Abstract
Hydrogen sulfide (H2S) is emerging as a physiological neuromodulator as well as a smooth muscle relaxant. We submit the first evidence that blood H2S levels are significantly lower in fasting blood obtained from type 2 diabetes patients compared with age-matched healthy subjects, and in streptozotocin-treated diabetic rats compared with control Sprague–Dawley rats. We further observed that supplementation with H2S or an endogenous precursor of H2S (l-cysteine) in culture medium prevents IL-8 and MCP-1 secretion in high-glucose–treated human U937 monocytes. These first observations led to the hypothesis that lower blood H2S levels may contribute to the vascular inflammation seen in diabetes. Antioxid. Redox Signal. 12, 1333–1338.
Introduction
Human blood contains a significant amount (10–100 μM) of H2S (18, 19). H2S is produced in vivo from l-cysteine by the action of two enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). Both of these enzymes are dependent on pyridoxal-5'- phosphate. Expression of CSE or CBS is tissue specific. CSE is expressed mainly in the thoracic aorta, portal vein, ileum, heart, liver, kidney, and vascular smooth muscle, whereas CBS is highly expressed in the central and peripheral nervous systems (1, 13, 18, 29). CBS is an H2S-producing enzyme in the brain, and it has been shown that H2S enhances the activity of N-methyl-d-aspartate (NMDA) receptors and facilitates the induction of hippocampal long-term potentiation (LTP), a synaptic model of memory (1, 15). This suggests that H2S is a neuromodulator as well as a smooth muscle relaxant (1, 13, 20). Recent studies have shown a third H2S-producing enzyme, 3-mercaptopyruvate sulfurtransferase (3MST), along with cysteine aminotransferase (CAT), which produces H2S in the brain as well as in the vascular endothelium (26, 27).
Recent studies have revealed several potential roles for H2S in pathophysiologic functions (1, 13, 18, 20, 22, 29). H2S inhibits oxidative stress, promotes stimulation of KATP channels, relaxation and vasodilation in vascular smooth muscle cells, and relaxation of the human corpus cavernosum smooth muscle (1, 2, 18, 20, 22, 29). In vivo, H2S has been shown to inhibit leukocyte endothelial cell interactions and ischemia/reperfusion injury in liver and heart in animal studies (18). Recent studies have shown that genetic deletion of the CSE enzyme in mice markedly reduces H2S levels in the serum, heart, aorta, and other tissues, and that mice lacking CSE display pronounced hypertension and diminished endothelium-dependent vasorelaxation (29). These studies suggest that H2S is a physiological neuromodulator, vasodilator, and regulator of blood pressure (1, 2, 13, 18, 29).
This study examined the hypothesis that diabetes is associated with reduced blood levels of H2S. We determined blood levels of H2S in type 2 diabetes patients and in a rat model of diabetes. In addition, the effect of H2S and LC, an endogenous precursor of H2S, on HG-induced secretion of IL-8 and MCP-1 in cultured U937 monocytic cells was examined.
Results, Discussion, and Future Directions
The mean age of diabetes patients (52 ± 3 years) was similar to that of normal subjects (54 ± 7 years). The mean HbA1C of diabetes patients was 8.3 ± 0.5 (mean ± SEM). The mean IL-8 levels were significantly higher (p < 0.05) in diabetes patients (11 ± 1.2 pg/ml) compared with those in normal subjects (2.9 ± 0.6 pg/ml). MCP-1 levels were not significantly different between diabetes patients (491 ± 31 pg/ml) and normal subjects (432 ± 55 pg/ml) in our study population.
