Abstract
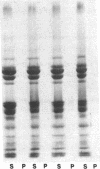
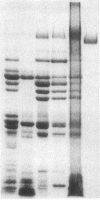
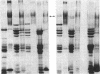
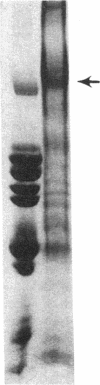
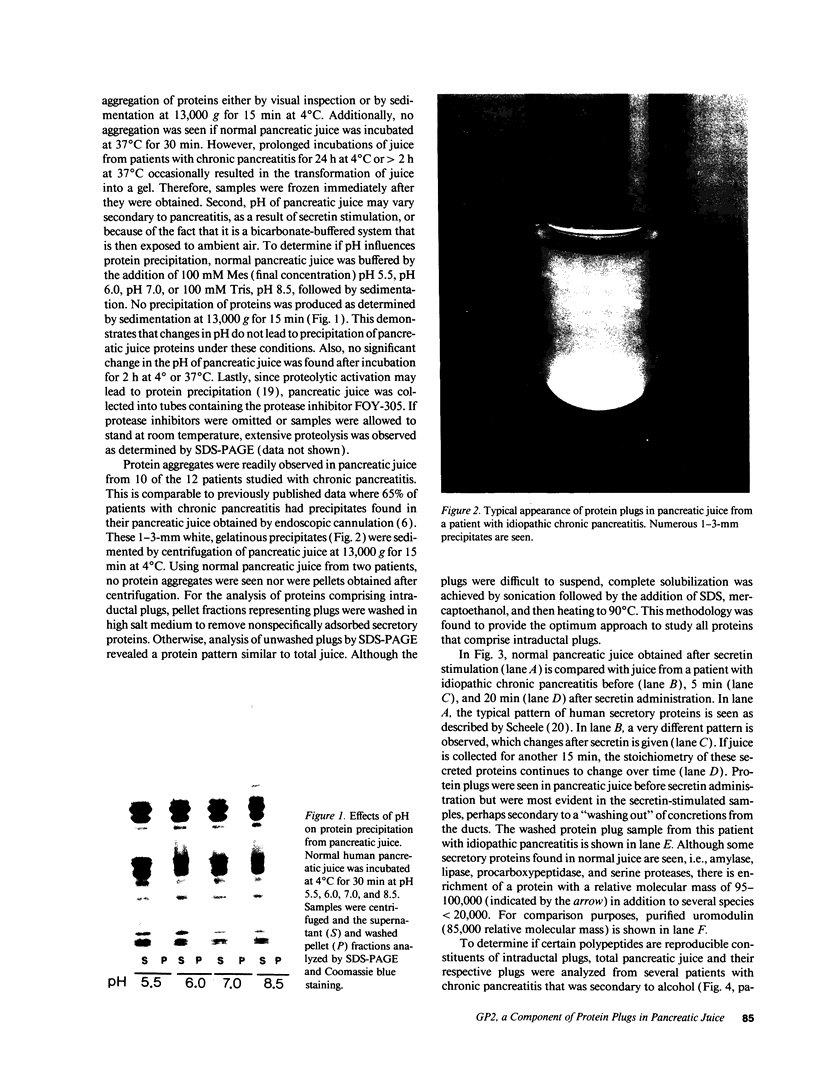
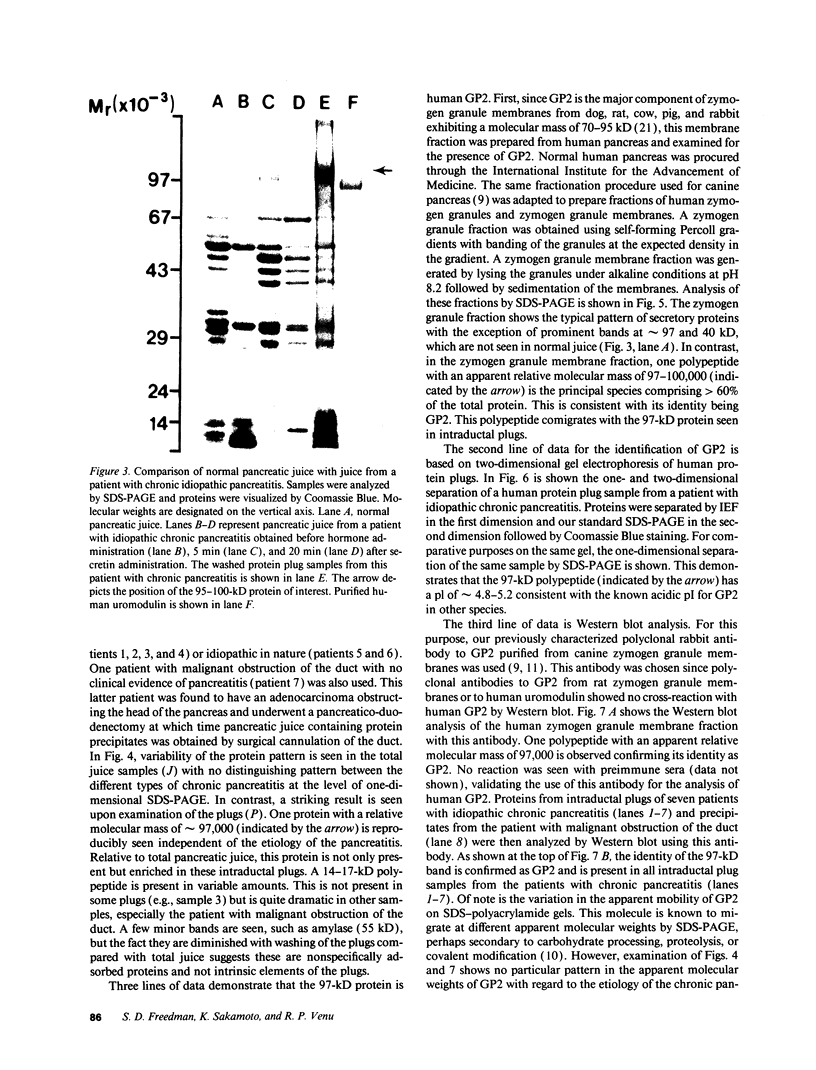
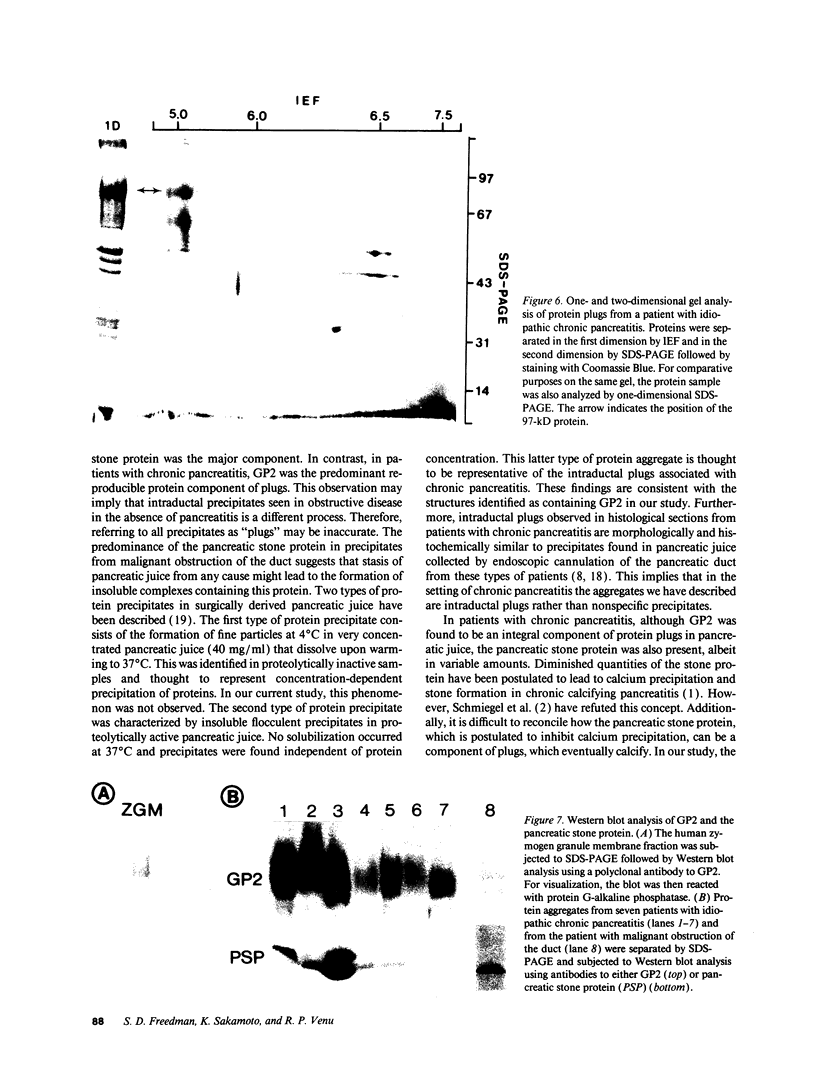
Protein plug obstruction of the pancreatic duct is one of the early events in chronic pancreatitis yet little is known about its pathogenesis. GP2, a protein in the exocrine pancreas, is a glycosyl phosphatidylinositol-anchored protein that is cleaved from the zymogen granule membrane and secreted into pancreatic juice. Since its homologue, uromodulin, is involved in renal cast formation, we asked the question whether GP2 might play a similar role in plug formation in chronic pancreatitis. The protein composition of intraductal plugs from patients with noncalcific chronic pancreatitis was examined. Plugs purified from pancreatic juice obtained by endoscopic cannulation were analyzed by SDS-PAGE. A 97-kD protein was found not only to be a reproducible constituent but also enriched within intraductal plugs. This protein was confirmed as GP2 by its localization to zymogen granule membranes, its isoelectric point, and by Western blotting. Although the pancreatic stone protein was identified in plugs, it was not a major reproducible component. These results demonstrate that GP2 is an integral component of plugs in pancreatic juice and suggest that GP2 may play a role in pancreatic plug formation that is analogous to the role played by uromodulin in the pathogenesis of renal casts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., White T. T. An alternate mechanism for the formation of protein plugs in chronic calcifying pancreatitis. Digestion. 1974;11(5-6):428–431. doi: 10.1159/000197611. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fukuoka S., Freedman S. D., Scheele G. A. A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2898–2902. doi: 10.1073/pnas.88.7.