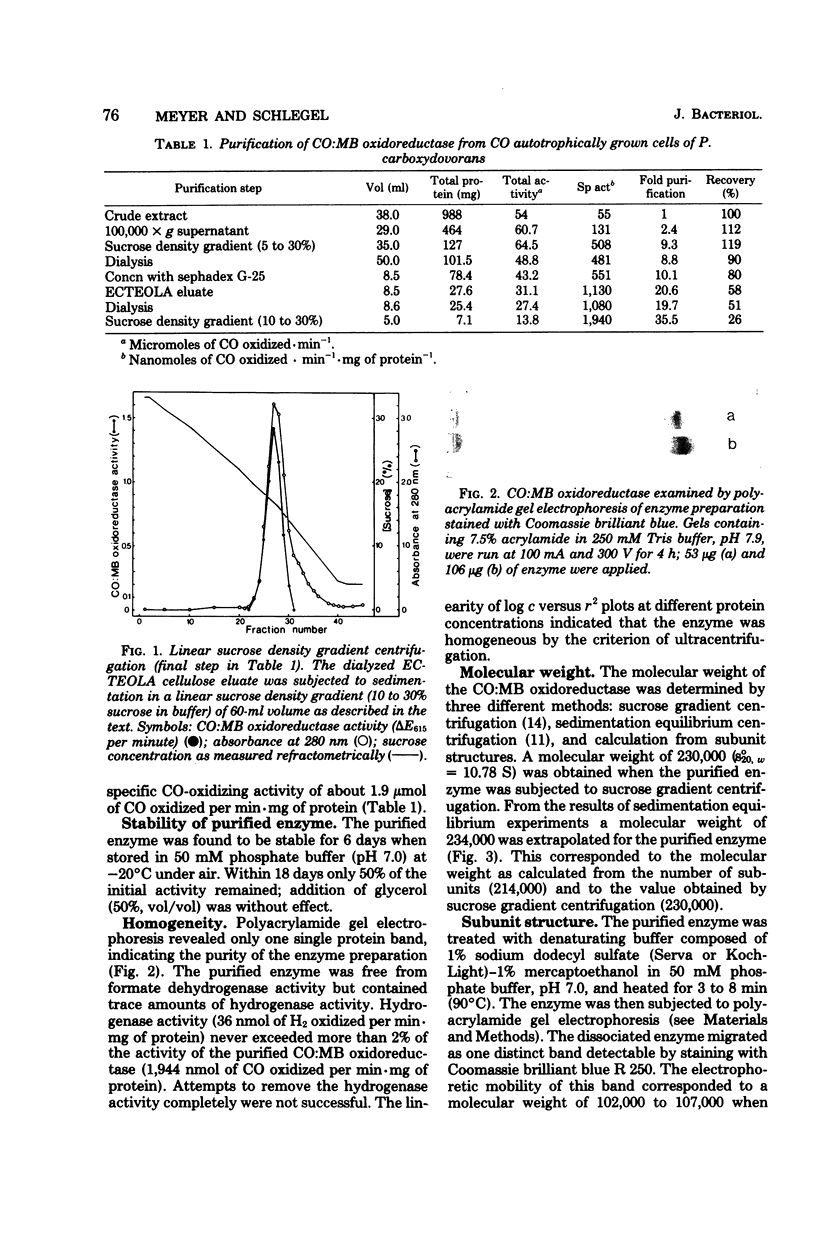
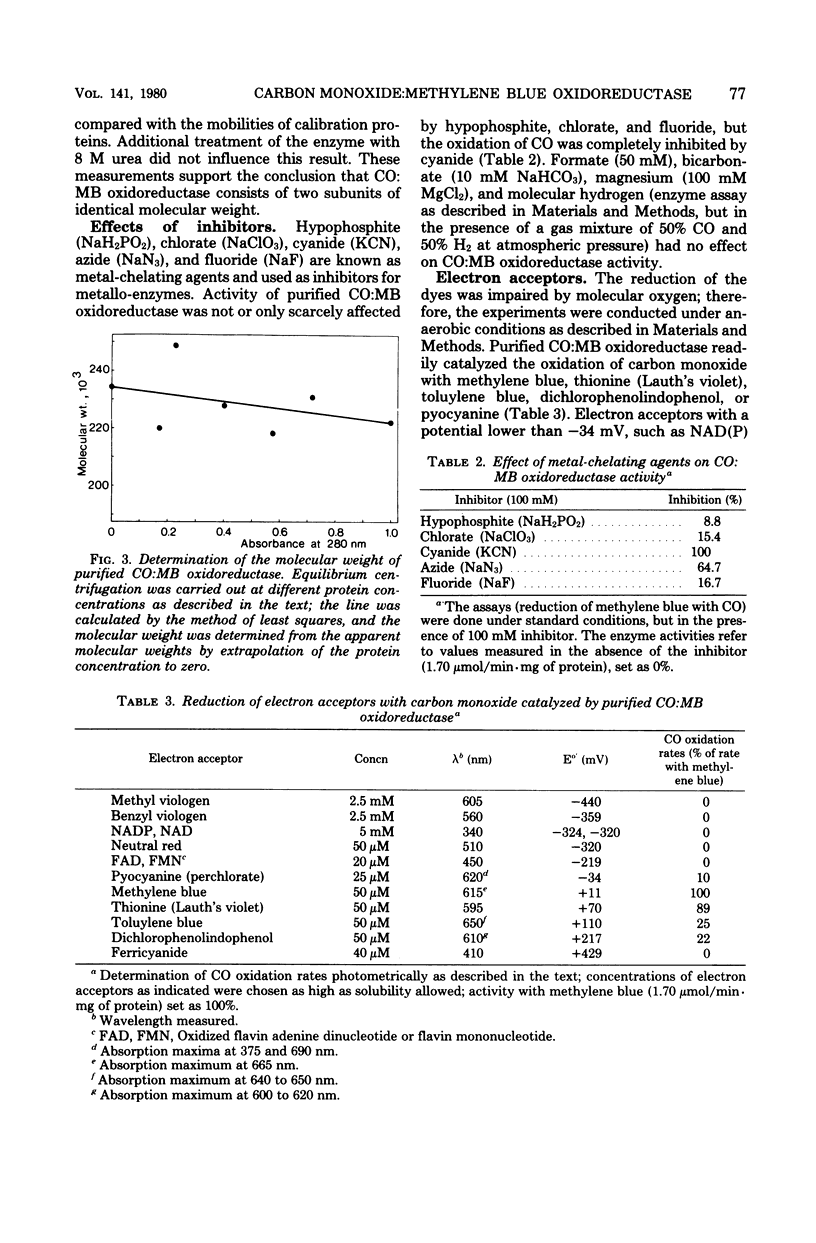
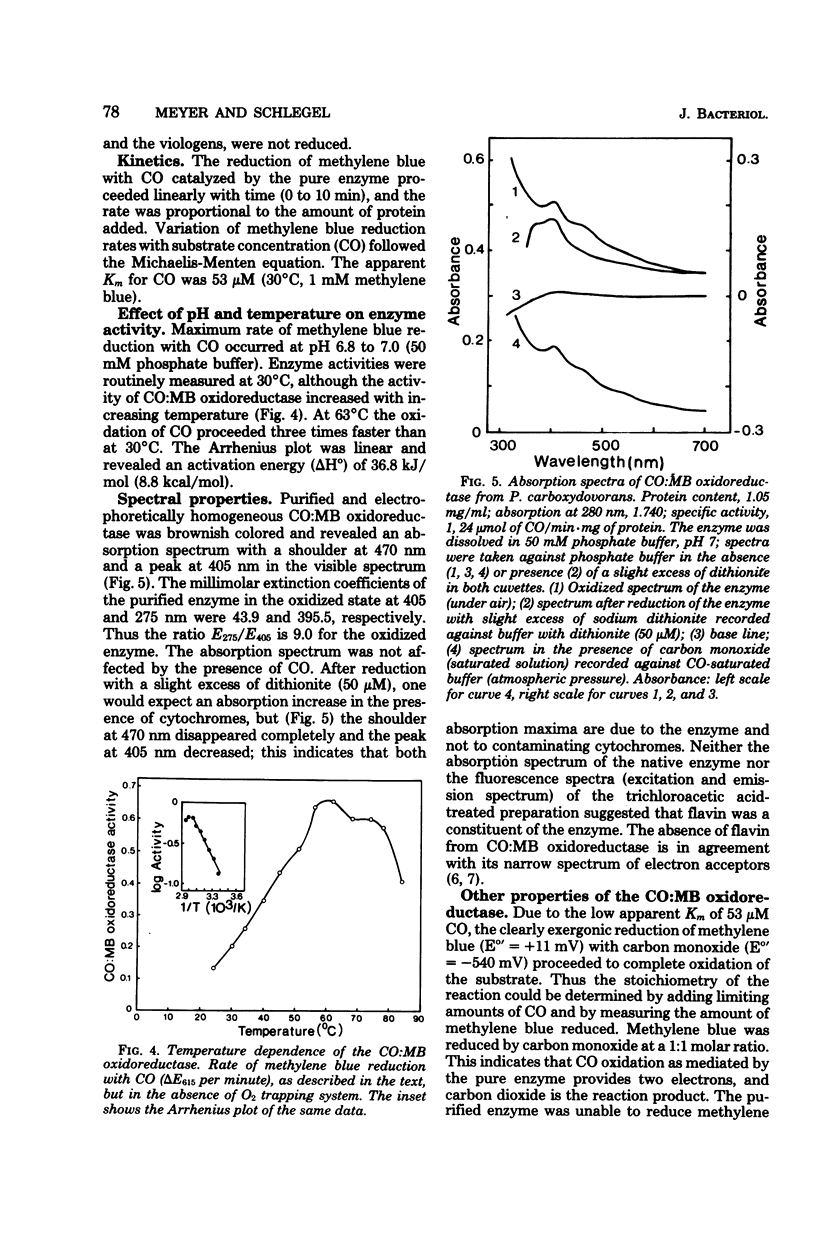
Abstract
The enzyme carbon monoxide:methylene blue oxidoreductase from CO autotrophically grown cells of Pseudomonas carboxydovorans strain OM5, was purified to homogeneity. The enzyme was obtained in 26% yield and was purified 36-fold. The enzyme was stable for at least 6 days, had a molecular weight of 230,000, gave a single protein and activity band on polyacrylamide gel electrophoresis, and was homogeneous by the criterion of sedimentation equilibrium. Sodium dodecyl sulfate gel electrophoresis revealed a single band of molecular weight 107,000. Carbon monoxide:methylene blue oxidoreductase did not catalyze reduction of pyridine or flavin nucleotides but catalyzed the oxidation of CO to CO2 in the presence of methylene