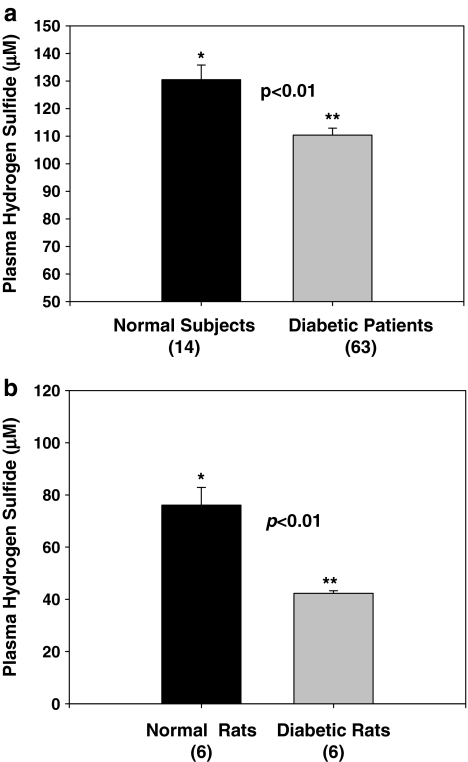
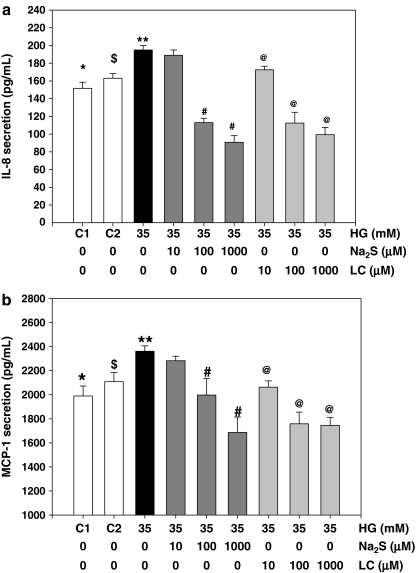
Diabetes patients have significantly lower blood levels of H2S compared with age-matched normal control subjects (Fig. 1a). In rat studies, a significantly lower plasma H2S level in diabetic rats was found compared with that in controls (Fig. 1b). At death, fasting blood HbA1 (16.98 ± 0.38% vs. 7.6 ± 0.2%) and MCP-1 (3,683 ± 423 vs. 2,164 ± 118 pg/ml) levels were significantly higher (p < 0.05) in diabetic rats compared with control nondiabetic rats. The effect of treatment with high glucose, without and with LC or H2S, on IL-8 and MCP-1 secretion in cultured monocytes is given in Fig. 2a and b. HG stimulated both MCP-1 and IL-8 secretion. Supplementation with exogenous H2S or LC, an endogenous precursor of H2S, inhibited the secretion of IL-8 (Fig. 2a) and MCP-1 (Fig. 2b) in HG-treated U937 monocytes. An osmolarity control for high glucose treatment (C2, consisting of 29 mM mannitol + 6 mM glucose) treatment did not show any effect on IL-8 or MCP-1 secretion (C2) in comparison with control (C1, 6 mM glucose).
FIG. 1.
Hydrogen sulfide levels in plasma of diabetes patients and normal subjects. Data (mean ± SEM) is from 63 type 2 diabetes patients and 14 age-matched normal volunteers (a) and streptozotocin-treated diabetic rats and control rats. Data (mean ± SEM) are from seven normal rats and six diabetic rats (b). Note a statistically significant decrease in hydrogen sulfide levels in diabetes patients in comparison to age-matched normal subjects and in diabetic rats in comparison to normal rats.
FIG. 2.
Effect of sodium sulfide and l-cysteine supplementation on IL-8 (a) and MCP-1 (b) secretion by monocytes cultured in high-glucose medium. Data (mean ± SEM) are from four different experiments. C1 is normal glucose control (6 mM glucose). C2 is an osmolarity control for high glucose and contains 6 mM glucose and 29 mM mannitol. Differences between * vs. **, $ vs. **, ** vs. #, and ** vs. @ were significant (p < 0.05).