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka S., Freedman S. D., Yu H., Sukhatme V. P., Scheele G. A. GP-2/THP gene family encodes self-binding glycosylphosphatidylinositol-anchored proteins in apical secretory compartments of pancreas and kidney. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1189–1193. doi: 10.1073/pnas.89.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerolami A., Marteau C., Matteo A., Sahel J., Portugal H., Pauli A. M., Pastor J., Sarles H. Calcium carbonate saturation in human pancreatic juice: possible role of ductal H+ secretion. Gastroenterology. 1989 Mar;96(3):881–884. [PubMed] [Google Scholar]
- Guy O., Robles-Diaz G., Adrich Z., Sahel J., Sarles H. Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology. 1983 Jan;84(1):102–107. [PubMed] [Google Scholar]
- Harada H., Hayashi T., Miki H., Miyake H., Ochi K., Kimura I., Takeda M., Tanaka J., Tanaka T. Histochemical studies on enzyme-digested protein plugs of patients with chronic pancreatitis: a preliminary report. Acta Med Okayama. 1983 Jun;37(3):227–231. doi: 10.18926/AMO/32430. [DOI] [PubMed] [Google Scholar]
- Harada H., Miyake H., Miki H., Kobayashi T., Sasaki T., Kimura I. Role of endoscopic elimination of protein plugs in the treatment of chronic pancreatitis. Gastroenterol Jpn. 1982 Oct;17(5):463–468. doi: 10.1007/BF02774724. [DOI] [PubMed] [Google Scholar]
- Harada H., Ueda O., Yasuoka M., Nakamura T., Hayashi T., Kobayashi T., Kimura I. Scanning electron-microscopic studies on protein plugs obtained from patients with chronic pancreatitis. Gastroenterol Jpn. 1982 Apr;17(2):98–101. doi: 10.1007/BF02774547. [DOI] [PubMed] [Google Scholar]
- Harada H., Ueda O., Yasuoka M., Nakamura T., Kunichika K., Ikubo I., Kochi F., Shigetoshi M., Yabe H., Hanafusa E. Histochemical studies on protein plugs obtained by endoscopic retrograde catheterization of the papilla. Gastroenterol Jpn. 1981 Dec;16(6):563–567. doi: 10.1007/BF02813790. [DOI] [PubMed] [Google Scholar]
