Abstract
The selective estrogen-receptor modulator (SERM) tamoxifen became the first U.S. Food and Drug Administration (FDA)-approved agent for reducing breast cancer risk but did not gain wide acceptance for prevention, largely because it increased endometrial cancer and thromboembolic events. The FDA approved the SERM raloxifene for breast cancer risk reduction following its demonstrated effectiveness in preventing invasive breast cancer in the Study of Tamoxifen and Raloxifene (STAR). Raloxifene caused less toxicity, including reduced thromboembolic events and endometrial cancer. In this paper, we detail a longer-term analysis of STAR (median follow-up of 81 months vs 47 months in the initial report). We performed this updated analysis in an effort to better understand how these two drugs differ, particularly in regard to their relative effects on noninvasive disease. STAR eligibility criteria included postmenopausal status and 5-year breast cancer risk of at least 1.66% (actual mean risk was 4.03%). STAR women were randomly assigned to receive either tamoxifen (20 mg/d) or raloxifene (60 mg/d) for 5 years. Of the originally randomized 19,747 women, 19,490 participated in the STAR follow-up described here. The risk ratio (RR; raloxifene:tamoxifen) for invasive breast cancer was 1.24 (95% confidence interval [CI], 1.05–1.47) and for noninvasive disease was 1.22 (95% CI, 0.95–1.59). Compared with the initial results, the RRs widened for invasive and narrowed for noninvasive breast cancer. Toxicity RRs (raloxifene:tamoxifen) were 0.55 (95% CI, 0.36–0.83; P = 0.003) for endometrial cancer (this difference was not significant in the initial results), 0.19 (95% CI, 0.12–0.29) for uterine hyperplasia, and 0.75 (95% CI, 0.60–0.93) for thromboembolic events. There were no significant mortality differences. Long-term, raloxifene retained 76% of the effectiveness of tamoxifen in preventing invasive disease and grew closer over time to tamoxifen in preventing noninvasive disease, with far less toxicity (e.g., highly significantly less endometrial cancer). These results have important public health implications and clarify that both raloxifene and tamoxifen are good preventive choices for postmenopausal women with elevated risk for breast cancer. These updated data should encourage widespread acceptance of raloxifene and a greater acceptance of tamoxifen for breast cancer risk reduction.
Keywords: Breast cancer, prevention, tamoxifen, raloxifene
Introduction
Despite improvements in the detection and treatment of breast cancer, this disease still accounted for 192,000 new cases and 40,000 deaths in the United States in 2009 (1). Therefore, the concept of preventing the development of invasive breast cancer remains an attractive one. The selective estrogen receptor modulator (SERM) tamoxifen has well-known benefits in the treatment of receptor-positive invasive breast cancer (2) and has been shown to be an effective chemoprevention therapy (3–6). However, in spite of its impressive efficacy in the prevention of breast cancer, tamoxifen has not been widely used for prevention due, in large part, to the increased risk of endometrial cancer and thromboembolic events associated with its use. Another SERM, raloxifene, has been shown to reduce the incidence of breast cancer in a series of clinical trials designed primarily to evaluate it for treatment and prevention of osteoporosis in postmenopausal women (7,8).
The National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol P-2, the Study of Tamoxifen and Raloxifene (STAR), directly compared tamoxifen with raloxifene in 19,747 healthy postmenopausal women at an increased risk for development of breast cancer. With 47 months of follow-up, the initial STAR results demonstrated no significant difference between the two trial arms in the incidence of invasive breast cancer, both with an estimated decreased incidence of approximately 50% (vs untreated women) (9). Raloxifene did not appear to be as effective as tamoxifen in reducing the incidence of noninvasive breast cancer (ductal carcinoma in situ [DCIS] and lobular carcinoma in situ [LCIS] combined). The toxicity and side-effect evaluations favored the raloxifene group, in which women had significantly fewer deep-vein thromboses and pulmonary emboli, cataracts, and hysterectomies for benign disease. The raloxifene group also had a nonsignificant reduction in endometrial cancer. This report provides updated STAR results.
Materials and Methods
STAR was a two-arm, randomized, double-blinded trial of tamoxifen versus raloxifene for the reduction of breast cancer incidence; participants and their physicians were unaware of the treatment that was being administered until the trial was unblinded in April 2006. All participants provided written informed consent that was reviewed and approved by the National Cancer Institute and the institutional review boards of all participating institutions. The details of the trial methodology, including the definition of endpoints and the methods used for randomization, schedule of patient follow-up, patient testing, and trial monitoring, are described in the initial report of 2006, for which the data were cut off as of December 31, 2005 (9). The update in the present report is based on a cut-off date of March 31, 2009, providing a median follow-up of 81 months. We focus here on updating findings for the primary endpoint (incidence of invasive breast cancer) and for all key secondary endpoints, including noninvasive breast cancer, endometrial and other cancers, and vascular-related events. In the original STAR report, no difference between treatment groups was noted for the secondary endpoints: ischemic heart disease, stroke, and osteoporotic fractures. Because our new analyses confirmed that this lack of differences continued in the longer term, these endpoints are not included in this report.
