Abstract
Pathogenic Leptospira spp. shed in the urine of reservoir hosts into freshwater can be transmitted to a susceptible host through skin abrasions or mucous membranes causing leptospirosis. The infection process involves the ability of leptospires to adhere to cell surface and extracellular matrix components, a crucial step for dissemination and colonization of host tissues. Therefore, the elucidation of novel mediators of host-pathogen interaction is important in the discovery of virulence factors involved in the pathogenesis of leptospirosis. In this study, we assess the functional roles of transmembrane outer membrane proteins OmpL36 (LIC13166), OmpL37 (LIC12263), and OmpL47 (LIC13050), which we recently identified on the leptospiral surface. We determine the capacity of these proteins to bind to host tissue components by enzyme-linked immunosorbent assay. OmpL37 binds elastin preferentially, exhibiting dose-dependent, saturating binding to human skin (Kd, 104±19 nM) and aortic elastin (Kd, 152±27 nM). It also binds fibrinogen (Kd, 244±15 nM), fibrinogen fragment D (Kd, 132±30 nM), plasma fibronectin (Kd, 359±68 nM), and murine laminin (Kd, 410±81 nM). The binding to human skin elastin by both recombinant OmpL37 and live Leptospira interrogans is specifically enhanced by rabbit antiserum for OmpL37, suggesting the involvement of OmpL37 in leptospiral binding to elastin and also the possibility that host-generated antibodies may promote rather than inhibit the adherence of leptospires to elastin-rich tissues. Further, we demonstrate that OmpL37 is recognized by acute and convalescent leptospirosis patient sera and also by Leptospira-infected hamster sera. Finally, OmpL37 protein is detected in pathogenic Leptospira serovars and not in saprophytic Leptospira. Thus, OmpL37 is a novel elastin-binding protein of pathogenic Leptospira that may be promoting attachment of Leptospira to host tissues.
Author Summary
Leptospirosis is a potentially fatal disease in humans and livestock caused by Leptospira bacteria. Effective antibiotic treatment depends on timely, accurate diagnosis. However, current diagnostic and vaccine options are limited by their specificity for the lipid-sugar coat of leptospires, which varies among 200 serum-reactive groups. We aim to understand how leptospires infect a host, and in turn, to develop broadly effective diagnostic and immunization products. We recently described OmpL37, a new protein on the surface of leptospires. Here, we show it is made by pathogenic strains, suggesting it can be a target for detecting and protecting against a wide range of Leptospira. Moreover, leptospirosis patients and hamsters infected with leptospires make antibodies against OmpL37. Purified OmpL37 binds host proteins, including human elastin, fibrinogen, fibronectin, and mouse laminin. Although other leptospiral proteins bind multiple host proteins, OmpL37 has novel preferential affinity for skin and aorta elastin, suggesting a role in a common route of transmission through abraded skin and exposed blood vessels. Indeed, OmpL37 binding and leptospiral attachment to elastin are both enhanced by OmpL37 antiserum, further implicating a possible role for OmpL37 during infection. Thus, OmpL37 may mediate host attachment and has potential clinical application with a broad range of Leptospira.
Introduction
Leptospirosis is a zoonosis caused by pathogenic Leptospira spp. transmitted from reservoir hosts (typically rodents) to humans via water contaminated by infected animals and has a significant impact on public health throughout the developing world [1]–[4]. Leptospirosis also has significant adverse effects on the agricultural industry by causing abortions, infertility, and death in livestock [5], [6]. After being shed in the urine of a reservoir host animal, leptospires can persist in freshwater or soil until contact with abraded skin or mucous membranes of a new host occurs. The resulting infection is potentially fatal, and is frequently characterized by jaundice, renal failure, and/or pulmonary hemorrhage [1], [4], [7]. Large outbreaks of leptospirosis occur in tropical and subtropical regions after heavy rainfall and the dispersal of leptospires in contaminated water [3], [8]. Current vaccines against leptospirosis target the lipopolysaccharide (LPS) coat of the leptospires, which is highly variable; this variation is thought to be the major antigenic determinant defining the differences between approximately 230 serovars that contribute to serovar-specific immunity [6], [9]. In contrast, vaccines directed towards well-conserved leptospiral outer membrane proteins (OMPs) [10], [11] would have an advantage in inducing cross-protective immunity [12].