- Havinga J. R., Slot J. W., Strous G. J. Membrane detachment and release of the major membrane glycoprotein of secretory granules in rat pancreatic exocrine cells. Eur J Cell Biol. 1985 Nov;39(1):70–76. [PubMed] [Google Scholar]
- Hoops T. C., Rindler M. J. Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm-Horsfall protein. J Biol Chem. 1991 Mar 5;266(7):4257–4263. [PubMed] [Google Scholar]
- MacDonald R. J., Ronzio R. A. Comparative analysis of zymogen granule membrane polypeptides. Biochem Biophys Res Commun. 1972 Oct 17;49(2):377–382. doi: 10.1016/0006-291x(72)90421-4. [DOI] [PubMed] [Google Scholar]
- McQueen E. G., Engel G. B. Factors determining the aggregation of urinary mucoprotein. J Clin Pathol. 1966 Jul;19(4):392–396. doi: 10.1136/jcp.19.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen E. G., Engel G. B. Factors determining the aggregation of urinary mucoprotein. J Clin Pathol. 1966 Jul;19(4):392–396. doi: 10.1136/jcp.19.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalto G., Bonicel J., Multigner L., Rovery M., Sarles H., De Caro A. Partial amino acid sequence of human pancreatic stone protein, a novel pancreatic secretory protein. Biochem J. 1986 Aug 15;238(1):227–232. doi: 10.1042/bj2380227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multigner L., Sarles H., Lombardo D., De Caro A. Pancreatic stone protein. II. Implication in stone formation during the course of chronic calcifying pancreatitis. Gastroenterology. 1985 Aug;89(2):387–391. doi: 10.1016/0016-5085(85)90341-5. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Sarles H., Payan H. Three-dimensional reconstruction of the pancreatic ducts in chronic pancreatitis. Gastroenterology. 1972 May;62(5):942–949. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Provansal-Cheylan M., Bernard J. P., Mariani A., Soehendra N., Cremer M., Sahel J., Sarles H. Occluded pancreatic endoprostheses--analysis of the clogging material. Endoscopy. 1989 Mar;21(2):63–69. doi: 10.1055/s-2007-1012902. [DOI] [PubMed] [Google Scholar]