Participant characteristics
Only women who were postmenopausal, at least 35 years of age, and who had a 5-year predicted breast cancer risk of at least 1.66% were eligible for STAR. The risk determination was based on the Gail model, as modified and applied in the Breast Cancer Prevention Trial (BCPT P-1) (10). Participants were also required to meet the following criteria: not taking either tamoxifen or raloxifene, hormone therapy, oral contraceptives, or androgens for at least 3 months before randomization; not currently taking warfarin or cholestyramine; no history of stroke, transient ischemic attack, pulmonary embolism, or deep-vein thrombosis; no atrial fibrillation, uncontrolled diabetes, or uncontrolled hypertension; no psychiatric condition that would interfere with adherence; a performance status that would not restrict normal activity; and no history of previous malignancy except basal cell or squamous cell carcinoma of the skin, carcinoma in situ of the cervix, or LCIS of the breast. Eligible women were randomly assigned to receive either 20 mg/d of tamoxifen plus placebo, or 60 mg/d of raloxifene plus placebo for 5 years; the placebo tablets were necessary to maintain the double blinding of treatment assignment because the formulations of tamoxifen and raloxifene tablets were dissimilar.
A total of 19,747 women were randomly assigned to one of the two groups between July 1, 1999, and November 4, 2004, and 19,471 of these women (9,726 tamoxifen; 9,745 raloxifene) were included in the analysis of the original report. Two hundred seventy-four women were not included due to lack of follow-up information (146 tamoxifen; 128 raloxifene). Two other women (in the raloxifene group) were excluded because they had received a prophylactic bilateral mastectomy before randomization and were not at risk for the development of invasive breast cancer. Since the time of the initial report, follow-up information was collected on 20 of the women (10 tamoxifen; 10 raloxifene) who lacked follow-up information at the time of the original report. One woman (in the raloxifene group) in the original report has been excluded from the follow-up analyses because she was discovered to have been diagnosed with invasive breast cancer before randomization. Therefore, this update report includes the findings for 19,490 women—9,736 in the tamoxifen group and 9,754 in the raloxifene group.
The characteristics of the participants included in the current analysis are shown in Table 1. The mean age at entry to the trial was 58.5 years (SD, 7.4). Nine percent of the women were younger than 50 years, 49.8% were between ages 50 and 59, 32.4% were between ages 60 and 69, and 8.8% were aged 70 years or older. The percentages of racial/ethnic groups were as follows: White = 93.5%, African American = 2.4%, Hispanic = 2.0%, and “other” = 2.1%. More than half (51.5%) of the participants had undergone a hysterectomy before entry to the study; over 70% had a first-degree female relative with a history of breast cancer; and 23% had a history of atypical hyperplasia of the breast. The mean 5-year predicted breast cancer risk at entry was 4.03% (SD, 2.2), subdivided as follows: 30.2% between 2.01 and 3.00%, 31.4% between 3.01 and 5.00, and 27.3% greater than 5.00%. The mean lifetime risk was 14.73% (SD, 7.4).
Table 1.
Participant characteristics at entry for women included in the analyses of the NSABP STAR Trial (P-2)
| Participant characteristics | Tamoxifen | Raloxifene | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age (years) | ||||
| ≤ 49 | 884 | 9.1 | 878 | 9.0 |
| 50–59 | 4,856 | 49.9 | 4,855 | 49.8 |
| 60–69 | 3,137 | 32.2 | 3,174 | 32.5 |
| ≥ 70 | 859 | 8.8 | 847 | 8.7 |
| Race/ethnicity | ||||
| White | 9,105 | 93.5 | 9,115 | 93.4 |
| African-American | 233 | 2.4 | 243 | 2.5 |
| Hispanic | 192 | 2.0 | 193 | 2.0 |
| Other | 206 | 2.1 | 203 | 2.1 |
| No. 10 relatives with breast cancer | ||||
| 0 | 2,838 | 29.1 | 2,791 | 28.6 |
| 1 | 5,046 | 51.8 | 5,135 | 52.6 |
| 2 | 1,532 | 15.7 | 1,561 | 16.0 |
| ≥ 3 | 320 | 3.3 | 267 | 2.7 |
| History of hysterectomy | ||||
| No | 4,739 | 48.7 | 4,717 | 48.4 |
| Yes | 4,997 | 51.3 | 5,037 | 51.6 |
| History of lobular carcinoma in situ | ||||
| No | 8,844 | 90.8 | 8,865 | 90.9 |
| Yes | 892 | 9.2 | 889 | 9.1 |
| History of breast atypical hyperplasia | ||||
| No | 7,545 | 77.5 | 7,513 | 77.0 |
| Yes | 2,191 | 22.5 | 2,241 | 23.0 |
| 5-year predicted breast cancer risk (%)* | ||||
| ≤ 2.00 | 1,055 | 10.8 | 1,102 | 11.3 |
| 2.01–3.00 | 2,993 | 30.7 | 2,893 | 29.7 |
| 3.01–5.00 | 3,042 | 31.2 | 3,086 | 31.6 |
| ≥ 5.01 | 2,646 | 27.2 | 2,673 | 27.4 |
| Total | 9,736 | 100.0 | 9,754 | 100.0 |
Abbreviation: NSABP STAR, National Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene
Determined by the Gail model.
The mean duration of treatment was 43.5 months (SD, 20.7) for the tamoxifen group and 46.8 months (SD, 20.0) for the raloxifene group. Participant adherence to 5 years of therapy was within the limits anticipated when the trial was designed. Also, since the original report and unblinding of treatment assignment, any woman who had not completed her 5-year course of tamoxifen was offered the option to switch to raloxifene for the remaining portion of her treatment course. A total of 879 women chose this option.