The leptospiral lifecycle involves interactions with host tissues at multiple stages of infection, including: (i) entering the host, (ii) evading its immune response, and (iii) adhering to tissues [13]–[15]. Identification and characterization of novel proteins that mediate the stage-specific interactions with the host are essential for the understanding of leptospiral pathogenesis, and in the development of diagnostic and protective antigens for leptospirosis. Pathogenic leptospires have been shown to bind to a variety of host ligands, including fibronectin, fibrinogen, collagen, laminin, elastin, and proteoglycans, indicating that cell surface and extracellular matrix (ECM)-binding OMPs, or adhesins, are likely to be expressed by the spirochetes [16]–[21]. It is possible that leptospires express distinct adhesins at different stages of infection, including initial attachment, dissemination, and colonization. Many leptospiral proteins, including LigA/B, Lsa21, Lsa27, Lsa63, Lsa24 (LfhA/LenA), LenB to F, LipL32, Lp95, TlyC, and LipL53, have been shown to have affinity for host ligands in vitro [18], [19], [21]–[32]. However, it is unclear to what extent these putative adhesins mediate interactions of leptospires with cell surface and ECM proteins. Only Lsa24, LigA/B, and Lsa63 have been tested for their capacity to inhibit leptospiral adherence to ECM proteins [18], [21], [24], [32]. In each case, only partial inhibition was observed, suggesting that additional fibronectin-, laminin-, collagen-, and elastin-binding proteins likely exist in Leptospira [18], [21], [24], [32].
In this study, we investigated whether the surface-exposed proteins in Leptospira, OmpL36 (LIC13166), OmpL37 (LIC12263), and OmpL47 (LIC13050) that we recently described [33] can bind to any host ligands. We now report that OmpL37 is the first leptospiral protein found to have pronounced specificity for human skin elastin. OmpL37 exhibits strong, saturating binding to skin elastin with one of the highest affinities of all leptospiral ligand-binding proteins described. In addition, OmpL37 binds efficiently to human aortic elastin, fibrinogen, fibrinogen fragment D, and to a lesser extent laminin and plasma fibronectin. OmpL47 also binds to laminin, plasma fibronectin, fibrinogen, fibrinogen fragment D, along with collagen type III, and aortic elastin, but showing much lower activities than OmpL37. OmpL36 shows no binding to any of the host tissue components investigated.
Elastin is a connective tissue component of ECM responsible for the elasticity and resilience of skin, lung, blood vessels, uterus, placenta, and other tissues [34]–[36]. These elastin-rich tissues are highly relevant to leptospirosis as infection includes entry through skin abrasions or mucous membranes, dissemination through the circulation, and attachment to vascular, renal, pulmonary, uterine, and other tissues. Until our discovery of the elastin-binding properties of OmpL37, only LigB was known to have the capacity to bind elastin and tropoelastin [21]. We also show that OmpL37 antiserum can enhance the binding to skin elastin by both live Leptospira and recombinant OmpL37, suggesting that leptospiral binding to elastin is at least partially mediated by OmpL37. Expression of OmpL37 during infection is confirmed with the recognition of OmpL37 by sera from Leptospira-infected hamsters and also acute and convalescent leptospirosis patients. While the gene for an OmpL37 homologue is present in saprophytic leptospires, OmpL37 is detectable only in pathogenic Leptospira serovars. Taken together, our data suggest that OmpL37 is an elastin-binding protein of Leptospira with potential roles in leptospirosis, including the attachment to elastin-rich tissues, such as the dermis, vasculature, and lungs.
Materials and Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of the Research and Development Committee, VA Greater Los Angeles Healthcare System, Research Service (PCC # 2008-121778).
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the VA Greater Los Angeles Healthcare System, Research Service (PCC # 2009-010088).
Bacterial strains and growth conditions
Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130 was isolated from a patient during a leptospirosis outbreak in Salvador, Brazil [8]. L. interrogans serovar Pomona strain PO-01, L. kirscheneri serovars Mazdok strain 5621 and Grippotyphosa strain RM52, L. borgpetersenii serovars Tarrasovi strain Perepelicin and Javanica strain Veldrat Bataviae 46, Leptonema illini strain 3055, Leptospira weilii serovar Celledoni strain Celledoni, L. wolbachii serovar Biflexa strain codice, L. inadai serovar Lyme strain 10, and L. biflexa serovar Patoc strain Patoc 1 were obtained from the National Leptospirosis Reference Center (National Animal Disease Center, Agricultural Research Service, U.S. Department of Agriculture, Ames, Iowa). Leptospires were cultivated in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium supplemented with 1% rabbit serum (Rockland Immunochemicals, Gilbertsville, PA) and 100 µg/ml 5-fluorouracil at 30°C [37].