blue, thionine, toluylene blue, dichlorophenolindophenol, or pyocyanine under strictly anaerobic conditions. The visible spectrum revealed maxima at 405 and 470 nm. The millimolar extinction coefficients were 43.9 (405 nm) and 395.5 (275 nm), respectively. Absorption at 470 nm decreased in the presence of dithionite, and the spectrum was not affected by the substrate CO. Maximum reaction rates were found at pH 7.0 and 63 degrees C; temperature dependence followed the Arrhenius equation, with an activation energy (delta H degree) of 36.8 kJ/mol (8.8 kcal/mol). The apparent Km was 53 microM for CO. The purified enzyme was incapable of oxidizing methane, methanol, or formaldehyde in the presence of methylene blue as electron acceptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. 3. Flavoproteins. Biochim Biophys Acta. 1971 Mar 2;226(2):269–284. doi: 10.1016/0005-2728(71)90094-6. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. I. Techniques for anaerobic spectrophotometry. Biochim Biophys Acta. 1971 Mar 2;226(2):241–258. doi: 10.1016/0005-2728(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Gitlitz P. H., Krasna A. I. Structural and catalytic properties of hydrogenase from Chromatium. Biochemistry. 1975 Jun 17;14(12):2561–2568. doi: 10.1021/bi00683a001. [DOI] [PubMed] [Google Scholar]
- Kirkconnell S., Hegeman G. D. Mechanism of oxidation of carbon monoxide by bacteria. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1584–1587. doi: 10.1016/0006-291x(78)91402-x. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Oxidation of carbon monoxide in cell extracts of Pseudomonas carboxydovorans. J Bacteriol. 1979 Feb;137(2):811–817. doi: 10.1128/jb.137.2.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