Statistical analyses
Analyses included all randomly assigned at-risk women for whom follow-up information was available. All analyses were based on the intention-to-treat principle and used the treatment assignment determined at randomization, regardless of the treatment status at the time of analysis. Rates per 1,000 person-years for each of the study endpoints were determined for each treatment group by dividing the number of events within each treatment group by the total number of event-specific person-years of follow-up within the group. Comparisons of rates between treatment groups were based on the risk ratio (RR) and the 95% CI for the RR. The RR was determined as the rate in the raloxifene group divided by the rate in the tamoxifen group. The 95% CI for each RR was determined assuming a Poisson distribution, conditioning on the total number of events and the person-years at risk. RRs for which the 95% CI did not include 1.00 were considered to be statistically significant. Plots of the cumulative incidence over time of follow-up were also developed. The cumulative incidence accounted for the competing risk of death (11). P-values to assess statistically significant differences between treatment group–specific cumulative incidence curves were determined by the log-rank test. All P-values are 2-sided using P < 0.05 to determine statistical significance. Analyses were performed using SAS version 9.1 software (SAS Institute, Inc).
Results
Breast cancer
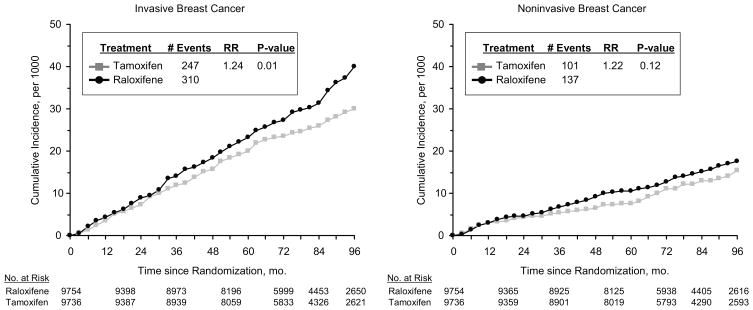
The updated findings for invasive breast cancer are shown in the left panel of Figure 1. In contrast with the results documented in the original report, there is now a significant difference between the treatment groups, with 310 cases of invasive breast cancer in the raloxifene group and 247 in the tamoxifen group. The invasive breast cancer RR (raloxifene:tamoxifen) is 1.24 (95% CI, 1.05–1.47), indicating that the rate in the raloxifene group is about 24% higher than the rate in the tamoxifen group. As demonstrated in the BCPT, compared with placebo, tamoxifen reduces the risk of invasive breast cancer by about 50% (3). Therefore, if there were no breast cancer RR effect from raloxifene, the expected rate of breast cancer in the raloxifene group would be about twice the rate in the tamoxifen group, yielding an RR of 2.00. Based on this information and the actual 1.24 RR observed in this study, one can extrapolate that raloxifene is about 76% as effective as tamoxifen in reducing breast cancer risk [{(2.00–1.24)/(2.00–1.00)} × 100 = 76%]. Then, compared with placebo, raloxifene would reduce the risk of invasive breast cancer by about 38% (50% × 76% = 38%), versus the 50% reduction seen with tamoxifen.
Fig. 1.
Cumulative incidence of invasive and noninvasive breast cancer.
The rate of invasive breast cancer by participant demographic characteristics is provided in Table 2. The number of events and the point estimates of the rate are higher in the raloxifene arm than in the tamoxifen arm for all categories of participant characteristics, and there is no indication of a quantitative interaction between treatment and any of the participant characteristics.
Table 2.
Annual rates of invasive breast cancer—NSABP STAR Trial (P-2)
| Participant characteristic at baseline | Number of events | Rate per 1000 | RR (95%CI) | ||||
|---|---|---|---|---|---|---|---|
| Tamoxifen | Raloxifene | Tamoxifen | Raloxifene | Difference* | RR† | ||
| Age at entry (years) | |||||||
| ≤ 49 | 10 | 15 | 1.84 | 2.80 | −0.96 | 1.53 | 0.64–3.80 |
| 50–59 | 125 | 155 | 4.09 | 5.03 | −0.94 | 1.23 | 0.97–1.57 |
| ≥ 60 | 112 | 140 | 4.47 | 5.48 | −1.01 | 1.22 | 0.95–1.58 |
| History of lobular carcinoma in situ | |||||||
| No | 197 | 253 | 3.54 | 4.50 | −0.96 | 1.27 | 1.05–1.54 |
| Yes | 50 | 57 | 9.14 | 10.34 | −1.20 | 1.13 | 0.76–1.69 |
| History of atypical hyperplasia | |||||||
| No | 187 | 218 | 3.90 | 4.52 | −0.62 | 1.16 | 0.95–1.42 |
| Yes | 60 | 92 | 4.58 | 6.79 | −2.21 | 1.48 | 1.06–2.09 |
| 5-year predicted breast cancer risk (%) | |||||||
| ≤ 3.00 | 61 | 81 | 2.39 | 3.21 | −0.82 | 1.34 | 0.95–1.90 |
| 3.01–5.00 | 84 | 91 | 4.43 | 4.63 | −0.20 | 1.05 | 0.77–1.42 |
| ≥ 5.01 | 102 | 138 | 6.13 | 8.17 | −2.04 | 1.33 | 1.02–1.74 |
| No. 10 relatives with breast cancer | |||||||
| 0 | 82 | 105 | 4.77 | 6.17 | −1.40 | 1.29 | 0.96–1.75 |
| 1 | 112 | 135 | 3.51 | 4.10 | −0.59 | 1.17 | 0.90–1.51 |
| ≥ 2 | 53 | 70 | 4.44 | 5.96 | −1.52 | 1.34 | 0.93–1.96 |
| Total | 247 | 310 | 4.04 | 5.02 | −0.98 | 1.24 | 1.05–1.47 |
Abbreviations: CI, confidence interval; NSABP STAR, National Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene; RR, risk ratio.
Rate in the tamoxifen group minus rate in the raloxifene group.
Risk ratio for women in the raloxifene group compared to women in the tamoxifen group.