This study demonstrates that diabetes is associated with lower circulating levels of hydrogen sulfide. This was shown in both in type 2 diabetes patients and in rats made diabetic by using streptozotocin. The streptozotocin-treated rat is a model of type 1 diabetes, whereas the patients examined in this study were type 2 diabetes patients. Even though both types of diabetes are associated with hyperglycemia and elevated proinflammatory cytokines, further studies are needed to establish low hydrogen sulfide levels in blood from a type 2 diabetic rat model and in type 1 diabetes patients.
A recent excellent review by Olsen et al. (25) examined the blood/plasma hydrogen sulfide measurements that have been reported over the past 30 years from the perspectives of analytic methods used by various authors. Several investigators have reported blood levels of 30–100 μM hydrogen sulfide (25). These numbers have been questioned in this review (25) because no obvious odor of H2S gas in the blood or in exhaled alveolar air, even though micromolar concentrations of H2S should result in H2S concentrations in exhaled air that can be detected by the human nose.
Second, Furne et al. (10) predicted that the high level of sulfide production necessary to sustain micromolar sulfide concentrations would consume cysteine at a rate nearly 100 times greater than the daily requirement of cysteine and methionine combined. Furne et al. also showed that whole-tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values (10). The method used by Zhu et al (30) measured not only the basal H2S but also H2S released from acid-labile sulfur and from bound sulfane sulfur (15). Hydrogen sulfide is gaining acceptance as a signaling molecule and has been shown to elicit a variety of biologic effects; however, the precise concentrations of H2S are present in tissues and are required for biologic effects is a matter of considerable debate.
A monocyte cell-culture model has been used to explore this understanding of the various mechanisms that contribute to the development of vascular inflammation and atherosclerosis (9, 24). The monocyte infiltration into subendothelial space that occurs through the combined actions of locally produced chemotactic cytokines and adhesion molecules expressed on the injured endothelial surface is critical to the development of vascular inflammation and the development of atherosclerosis. Previous studies have shown that activation with LPS is needed to elicit proinflammatory cytokine secretion in response to high-glucose treatment of monocytes (11, 16).
We focused our study on MCP-1 and IL-8 because these cytokines do not need activation by LPS to elicit a secretion response to HG treatment in monocytes. In this study, cells were exposed to a high-glucose concentration of 35 mM. Many previous studies have reported that glucose concentrations as high as 50 mM have been found in the blood of patients with uncontrolled diabetes (5). It is true that blood glucose levels in patients are not likely to stay as high as 35 mM for 24 h. However, tissue damage in diabetes patients occurs over many years of countless hyperglycemic episodes. Thus, the glucose concentration of 35 mM used in this cell-culture study does not seem unreasonable.
Many diabetes patients have elevated blood levels of the proinflammatory cytokines, such as IL-8 and MCP-1 (9, 12). These proinflammatory cytokines are markers of vascular inflammation, a known risk factor for cardiovascular disease (9, 12). Darmaun et al. (7), by using an isotope method, provided evidence that an alteration in glutathione and cysteine homeostasis occurs in vivo in adolescents with poorly controlled type 1 diabetes (6, 7). The glutathione and cysteine depletion are likely to arise from increased glutathione use. Along with a host of proteins, l-cysteine is a precursor of glutathione, which is considered pivotal for the reduction of cellular oxidative stress (8, 18, 21). Dietary supplementation of N-acetylcysteine or whey protein and α-lactoalbumin (cysteine-rich proteins) reduces the oxidative stress and insulin resistance induced by sucrose or fructose in rats (3, 4, 28). N-Acetylcysteine (NAC) supplementation in drinking water, and naturally formed cysteine-containing compounds in garlic and onion plants can reduce blood glucose levels in streptozotocin-treated diabetic mice (14). Oral supplementation with l-cysteine decreases blood levels of glucose, glycated hemoglobin, C-reactive protein, MCP-1, and protein oxidation, and inhibits phosphorylation of NF-κB and Akt in the livers of ZDF rats, an animal model of type 2 diabetes (17). These studies indicate that l-cysteine is essential in diabetes (3, 4, 19, 17, 21, 27). H2S can be formed from LC in the body (1, 18, 29). It is known that LC and H2S can act as antioxidants and reduce oxidative stress in cell-culture studies (19). Our finding of decreased levels of IL-8 and MCP-1 secretion in H2S- or LC-supplemented monocytes is novel. Thus, supplementation with LC may replenish blood H2S, thereby lowering circulating levels of proinflammatory cytokines. LC supplementation is also known to increase cellular GSH and decrease oxidative stress, both of which are known to reduce proinflammatory cytokines secretion (6, 7, 21). This study does not rule out the role played by many other factors, such as hypoglycemic drugs, hyperglycemia, hyperlipidemia, or oxidative stress in influencing levels of proinflammatory cytokines in diabetes.