Antibodies and serum samples
The polyclonal rabbit sera specific for OmpL37, OmpL47, and OmpL54 have been described previously [33]. Immunoglobulins G (IgG) from OmpL37 and OmpL47 antisera were purified by Melon Gel IgG spin purification kit (Thermo Scientific, Rockford, IL) according to manufacturer's instructions. LipL32 monoclonal antibody 1D9 [38], [39] was a kind gift from Dr. José Antonio Guimarães Aleixo (Universidade Federal De Pelotas, Pelotas, Brazil). Pooled sera from infected Syrian hamsters (Harlan Laboratories) were obtained ten days following intradermal challenge of one month-old animals with L. interrogans L1-130. As a negative control, a serum sample from a hamster injected intradermally with EMJH was collected after 12 days. Patient sera from leptospirosis outbreaks in 1996 and 1997 in Salvador, Brazil, were kindly provided by Dr. Albert I. Ko (Oswaldo Cruz Foundation, Salvador, Bahia, Brazil). Acute and convalescent samples from the same patients were prepared by pooling sera from 13 individuals with laboratory-confirmed leptospirosis. Normal human serum was obtained from Thermo Scientific and Millipore (Billerica, MA).
Cloning, expression, and purification of recombinant LIC10091
The gene encoding potential outer membrane lipoprotein, LIC10091 (LipL40) [40], was amplified from Fiocruz L1-130 DNA using forward primer, 5′- TTCGCATATGAAAACGCCTCCTCCTAAAG -3′, and reverse primer, 5′- TAAAATCTCGAGTTTCAAAACTTCTACGGGC- 3′, by PCR conditions as described for ompL36, ompL37, and ompL47 [33]. PCR product was digested with NdeI and XhoI (New England BioLabs, Ipswich, MA), cloned into NdeI- and XhoI- digested expression vector, pET-20b(+) (Novagen, San Diego, CA), and purified as previously described for OmpL36, OmpL37, and OmpL47 [33].
Gel electrophoresis and immunoblotting
Protein samples were boiled for 5 min in Novex NuPAGE sample buffer (Invitrogen, Carlsbad, CA) in the presence of 2.5% β-mercapthoethanol and separated in Bis-Tris 4–12% polyacrylamide gradient NuPAGE gels (Invitrogen).
For immunoblotting, proteins were transferred to a polyvinylidene difluoride (PVDF) Immobilon-P membrane (Millipore) and probed with rabbit polyclonal antisera, Syrian hamster sera, or leptospirosis patient sera. Bound antibodies were detected using horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (GE Lifesciences, Buckinghamshire, England), anti-Syrian hamster IgG (Jackson Immuno Research, West Groove, PA), or anti-human IgG (Sigma-Aldrich, St. Louis, MO), respectively. Immunoblots were visualized by enhanced chemiluminescence reagents according to the manufacturer's instructions (Thermo Scientific).