In our original report, the difference between treatment groups for the rate of noninvasive breast cancer was borderline for statistical significance (RR = 1.40; 95% CI, 0.98–2.00; P = 0.052). Currently, the difference between treatment groups for this event is less than originally seen (right panel of Fig. 1). There are 137 cases in the raloxifene group compared with 111 in the tamoxifen group, for an RR of 1.22 (95% CI, 0.95–1.59). The difference between treatment groups in noninvasive breast cancer appears to be limited to cases of pure DCIS or cases of mixed DCIS and LCIS (top portion of Table 3). There was no difference between the groups for pure LCIS cases; the numbers of women diagnosed with this condition were 33 (tamoxifen) and 34 (raloxifene; RR = 1.02; 95% CI, 0.61–1.70). In parallel with the analysis presented above for invasive breast cancer, tamoxifen has been shown previously to reduce the risk of noninvasive breast cancer by about 50%. Therefore, if there were no noninvasive breast cancer risk reduction effect of raloxifene, the expected rate of noninvasive breast cancer in the raloxifene group would be about twice the rate in the tamoxifen group, yielding an RR (raloxifene:tamoxifen) of 2.00. Based on this information and the actual 1.22 RR observed in this study, one can extrapolate that raloxifene is about 78% as effective as tamoxifen in reducing noninvasive breast cancer risk [{(2.00–1.22)/(2.00–1.00)} × 100 = 78%]. Then, compared with placebo, raloxifene reduces the risk of noninvasive breast cancer by about 39% (50% × 78% = 39%).
Table 3.
Annual rates of noninvasive breast cancer and uterine disease/hysterectomy—NSABP STAR Trial (P-2)
| Disease/uterine event type | Events, n |
Rate per 1,000 |
RR† | RR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Tamoxifen | Raloxifene | Tamoxifen | Raloxifene | Difference* | |||
| Noninvasive breast cancer | |||||||
| DCIS | 70 | 86 | 1.15 | 1.40 | −0.25 | 1.22 | 0.88–1.69 |
| LCIS | 33 | 34 | 0.54 | 0.55 | −0.01 | 1.02 | 0.61–1.70 |
| Mixed | 8 | 17 | 0.13 | 0.28 | −0.15 | 2.11 | 0.86–5.64 |
| Total | 111 | 137 | 1.83 | 2.23 | −0.40 | 1.22 | 0.95–1.59 |
| Uterine disease and hysterectomy‡ | |||||||
| Invasive Cancer | 65 | 37 | 2.25 | 1.23 | 1.02 | 0.55 | 0.36–0.83 |
| Hyperplasia§ | 126 | 25 | 4.40 | 0.84 | 3.56 | 0.19 | 0.12–0.29 |
| Without atypia§ | 104 | 21 | 3.63 | 0.70 | 2.93 | 0.19 | 0.11–0.31 |
| With atypia§ | 22 | 4 | 0.77 | 0.13 | 0.64 | 0.17 | 0.04–0.51 |
| Hysterectomy during follow-up | 349 | 162 | 12.08 | 5.41 | 6.67 | 0.45 | 0.37–0.54 |
Abbreviations, CI, confidence interval; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ; NSABP STAR, National Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene; RR, risk ratio.
Rate in the tamoxifen group minus rate in the raloxifene group.
Risk ratio for women in the raloxifene group compared with women in the tamoxifen group.
Women at risk were those with an intact uterus at entry (see Table 1).
Among women not diagnosed with uterine cancer.
Uterine disease
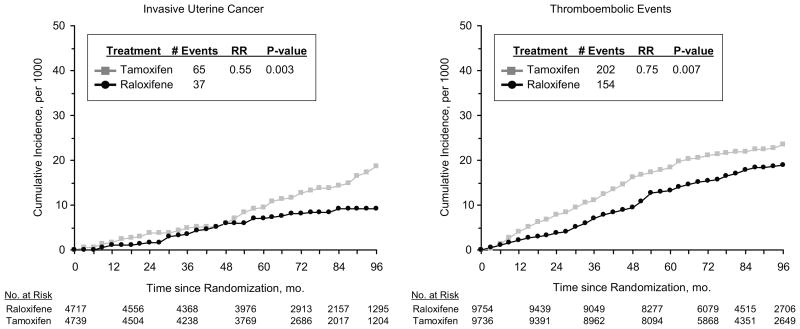
Invasive uterine cancer and uterine hyperplasia are well-established toxicities associated with tamoxifen treatment. When compared with tamoxifen, raloxifene does not have such a profile (bottom portion of Table 3). The incidence of invasive uterine cancer is significantly lower in the raloxifene group (P = 0.003; left panel of Fig. 2). The annual average rate per 1,000 was 2.25 in the tamoxifen group compared with 1.23 in the raloxifene group (RR = 0.55; 95% CI, 0.36–0.83). In our original report, the difference between treatment groups for the rate of invasive uterine cancer was not statistically significant. The average annual incidence rate of uterine hyperplasia, the majority of which was hyperplasia without atypia, was 5 times higher in the tamoxifen group (4.40 per 1,000) than in the raloxifene group (0.84 per 1,000; RR = 0.19; 95% CI, 0.12–0.29). The number of hysterectomies performed in the tamoxifen group (349), including those done for benign disease, was more than double that performed in the raloxifene group (162; RR = 0.45; 95% CI, 0.37–0.54).
Fig. 2.
Cumulative incidence of invasive uterine cancer and thromboembolic events.
Other cancers
Comparisons between treatment groups of the average annual rates of invasive cancer of sites other than the breast or uterus are presented in Table 4. These data are consistent with those in the original report, which also showed no significant differences for cancer in non-breast or non-uterus sites.
Table 4.