In conclusion, this study demonstrates for the first time that diabetes is associated with lower circulating levels of hydrogen sulfide, which may play a role in vascular inflammation. H2S or l-cysteine, an endogenous precursor of H2S, can significantly inhibit the effect of hyperglycemia on MCP-1 and IL-8 secretion in a cell-culture model. Whether LC supplementation replenishes blood levels of H2S and diminishes proinflammatory cytokines levels in diabetes requires investigation at the clinical level to determine whether LC- or H2S-generating drug supplementation can be used as an adjuvant therapy to prevent or delay the excess vascular disease observed among the diabetes patient population.
Materials and Methods
Diabetes patients and normal volunteers
The protocol followed the guidelines approved by the Institutional Review Board for the Protection of Human Research Subjects. Blood from overnight-fasted patients was collected into tubes with EDTA. The EDTA blood was centrifuged, and the clear plasma saved for H2S, MCP-1, and IL-8 assays. All subjects were type 2 diabetes patients (n = 63) and age-matched normal volunteers (n = 14).
Animal studies
All of the procedures were carried out in accordance with the ethical standards of the institution after approval by the institutional Animal Welfare Committee. Male Sprague–Dawley rats were purchased at 49–52 days of age (200–220 g) from Harlan (Indianapolis, IN) and allowed 2 days for environmental and trainer-handling acclimation. The rats were weighed and then fasted overnight before intraperitoneal injection of 65 mg/kg streptozotocin in citrate buffer (pH, 4.5). Control rats were injected with citrate buffer alone to serve as a normal control group (group 1). The rats were tested for hyperglycemia by measuring their blood glucose concentration at 3 and 7 days after the streptozotocin injections. Blood for the blood glucose was obtained via tail incision and measured by using an Advantage Accu-chek glucometer (Boehringer Mannheim Corp., Indianapolis, IN). The rats that became hyperglycemic (blood glucose >300 mg/dl) were randomly divided into two groups: group 2, diabetic rats. The rats were maintained under standard housing conditions at 22 ± 2°C with 12:12-h light/dark cycles with a standard 8640 lab chow diet (Harlan, Indianapolis, IN). At the end of 7 weeks, the rats were fasted overnight and then killed for analysis by exposure to halothane (2-bromo-2-chloro-1,1,1-trifluoroethane). At the time of death, seven rats were in the control, and six in the diabetic group. Blood was collected via heart puncture with a 19½-gauge needle into EDTA Vacutainer tubes. EDTA blood was centrifuged; the clear plasma was saved, and buffy coat layers were discarded.
Human monocytes and treatment with high glucose (HG), LC, or H2S
U937 monocytes (two million cells/ml) were suspended in RPMI media containing 6 mM glucose. This medium is prepared by using glucose-free medium (Sigma Catalog number R1383) and was then mixed with glucose and serum and antibiotics. These cells were treated with LC or H2S with high-glucose medium (+29 mM glucose giving a final concentration of 35 mM) without and with LC or H2S. The concentrations of LC and H2S are given in the figures (10, 100, and 1,000 μM); treatments were carried out at 37°C for 24 h. Na2S was used as a source of H2S (19). Cells were pretreated with LC or hydrogen sulfide for 60 min before the treatment with high-glucose medium. Mannitol was used as an osmolarity control. All experiments were repeated 4 times.