Enzyme-linked immunosorbent assay (ELISA) of binding to host ligands by OmpL proteins
Host ligands included human plasma fibronectin (Sigma-Aldrich), human plasma fibronectin 30-kDa proteolytic fragment (heparin-binding domain, Sigma-Aldrich), human plasma fibronectin 45-kDa proteolytic fragment (gelatin-binding domain, Sigma-Aldrich), human fibroblast fibronectin (Calbiochem, La Jolla, CA), human plasma fibrinogen (HYPHEN BioMed, France), human plasma fibrinogen fragment D (HYPHEN BioMed), murine laminin (Sigma-Aldrich), bovine skin collagen type I (Sigma-Aldrich), human placenta collagen type III (Sigma-Aldrich), human placenta collagen type IV (Sigma-Aldrich), soluble human skin elastin (Elastin Products Company, Owensville, MO), soluble human aorta elastin (Sigma-Aldrich), bovine kidney heparan sulfate (Sigma-Aldrich), shark cartilage chondroitin sulfate (Sigma-Aldrich), fetal calf serum fetuin (Sigma-Aldrich), and bovine serum albumin (BSA, Sigma-Aldrich). Ultra-high binding Immulon 4HBX microtiter plates (Thermo Scientific) were coated with 1 µg of host ligand in 0.1 ml of phosphate buffered saline (PBS), pH 7.2, and incubated overnight at 4°C. Fresh (stored for less than 4 months at 4°C) recombinant OmpL36, OmpL37, OmpL47 [33], and LIC10091 (used as a negative control) binding to individual ligands was assessed by ELISA. Briefly, non-specific binding sites were blocked with Protein-Free Blocking buffer (PFBb; Thermo Scientific) for 1 h at room temperature and 1 µg of recombinant protein in 0.1 ml of PFBb was added per well and incubated for 1 h at 37°C. For assays of ligand binding as a function of leptospiral protein concentration, serial dilutions of recombinant OmpL37 and OmpL36 (negative control) ranging from 0 to 2 µM in 0.1 ml of PFBb were added to wells and incubated for 1 h at 37°C. Wells were washed three times with PBS, pH 7.2, and bound protein was detected by probing with anti-His Tag monoclonal antibody (5 Prime, Gaithersburg, MD), developing with HRP-conjugated anti-mouse IgG (Novagen) and a tetramethyl benzidine substrate (Thermo Scientific), and recording by spectrophotometry at 450 nm. For saturating binding the apparent dissociation constant (Kd) was estimated as the concentration of OmpL37 resulting in half-maximal binding.
For assays assessing effects of antibodies on binding of recombinant OmpL37 to skin elastin, recombinant OmpL37 was pre-incubated either with serum against OmpL37 or OmpL47 (negative control) at 1∶2500 dilution or purified IgG at 1∶40 to 1∶640 serial dilutions in PFBb at room temperature for 30 min. In addition, OmpL37 was pre-incubated with convalescent leptospirosis patient sera or Leptospira-infected hamster sera at 1∶640 to 1∶10,240 serial dilutions in PFBb at room temperature for 1 h. Then mixtures containing 0.5 µg of OmpL37 in 0.1 ml of PFBb were added to microtiter wells, incubated for 1 h at 37°C, and bound protein detected as described above.
ELISA of immobilized OmpL37 binding to freely soluble host ligands
Oneµg of recombinant OmpL37 or OmpL36 (negative control) in 0.1 ml PBS, pH 7.2, was used to coat Immulon 4HBX microtiter wells overnight at 4°C. Non-specific binding sites were blocked with PFBb and 1 µg of human plasma fibronectin, human plasma fibrinogen, human plasma fibrinogen fragment D, murine laminin, bovine skin collagen type I, human collagen type IV, or human skin elastin in 0.1 ml of PFBb was added and incubated for 1 h at room temperature. After three washes with PBS, pH 7.2, OmpL37-bound host ligands were detected by probing with anti-human fibronectin rabbit antibody (Sigma-Aldrich), anti-human fibrinogen rabbit IgG (HYPHEN BioMed), anti-laminin rabbit IgG (Sigma-Aldrich), anti-collagen type I monoclonal antibody (Clone COL-1, Sigma-Aldrich), anti-collagen type IV monoclonal antibody (Clone COL-94, Sigma-Aldrich), or anti-elastin rabbit IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), then adding HRP-conjugated anti-mouse IgG (Novagen) or HRP-conjugated anti-rabbit IgG (GE Lifesciences) and developing as described above.
ELISA of leptospiral binding to skin elastin
Microtiter plates were coated with human skin elastin and non-specific binding sites were blocked as described above. L. interrogans cultures were harvested by centrifugation at 2000× g for 15 min at room temperature and resuspended in PBS-5 mM MgCl2 to a final concentration of 1×109 cells/ml. To assess the effect of the OmpL37 antibodies on leptospiral binding to elastin, serial dilutions (1∶40 to 1∶1280) of anti-OmpL37 or anti-OmpL54 (used as a negative control) were mixed with the leptospires or PBS-5 mM MgCl2 and 1×108 cells in 0.1 ml of PBS-5 mM MgCl2 were added to the microtiter wells. For experiments assessing the inhibition of leptospiral binding by recombinant OmpL37, prior to the addition of leptospires, 0.5 µM of recombinant protein in 0.1 ml of PFBb was either added directly to elastin-coated microtiter wells or pre-incubated with anti-OmpL37 or anti-OmpL47 (negative control) at a 1∶500 dilution for 30 min at room temperature and added to the microtiter wells. Plates were incubated for 1 h at 37°C, and washed three times with PBS. After addition of leptospires, plates were incubated at 30°C for 90 min, unbound leptospires were removed by four washes with PBS-5 mM MgCl2, and adherent cells were fixed with methanol at −20°C for 10 min. Elastin-bound leptospires were detected by probing with LipL32 monoclonal antibody 1D9 and developing as described above.