Annual rates of site-specific invasive cancer cases other than breast and uterine cancer—NSABP STAR Trial (P-2)
| Site of cancer | Events, n | Rate per 1000 | RR† | RR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Tamoxifen | Raloxifene | Tamoxifen | Raloxifene | Difference* | |||
| Adrenal gland | 0 | 1 | 0 | 0.02 | −0.02 | — | — |
| Bone/cartilage/connective tissue | 3 | 4 | 0.05 | 0.06 | −0.01 | 1.32 | 0.22–8.98 |
| Buccal cavity and pharynx | 4 | 6 | 0.06 | 0.10 | −0.04 | 1.48 | 0.35–7.13 |
| Cervix | 3 | 0 | 0.05 | 0 | 0.05 | — | — |
| Colorectal | 48 | 45 | 0.78 | 0.72 | 0.06 | 0.93 | 0.60 to 1.42 |
| Esophagus | 2 | 0 | 0.03 | 0 | 0.03 | — | — |
| Eye | 1 | 1 | 0.02 | 0.02 | 0 | 0.99 | 0.01–77.48 |
| Gallbladder | 5 | 2 | 0.08 | 0.03 | 0.05 | 0.39 | 0.04–2.41 |
| Kidney | 14 | 21 | 0.23 | 0.34 | −0.11 | 1.48 | 0.72–3.15 |
| Larynx | 0 | 1 | 0 | 0.02 | −0.02 | — | — |
| Leukemia/other lymph/hemato | 60 | 53 | 0.97 | 0.85 | 0.12 | 0.87 | 0.59–1.28 |
| Liver | 7 | 2 | 0.11 | 0.03 | 0.08 | 0.28 | 0.03–1.48 |
| Lung, trachea, bronchus | 57 | 64 | 0.92 | 1.02 | −0.10 | 1.11 | 0.76–1.61 |
| Nasal/middle ear/sinuses | 1 | 1 | 0.02 | 0.02 | 0 | 0.99 | 0.01–77.48 |
| Nervous system | 9 | 10 | 0.15 | 0.16 | −0.01 | 1.10 | 0.40–3.05 |
| Other gyn | 2 | 2 | 0.03 | 0.03 | 0 | 0.99 | 0.07–13.62 |
| Ovary | 21 | 34 | 0.50 | 0.79 | −0.29 | 1.58 | 0.89–2.86 |
| Pancreas | 12 | 11 | 0.19 | 0.18 | 0.01 | 0.90 | 0.36–2.24 |
| Retroperitoneum | 7 | 4 | 0.11 | 0.06 | 0.05 | 0.56 | 0.12–2.22 |
| Skin | 25 | 24 | 0.40 | 0.38 | 0.02 | 0.95 | 0.52–1.73 |
| Small intestine | 0 | 2 | 0 | 0.03 | −0.03 | — | — |
| Spleen | 0 | 2 | 0 | 0.03 | −0.03 | — | — |
| Stomach | 5 | 1 | 0.08 | 0.02 | 0.06 | 0.20 | 0.004–1.76 |
| Thyroid gland | 18 | 32 | 0.29 | 0.51 | −0.22 | 1.76 | 0.96–3.32 |
| Urinary bladder | 15 | 12 | 0.24 | 0.19 | 0.05 | 0.79 | 0.34–1.81 |
| Site unspecified/unspecified nature | 15 | 19 | 0.24 | 0.30 | −0.06 | 1.25 | 0.60–2.64 |
| Secondary/uncertain | 4 | 5 | 0.06 | 0.08 | −0.02 | 1.23 | 0.27–6.22 |
Abbreviations: CI, confidence interval; gyn, gynecologic; hemato, hematopoietic; lymph, lymphatic; NSABP STAR, National Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene; RR, risk ratio.
Rate in the tamoxifen group minus rate in the raloxifene group.
Risk ratio for women in the raloxifene group compared with women in the tamoxifen group.
Thromboembolic events
Pulmonary embolism and deep-vein thrombosis are other toxicities with a well-recognized association with tamoxifen treatment. The incidence of such events was significantly elevated in the tamoxifen group compared with the raloxifene group (P = 0.007; right panel of Fig. 2 and top of Table 5). The average annual rates of thromboembolic events were 3.30 per 1,000 (tamoxifen) and 2.47 per 1,000 (raloxifene; RR = 0.75; 95% CI, 0.60–0.93).
Table 5.
Rates of thromboembolic events, cataracts and cataracts surgery—NSABP STAR Trial (P-2)
| Type of Event | Events, n | Rate per 1,000 | RR† | RR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Tamoxifen | Raloxifene | Tamoxifen | Raloxifene | Difference* | |||
| Thromboembolic events | |||||||
| Thromboembolic events | 202 | 154 | 3.30 | 2.47 | 0.83 | 0.75 | 0.60–0.93 |
| Pulmonary embolism | 84 | 68 | 1.36 | 1.09 | 0.27 | 0.80 | 0.57–1.11 |
| Deep-vein thrombosis | 118 | 86 | 1.93 | 1.38 | 0.55 | 0.72 | 0.54–0.95 |
| Cataracts and Cataract Surgery | |||||||
| Developed cataracts during follow-up‡ | 739 | 603 | 14.58 | 11.69 | 2.89 | 0.80 | 0.72–0.89 |
| Developed cataracts and had cataract surgery‡ | 575 | 462 | 11.18 | 8.85 | 2.33 | 0.79 | 0.70–0.90 |
Abbreviations: CI, confidence interval; NSABP STAR, National Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene; RR, risk ratio.
Rate in the tamoxifen group minus rate in the raloxifene group.
Risk ratio for women in the raloxifene group compared to women in the tamoxifen group.