Plasma H2S was determined by the method of Zhu et al. (30). Interleukin (IL)-8 and monocyte chemoattractant protein-1 (MCP-1) levels in the supernatant of treated cells and in the plasma of human and MCP-1 in the plasma of rats were determined by using ELISA kits from Fisher Thermo Scientific Co. (Rockford, IL) and from R & D Systems (Minneapolis, MN). We did not assay levels of IL-8 in the blood of diabetic rats because a rat-specific kit for IL-8 is not commercially available. All appropriate controls and standards as specified by the manufacturers were used; the data are expressed as cytokine secreted per two million cells or per milliliter of plasma. In the cytokine assay, control plasma samples were analyzed each time to check the variation from plate to plate on different days of analyses. The assays were repeated if the variation in control plasma value from day to day was >7%. Information on age and HbA1c was collected from patients' medical records in the clinic. Glycosylated hemoglobin in rats was determined by using Glyco-Tek Affinity column kits and reagents (catalog number 5351) purchased from Helena Laboratories (Beaumont, TX). This Glycated-hemoglobin method assesses HbA1. All chemicals were purchased from Sigma Chemicals (St. Louis, MO) unless otherwise mentioned. Data in figures is given as mean ± SEM. Data between different groups were analyzed statistically by using ANOVA on Ranks with Sigma Plot and Sigma Stat statistical software (SPSS, Chicago, IL). For cell-culture studies, Student's t test was used for data analyses. A value of p < 0.05 was considered significant.
Abbreviations Used
- HbA1
total glycosylated hemoglobin
- HbA1c
glycated hemoglobin
- HG
high glucose
- H2S
hydrogen sulfide
- IL-8
interleukin-8
- LC
l-cysteine
- MCP-1
monocyte chemoattractant protein-1
Acknowledgments
The authors are supported by grants from NIDDK and the Office of Dietary Supplements of the National Institutes of Health (RO1 DK064797 and RO1 DK072433). The authors thank Ms. Georgia Morgan for excellent editing of this article. None of the authors has any financial interest in the publication of this article.
References
- 1.Abe K. Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianca RDDV. Sorrentino R. Maffia P. Mirone V. Imbimbo C. Fusco F. Palma RD. Ignarro LJ. Cirino G. Hydrogen sulfide as a mediator of human corpus cavernosum smoth-muscle relaxation. Proc Natl Acad Sci USA. 2009;106:4513–4518. doi: 10.1073/pnas.0807974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blouet C. Mariotti F. Mikogami T. Tome D. Huneau JF. Meal cysteine improves postprandial glucose control in rats fed a high-sucrose meal. J Nutr Biochem. 2007;18:519–524. doi: 10.1016/j.jnutbio.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Blouet CB. Mariotti F. Azzout-Marniche D. Mathe V. Mikogami T. Tome D. Huneau JF. Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Radic Biol Med. 2007;42:1089–1097. doi: 10.1016/j.freeradbiomed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Candiloros H. Muller S. Zeghari N. Donner M. Drouin P. Ziegler O. Decreased erythrocyte membrane fluidity in poorly controlled IDDM: influence of ketone bodies. Diabetes Care. 1995;18:549–551. doi: 10.2337/diacare.18.4.549. [DOI] [PubMed] [Google Scholar]
- 6.Darmaun D. Smith SD. Sweeten S. Hartman BK. Welch C. Mauras N. Poorly controlled type 1 diabetes is associated with altered glutathione homeostasis in adolescents: apparent resistance to N-acetylcysteine supplementation. Pediatr Diabetes. 2008;9:577–582. doi: 10.1111/j.1399-5448.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 7.Darmaun D. Smith SD. Sweeten S. Sager BK. Welch S. Muras N. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes. Diabetes. 2005;54:190–196. doi: 10.2337/diabetes.54.1.190. [DOI] [PubMed] [Google Scholar]