- Renner I. G., Rinderknecht H., Douglas A. P. Profiles of pure pancreatic secretions in patients with acute pancreatitis: the possible role of proteolytic enzymes in pathogenesis. Gastroenterology. 1978 Dec;75(6):1090–1098. [PubMed] [Google Scholar]
- Rindler M. J., Hoops T. C. The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol. 1990 Oct;53(1):154–163. [PubMed] [Google Scholar]
- Rouimi P., Bonicel J., Rovery M., De Caro A. Cleavage of the Arg-Ile bond in the native polypeptide chain of human pancreatic stone protein. FEBS Lett. 1987 Jun 1;216(2):195–199. doi: 10.1016/0014-5793(87)80688-9. [DOI] [PubMed] [Google Scholar]
- Sahel J., Sarles H. Modifications of pure human pancreatic juice induced by chronic alcohol consumption. Dig Dis Sci. 1979 Dec;24(12):897–905. doi: 10.1007/BF01311942. [DOI] [PubMed] [Google Scholar]
- Schmiegel W., Burchert M., Kalthoff H., Roeder C., Bützow G., Grimm H., Kremer B., Soehendra N., Schreiber H. W., Thiele H. G. Immunochemical characterization and quantitative distribution of pancreatic stone protein in sera and pancreatic secretions in pancreatic disorders. Gastroenterology. 1990 Nov;99(5):1421–1430. doi: 10.1016/0016-5085(90)91171-2. [DOI] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi T., Fujii Y., Takeda M., Tanaka J., Harada H., Oka H. A case of chronic pancreatitis successfully treated by endoscopic removal of protein plugs. Acta Med Okayama. 1984 Apr;38(2):169–174. doi: 10.18926/AMO/30336. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C. Uromucoid (Tamm-Horsfall glycoprotein) forms different polymeric arrangements on a filter surface under different physicochemical conditions. Clin Chim Acta. 1987 Feb 15;162(3):329–340. doi: 10.1016/0009-8981(87)90052-0. [DOI] [PubMed] [Google Scholar]