Women at risk were those with no prior history of cataracts at entry (8,341 and 8,336 tamoxifen and raloxifene participants, respectively).
Cataracts
When compared with the results in the placebo group in the BCPT, tamoxifen increased the incidence of cataract development and cataract surgery (3). Raloxifene does not have this effect. In the original report of STAR, cataract events were significantly elevated in the tamoxifen group compared with the raloxifene group, and these differences persisted in the current analysis (bottom of Table 5). The rate of cataract development (RR = 0.80; 95% CI, 0.72–0.89) and the rate of cataract surgery (RR = 0.79; 95% CI, 0.70–0.90) are about 20% less in the raloxifene group than in the tamoxifen group.
Mortality
The number of deaths observed during follow-up is shown in Table 6. There is no statistically significant mortality difference between the treatment groups. Overall, 236 deaths occurred in the tamoxifen group and 202 deaths in the raloxifene group, for an RR of 0.84, which was not statistically significant (95% CI, 0.70–1.02). When the differences between treatment groups are compared by specific causes of death, the data are consistent with variation due to chance.
Table 6.
Distribution of Deaths - NSABP STAR Trial (P-2)
| Cause of death | Deaths, n |
|
|---|---|---|
| Tamoxifen | Raloxifene | |
| Cancer | 101 | 86 |
| Bladder | 1 | 3 |
| Bone, articular cartilage and connective tissue | 1 | 1 |
| Brain | 6 | 4 |
| Breast | 11 | 4 |
| Colon | 4 | 3 |
| Endocrine gland | 0 | 1 |
| Gallbladder | 2 | 1 |
| Kidney | 1 | 1 |
| Liver | 7 | 1 |
| Lung | 25 | 28 |
| Lymphatic/hematopoietic | 12 | 11 |
| Oral | 2 | 1 |
| Ovary | 8 | 7 |
| Pancreas | 7 | 5 |
| Peritoneum | 2 | 0 |
| Skin | 2 | 0 |
| Spleen | 0 | 1 |
| Stomach | 2 | 1 |
| Thyroid | 1 | 0 |
| Uterus | 2 | 2 |
| Other, uncertain, and unspecified sites | 5 | 11 |
| Circulatory/vascular disease | 42 | 42 |
| Aortic | 1 | 2 |
| Atherosclerosis | 0 | 1 |
| Cerebrovascular disease, unspecified | 1 | 0 |
| Hypertensive disease | 1 | 4 |
| Ischemic heart disease | 13 | 8 |
| Other heart disease | 9 | 14 |
| Peripheral vascular disease, unspecified | 0 | 1 |
| Polyarteritis nodosa | 0 | 1 |
| Pulmonary embolism | 3 | 2 |
| Primary pulmonary hypertension | 1 | 0 |
| Stroke | 13 | 9 |
| Other | 93 | 74 |
| Accident, auto | 3 | 4 |
| Accident, fire | 1 | 0 |
| Alcohol dependence syndrome | 1 | 1 |
| Asphyxiation and strangulation | 1 | 0 |
| Complications of surgery | 0 | 1 |
| Dementia | 0 | 1 |
| Diabetes | 1 | 3 |
| Disorders of metabolism | 1 | 0 |
| Emphysema | 1 | 0 |
| Injury, intracranial | 2 | 2 |
| Injury, other | 1 | 0 |
| Interferon toxicity | 0 | 1 |
| Intestinal infectious disease | 0 | 1 |
| Other conditions of the blood | 0 | 2 |
| Other conditions of the brain/neurological system | 7 | 3 |
| Other diseases of the digestive system | 7 | 6 |
| Other Diseases of the urinary system | 2 | 1 |
| Other respiratory disease | 13 | 7 |
| Pneumonia | 2 | 4 |
| Poisoning | 2 | 0 |
| Septicemia | 4 | 3 |
| Skin infections | 0 | 1 |
| Symptoms, signs, and ill-defined conditions | 2 | 3 |
| Unknown | 42 | 30 |
| Total deaths (rate per 1,000) | 236 (3.81) | 202 (3.22) |
| Risk ratio (95% CI) | 0.84 (0.70–1.02) | |
Abbreviations: CI, confidence interval; NSABP STAR, National Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene
Discussion
Tamoxifen has been shown to reduce the risk of contralateral breast cancer in women with invasive breast cancer and DCIS (12,13). The benefit appears to be very durable. After 2 to 5 years of adjuvant tamoxifen, the contralateral breast cancer reduction continued through at least 15 years of follow-up (2,14). In primary prevention trials of tamoxifen in women at risk for the future development of breast cancer, 5 to 8 years of tamoxifen significantly reduced the incidence of invasive breast cancer, and this benefit persisted for at least 7 to 12 years (6, 15, 16).
