the gradual slowing in their APR. The consequence of this switch to active treatment is that the degree of progression observed in the ‘placebo’ group is less than would have been observed had those patients been maintained solely on MTX, thereby underestimating the relative treatment effect. The extent of this discrepancy can be estimated using the method devised by Strand and Sharp15 for estimating APRs. By dividing the baseline mean mTSS (32.5) by the mean disease duration (11.7), the predicted progression for the placebo group over 2 years was 5.55. However, the observed progression was much lower (2.81), suggesting that the switch to rituximab had a large influence on the progression of joint damage in this control group. Consequently, the relative treatment effect size cannot be accurately measured. Nevertheless, using the predicted and observed progression in the placebo group a reduction in joint damage of 59–79% for rituximab plus MTX compared with placebo plus MTX could be estimated. Given the estimated nature of this effect, together with the lack of available radiographic data in similar patient populations, comparisons of this effect size with other biological agents used for RA would not be appropriate.
In conclusion, this 2-year analysis demonstrates that rituximab plus MTX has significant and sustained effects on the inhibition of joint damage in a population of patients with active RA who had previously experienced an inadequate response to TNF inhibitors.
Acknowledgments
Writing assistance was provided by Claire Snowball (Adelphi Communications Ltd) in consultation with the authors, Roche Products Ltd and Genentech.
Footnotes
Funding: This study was sponsored by F Hoffmann-La Roche Ltd, Genentech, Inc and Biogen Idec, Inc. A portion of this work (Stanford University) was supported in part by a grant from the National Institutes of Health National Center for Research Resources (5 M01 RR000070).
Competing interests: SC has received consulting and speaker fees and research grants from Genentech and Biogen Idec. PE and PPT have received consulting and speaker fees and research grants from Roche. EK has received consulting and speaker fees from Roche and Genentech and research grants from Roche. MCG has received speaker fees and research grant support from Roche and has served as a consultant for Roche, Biogen Idec and Genentech. DH and MWC are employees of Biogen Idec. TS is an employee and owns shares in Roche Products Ltd. CP has received consulting and speaker fees from Genentech and Biogen Idec and is an employee of Synarc Inc.
Ethics approval: This study was conducted with the approval of the protocol for this study and any accompanying material provided to the patient (eg, patient information sheets and descriptions of the study used to obtain informed consent) were submitted by the investigator to the associated independent ethics committee (IEC) or institutional review board (IRB). Approval from the committee was obtained before starting the study, and was documented in a letter to the investigator specifying the date on which the committee met and granted the approval. Any modifications made to the protocol after receipt of the IEC/IRB approval were also to be submitted by the investigator to the committee in accordance with local procedures and regulatory requirements.
References
- 1.Pincus T, Callahan LF. The ‘side effects’ of rheumatoid arthritis: joint destruction, disability and early mortality. Br J Rheumatol 1993;32(Suppl 1):28–37 [PubMed] [Google Scholar]
- 2.Pincus T, Callahan LF, Sale WG, et al. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum 1984;27:864–72 [DOI] [PubMed] [Google Scholar]
- 3.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37 [DOI] [PubMed] [Google Scholar]
- 4.St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432–43 [DOI] [PubMed] [Google Scholar]
- 5.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81 [DOI] [PubMed] [Google Scholar]
- 6.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11 [DOI] [PubMed] [Google Scholar]
- 7.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 2007;66:1162–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 2006;144:865–76 [DOI] [PubMed] [Google Scholar]
- 9.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793–806 [DOI] [PubMed] [Google Scholar]
- 10.Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis 2009;68:216–21 [DOI] [PubMed] [Google Scholar]
- 11.Genant HK. Methods of assessing radiographic change in rheumatoid arthritis. Am J Med 1983;75(6A):35–47 [DOI] [PubMed] [Google Scholar]
- 12.Genant HK, Jiang Y, Peterfy C, et al. Assessment of rheumatoid arthritis using a modified scoring method on digitized and original radiographs. Arthritis Rheum 1998;41:1583–90 [DOI] [PubMed] [Google Scholar]
- 13.Genant HK, Peterfy CG, Westhovens R, et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann Rheum Dis 2008;67:1084–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genovese MC, Bathon JM, Fleischmann RM, et al. Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol 2005;32:1232–42 [PubMed] [Google Scholar]
- 15.Strand V, Sharp JT. Radiographic data from recent randomized controlled trials in rheumatoid arthritis: what have we learned? Arthritis Rheum 2003;48:21–34 [DOI] [PubMed] [Google Scholar]