To measure the effect of OmpL37 antibodies on the binding of immobilized leptospires to freely soluble skin elastin, 1×108 of L. interrogans in PBS-5 mM MgCl2 were allowed to adhere to Immulon 4HBX microtiter wells for 90 min at 30°C, washed twice with PBS-5 mM MgCl2, and non-specific binding sites were blocked with PFBb for 30 min at room temperature. Rabbit sera for OmpL37 and OmpL47 (negative control) diluted 1∶40 and 1∶2560 in PBS-5 mM MgCl2 were added to the wells and incubated for 90 min at room temperature, followed by three washes with PBS-5 mM MgCl2. One µg of human skin elastin in 0.1 ml PBS-5 mM MgCl2 was added and incubated overnight at 4°C. Wells were washed three times with PBS, pH 7.2, and cell-bound complexes were fixed with methanol at −20°C for 10 min, followed by additional blocking with PFBb. Leptospira-bound elastin was detected by probing with anti-elastin mouse serum (Novus Biologicals, Littleton, CO) and developing as described above.
Results
Recombinant OmpL37 binds host tissue components
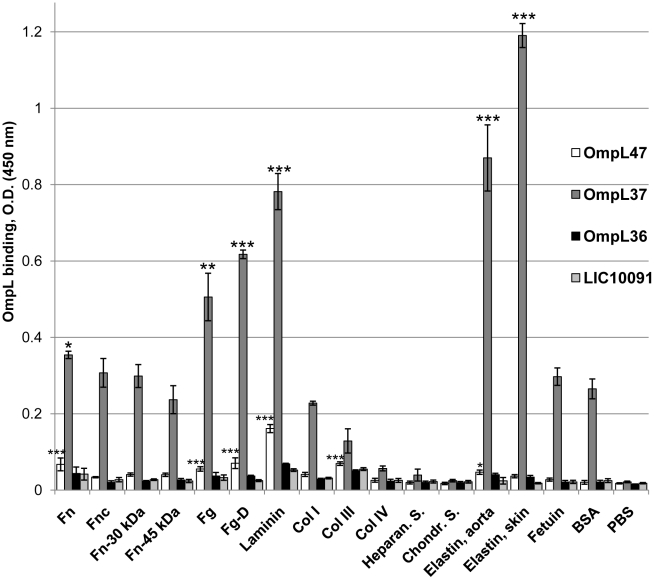
To investigate the capacities of our recently described surface-exposed transmembrane OmpL proteins [33] to interact with host tissue ligands, soluble recombinant OmpL36, OmpL37, and OmpL47 were assessed for binding to immobilized host components. BSA and the highly glycosylated serum protein, fetuin, were used as controls for non-specific binding. Soluble recombinant LIC10091 was used as a non-binding protein control. OmpL37 exhibited significant binding to ECM components, such as human skin (P<0.001 compared to BSA) and aorta elastin (P<0.001), laminin (P<0.001), fibrinogen (P<0.01), fibrinogen fragment D (P<0.001), and plasma fibronectin (P<0.05) (Fig. 1). OmpL37 binding to the 30-kDa and 45-kDa fragments of plasma fibronectin, fibroblast fibronectin, collagen types I, III, and IV, heparan sulfate, and chondroitin sulfate was not statistically significant (Fig. 1). OmpL47 also showed significant binding to laminin (P<0.001), fibrinogen (P<0.001), fibrinogen fragment D (P<0.001), plasma fibronectin (P<0.001), collagen III (P<0.001), and aorta elastin (P<0.05). However, the OmpL47 activities were much lower than those observed for OmpL37 and were not investigated further. None of the other recombinant proteins exhibited significant binding to any of the host tissue components investigated (Fig. 1).
Figure 1. Binding of recombinant Omp36, OmpL37, and OmpL47 to host tissue components.