Raloxifene has also been shown to reduce the incidence of primary invasive breast cancer (compared with placebo). The Multiple Outcomes of Raloxifene Evaluation (MORE) trial randomized 7,704 postmenopausal women with osteoporosis; with a median follow-up of 45 months, raloxifene (given for 4 years) reduced the incidence of breast cancer by 76% (RR = 0.24; 95% CI, 0.13–0.44) (7). In the Raloxifene Use for the Heart (RUTH) trial, 10,101 postmenopausal women with coronary heart disease or multiple risk factors for this disease were assigned to either raloxifene (60 mg/d) or placebo. With 5.6 years median follow-up, raloxifene reduced the incidence of invasive breast cancer by a significant 44% (hazard ratio [HR] = 0.56; 95% CI, 0.38–0.83) (17). As detailed in the initial report of STAR, after a median follow-up of 47 months, raloxifene was as effective as tamoxifen in reducing the risk of invasive breast cancer. The updated results reported here demonstrate that, after a median follow-up of 81 months, which represents 60 months of treatment plus an additional 21 months of follow-up, raloxifene no longer appears to be as effective as tamoxifen in preventing primary invasive breast cancer. Raloxifene does appear, however, to retain approximately 76% of tamoxifen’s effectiveness, which represents as much as a 38% reduction in invasive breast cancer (compared with an untreated group). The initial STAR report also suggested that raloxifene may not be as effective as tamoxifen in preventing the development of noninvasive breast cancers (LCIS and DCIS combined). The updated results show that the difference between the treatment groups has narrowed, and, much like its effect against invasive breast cancer, raloxifene is about 78% as effective as tamoxifen in reducing the risk of noninvasive breast cancer. Patients with a history of LCIS or atypical hyperplasia of the breast have a 4-fold to 10-fold increased risk of subsequent invasive disease, and tamoxifen and raloxifene were equally effective in reducing this risk in the initially reported STAR results. The current analyses indicate that this equality is no longer the case for STAR women with a history of atypical hyperplasia (RR = 1.48; 95% CI=1.06–2.09), although results for the LCIS group remain similar to those reported originally (RR = 1.13; 95% CI, 0.76–1.69).
Only a slight difference was evident between treatment groups in the cumulative incidence of both invasive and noninvasive breast cancer (Fig. 1) through the first 20 months of the study. After 30 months, a clear separation of the treatment curves was observed, with a higher cumulative incidence of both invasive and noninvasive breast cancer in the raloxifene group. Why are we seeing this apparent diminution of raloxifene’s benefits with longer follow-up? When the initial STAR results were published, all participants were notified of the results, and women who were still receiving tamoxifen were offered the option of crossing over to raloxifene therapy for the remainder of their 5 years of treatment. Only 879 women (9%) chose this option. The cross-over is unlikely to fully explain our updated findings.
Is nonadherence with the medication an issue? Only about 2% of orally administered raloxifene becomes bioavailable, and the biological half-life of raloxifene is much shorter than that of tamoxifen. Missing a day or 2 of raloxifene may result in a greater reduction of effectiveness than will similarly skipped doses of tamoxifen. However, overall adherence to protocol medication, as measured by pill counts, was similar in the two groups, and the protocol medication drop-off rates were higher in the tamoxifen group (38.9% vs 27.4%), which indicates that nonadherence or drop-offs in the raloxifene group do not provide the answer. Raloxifene may simply be less potent than is tamoxifen. It was originally developed as a drug to treat breast cancer but was less effective than was tamoxifen in that setting as well (18).
The superiority of tamoxifen over raloxifene in reducing breast cancer risk comes with a cost: significantly more endometrial cancers, hysterectomies for benign disease, thromboembolic events, and cataracts. These toxicities may be acceptable for the treatment of breast cancer but have proved to be a barrier to the use of tamoxifen for preventing primary breast cancers. It is important to point out that, unlike raloxifene, tamoxifen is approved for use in premenopausal women, and the BCPT (NSABP P-1) showed no excessive risk of endometrial cancers or thromboembolic events in the tamoxifen-treated premenopausal group compared with the placebo group. For premenopausal women at increased risk, particularly those with biopsy-proven risk factors such as LCIS or atypical hyperplasia, tamoxifen has a positive risk/benefit ratio and should be presented as a treatment option. A similar risk/benefit ratio may exist in younger postmenopausal women with elevated Gail scores and a prior hysterectomy.
Our results demonstrate that raloxifene (compared with tamoxifen) retains substantial benefit in reducing the risk of invasive breast cancer with fewer life-threatening side effects, including significantly fewer endometrial cancers, and these results are in keeping with those in the placebo-controlled raloxifene trials. We saw no significant increases in other primary cancers, although there were numerically more ovarian cancers and thyroid cancers. Neither of these tumors was noted to be of concern in the other raloxifene trials, but we plan to continue to follow STAR patients with particular attention to all potential long-term side effects.
The 5-year duration of therapy in STAR was a carryover from the P-1 trial of tamoxifen versus placebo, in which 5 years of tamoxifen was chosen based on the duration of treatment in adjuvant trials. In the combined results of MORE and the Continuing Outcomes Relevant to Evista (CORE) trial, which involved as much as 8 years of raloxifene therapy, a 66% reduction in the incidence of invasive breast cancer was seen in the raloxifene-treated group compared with the placebo group (HR = 0.34; 95% CI, 0.22–0.50). The women in the MORE/CORE studies were not selected based on breast cancer risk, and the majority had Gail scores below 1.66%, although some high-risk women were included.
Laboratory studies demonstrate that the antitumor actions of raloxifene and related hydroxylated SERMs depend on the duration of administration (19–21). In other words, longer administration periods are necessary to control tumorigenesis with short-acting SERMs with poor bioavailability (20). It may be that the long-term benefit of tamoxifen in controlling tumorigenesis after stopping the 5-year treatment continues because of the development of a sophisticated (phase II) antihormone-resistant disease that is vulnerable to the apoptotic actions of physiologic estrogen (22). In contrast, the evolution of acquired antihormone resistance may not advance as quickly with raloxifene as with tamoxifen, and raloxifene only remains therapeutically effective as long as it is given (8). It is unlikely that the optimual duration of raloxifene for chemoprevention will be evaluated in a breast cancer prevention setting; however, the use of raloxifene in treating and preventing osteoporosis is approved for an indefinite period of time. Therefore, continuing raloxifene therapy beyond 5 years might be an approach that would preserve its chemopreventive activity.