- 8.Dröge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Phil Trans Royal Soc. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egashira K. Molecular mechanisms mediating inflammation in vascular disease: special reference to monocyte chemoattractant protein-1. Hypertension. 2003;41:834–841. doi: 10.1161/01.HYP.0000051642.65283.36. [DOI] [PubMed] [Google Scholar]
- 10.Furne J. Saeed A. Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accreted values. Am J Physiol Regular Integr Comp Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 11.Hâncu N. Netea MG. Baciu I. High glucose concentrations increase the tumor necrosis factor-alpha production capacity by human peripheral blood mononuclear cells. Rom J Physiol. 1998;35:325–330. [PubMed] [Google Scholar]
- 12.Harnandez C. Segura RM. Fonollos A. Carrasco E. Francisco G. Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Med. 2005;22:719–722. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 13.Hosoki R. Matsuki N. Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CC. Yen HF. Yin MC. Tsai CM. Hsieh CH. Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J Nutr. 2004;134:3245–3249. doi: 10.1093/jn/134.12.3245. [DOI] [PubMed] [Google Scholar]
- 15.Ishigami M. Hiraki K. Umemura K. Ogasawara Y. Ishii K. Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 16.Iwata H. Soga Y. Meguro M. Yoshizawa S. Okada Y. Iwamoto Y. Yamashita A. Takashiba S. Nishimura F. High glucose up-regulates lipopolysaccharide-stimulated inflammatory cytokine production via c-jun N-terminal kinase in the monocytic cell line THP-1. J Endotoxin Res. 2007;13:227–234. doi: 10.1177/0968051907082608. [DOI] [PubMed] [Google Scholar]
- 17.Jain SK. Velusamy T. Croad JL. Rains JL. Bull R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, oxidative stress and inhibits NFkB activation in the livers of Zucker diabetic rats. Free Radic Biol Med. 2009;46:1633–1638. doi: 10.1016/j.freeradbiomed.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha S. Calvert JW. Durankski MR. Ramachandran A. Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1124. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 21.Kimura Y. Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y. Dargusch R. Schubert D. Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 23.McDonnell CM. Pedreira CC. Vadamalayan B. Cameron FJ. Werther GA. Diabetic ketoacidosis, hyperosmolarity and hypernatremia: are high carbohydrate drinks worsening initial presentation? Pediatr Diabetes. 2005;6:90–94. doi: 10.1111/j.1399-543X.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 24.Lefer DJ. Granger DN. Monocyte rolling in early atherogenesis: vital role in lesion development. Circ Res. 1999;84:1353–1355. doi: 10.1161/01.res.84.11.1353. [DOI] [PubMed] [Google Scholar]
- 25.Olson KE. Is hydrogen sulfide a circulating gasotransmitter in vertebrate blood? Biochim Biophys Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya N. Mikami Y. Kimura Y. Nagahara N. Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. doi: 10.1093/jb/mvp111. (in press, 2009). [DOI] [PubMed] [Google Scholar]
- 28.Song D. Hutchings S. Pang CCY. Chronic N-acetylcysteine prevents fructose-induced insulin resistance and hypertension in rats. Eur J Pharmacol. 2005;508:205–210. doi: 10.1016/j.ejphar.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Yang G. Wu L. Jiang B. Yang W. Qi J. Cao K. Meng Q. Mustafa AK. Mu W. Zhang S. Snyder SH. Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu YZ. Wang ZJ. HO P. Loke YY. Zhu YC. Huang SH. Tan CS. Whiteman M. Lu J. Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]