Large randomized cancer-prevention trials with long-term clinical follow-up of a carefully characterized population of individuals provide a valuable resource beyond the primary aims of the study. In the NSABP STAR (P-2) and BCPT (P-1), baseline blood samples have been collected and stored from more than 30,000 women at an increased risk for breast cancer, as have tumor specimens from breast cancer events. Various studies have already been conducted using these resources, and others are underway, including a genome-wide-association study by NSABP in collaboration with the National Institutes of Health Pharmacogenetics Research Network (PGRN) and the RIKEN Yokohama Institute Center for Genomic Medicine; this study includes a detailed evaluation of cytochrome P450–2D6 (CYP2D6) status ([refs. 23–27; access to these data and specimens is not restricted to NSABP members; the pathology section of the NSABP Web site (http://www.nsabp.pitt.edu) describes the process by which one can submit applications for such projects).
In conclusion, with a median follow-up of 81 months, our long-term, updated results show that raloxifene retained 76% of the effectiveness of tamoxifen in preventing invasive disease, and this level of effectiveness grew closer over time to that of tamoxifen (78% as effective) in preventing noninvasive disease, and that raloxifene remained far less toxic (e.g., now with highly statistically significantly fewer endometrial cancers). These relative effects of the drugs in the longer term—including greater potency of tamoxifen in preventing invasive and noninvasive disease and significantly less endometrial toxicity with raloxifene—are more consistent with the profiles that were expected on the basis of findings from other published studies. With deep public-health implications, these results help to clarify that both raloxifene and tamoxifen are good preventive choices for higher-risk postmenopausal women, depending largely on a woman’s personal risk factors for breast cancer. For postmenopausal women with elevated risk, these results should encourage widespread acceptance of raloxifene for breast cancer risk reduction, especially in women with an intact uterus who also face a risk of osteoporosis and fracture. The results should also promote greater acceptance of tamoxifen (given its greater efficacy) by premenopausal women who are at very high risk for breast cancer. Such increased acceptances of both SERMs for breast cancer risk reduction ultimately would reduce the public health burden of the disease.
Acknowledgments
The authors wish to thank Barbara G. Good, PhD, and Wendy L. Rea for editorial assistance.
Funding from Public Health Service grantsU10-CA-12027, U10-CA-69651, U10-CA-37377, and U10-CA-69974 from the National Cancer Institute, Department of Health and Human Services.
Footnotes
Notes: Clinical Trial Registration for NSABP P-2: NCT00003906. The work described in this manuscript is original research and has not been previously published. The following recent related work has been published: Vogel V, Costantino J, Wickerham DL, et al. “Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial,” JAMA 2006;295:2727 41, and Land SR, Wickerham DL, Costantino J, et al. “Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial,” JAMA 2006;295:2742 51. For earlier related publications, please see the references. The authors retain the right to provide a copy of the final manuscript to the NIH upon acceptance for public archiving in PubMed Central no later than 12 months after publication.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-1): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 6.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 7.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 8.Martino S, Cauley JA, Barrett-Conner E, et al. Continuing Outcomes Relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–61. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 9.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 10.Costantino JP, Gail MH, Pee D, et al. Validation studies for models to project the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–8. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 11.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11:813–29. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 13.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–68. doi: 10.1016/S0140-6736(04)16981-X. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Costantino J, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 16.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer–96-month follow-up of the randomized IBIS-1 trial. J Natl Cancer Inst. 2007;99:272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Conner E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 18.Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of Ly156758 in metastatic breast cancer. Oncology. 1998;45:344–5. doi: 10.1159/000226637. [DOI] [PubMed] [Google Scholar]
- 19.Gottardis MM, Jordan VC. The antitumor actions of keoxifene (raloxifene) and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987;47:4020–4. [PubMed] [Google Scholar]
- 20.Jordan VC, Allen KE. Evaluation of the antitumor activity of the nonsteroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16:239–51. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- 21.Jordan VC, Gosden B. Inhibition of the uterotrophic activity of estrogens and antiestrogens by the short acting antiestrogen LY117018. Endocrinology. 1983;113:463–8. doi: 10.1210/endo-113-2-463. [DOI] [PubMed] [Google Scholar]
- 22.Jordan VC. The 38th David A. Karnofsky lecture: The paradoxical actions of estrogen in breast cancer—survival or death? J Clin Oncol. 2008;26:3073–82. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 23.Abramson N, Costantino JP, Garber JE, Berliner N, Wickerham DL, Wolmark N. Effect of Factor V Leiden and prothrombin G20210→A mutations on thromboembolic risk in the National Surgical Adjuvant Breast and Bowel Project cancer prevention trial. J Natl Cancer Inst. 2006;98:904–10. doi: 10.1093/jnci/djj262. [DOI] [PubMed] [Google Scholar]
- 24.Cushman M, Costantino JP, Tracy RP, et al. Tamoxifen and cardiac risk factors in healthy women: Suggestion of an anti-inflammatory effect. Arterioscler Thromb Vasc Biol. 2001;21:255–61. doi: 10.1161/01.atv.21.2.255. [DOI] [PubMed] [Google Scholar]
- 25.King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P-1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–6. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 26.Cushman M, Costantino JP, Bovill EG, et al. Effect of tamoxifen on venous thrombosis risk factors in women without cancer: The breast cancer prevention trial. Br J Haematol. 2003;120:109–16. doi: 10.1046/j.1365-2141.2003.03976.x. [DOI] [PubMed] [Google Scholar]
- 27.Beattie MS, Costantino JP, Cummings SR, et al. Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP breast cancer prevention trial (P-1) J Natl Cancer Inst. 2006;98:110–5. doi: 10.1093/jnci/djj011. [DOI] [PubMed] [Google Scholar]