Microtiter wells were coated with 1 µg of plasma fibronectin (Fn), fibroblast cellular fibronectin (Fnc), heparin-binding domain of plasma fibronectin (Fn-30 kDa), gelatin-binding domain of plasma fibronectin (Fn-45 kDa), plasma fibrinogen (Fg), plasma fibrinogen fragment D (Fg-D), laminin (Lm), collagen type I (Col I), collagen type III (Col III), collagen type IV (Col IV), kidney heparan sulfate (Heparan. S.), cartilage chondroitin sulfate (Chondr. S.), aorta elastin, skin elastin, fetuin, and BSA. One microgram of recombinant protein was added per well and binding was measured by ELISA. Data represent the mean absorbance at 450 nm ± the standard deviation of three independent experiments. The binding of recombinant proteins to tissue components was compared to their binding to BSA by Student's two-tailed t test (*** P<0.001, ** P<0.01, * P<0.05).
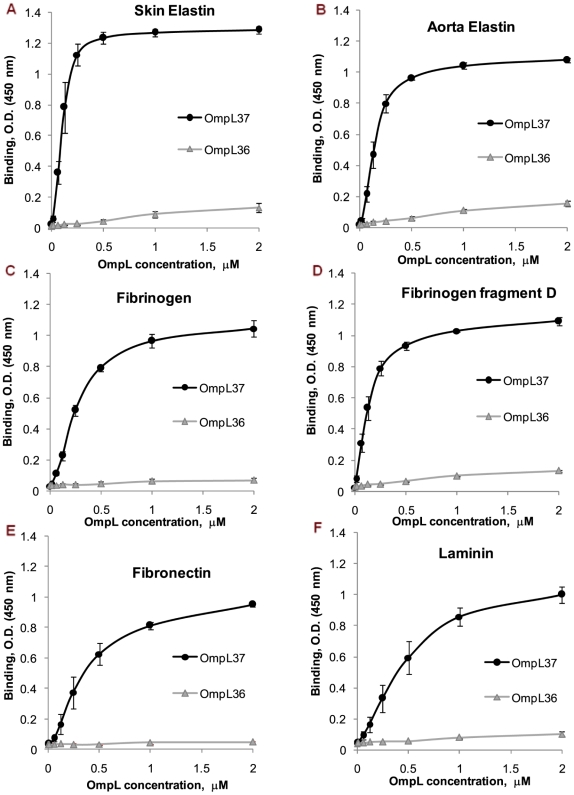
In order to compare binding affinities, the interaction with immobilized skin and aorta elastin, fibrinogen, fibrinogen fragment D, plasma fibronectin, plasma fibronectin 30-kDa and 45-kDa fragments, and laminin was measured as a function of OmpL37 concentration from 0 to 2 µM (Fig. 2 and Table 1). OmpL37 exhibited very strong, saturating binding to skin elastin (Kd , 104±19 nM), aorta elastin (Kd , 152±27 nM), fibrinogen (Kd , 244±15 nM), and fibrinogen fragment D (Kd , 132±30 nM) as estimated by ELISA from three independent experiments. OmpL37 binding to plasma fibronectin (Kd , 359±68 nM), its 30-kDa (Kd , 408±94 nM) and 45-kDa fragment (Kd , 460±70 nM), and laminin (Kd , 410±81 nM) were noticeably lower (Fig. 2 and Table 1). OmpL36 was used as a negative control based on our observation that OmpL36 does not bind to host ligands (Fig. 1). The comparison of apparent Kds for the saturation binding of host proteins by recombinant OmpL37 is summarized in Table 1.
Figure 2. Binding to skin and aorta elastin, fibrinogen, fibrinogen fragment D, fibronectin, and laminin as a function of OmpL37 concentration.
Binding of OmpL37 (concentration ranging from 0 to 2 µM) to 1 µg of immobilized (A) human skin elastin, (B) human aorta elastin, (C) human plasma fibrinogen, (D) human plasma fibrinogen fragment D, (E) human plasma fibronectin, and (F) murine laminin was measured by ELISA. The mean optical density at 450 nm ± the standard deviation of three independent experiments is shown at each point. The apparent Kd for saturating binding was estimated as the concentration of recombinant OmpL37 resulting in half-maximal binding (see text and Table 1). OmpL36 served as a negative control.
Table 1. Host tissue component binding by OmpL37.
| Mean Kd a ± SD | ||||||||
| Elastin, skinb | Elastin, aorta | Fibrinogen | Fg-D | Fn | Fn-30 kDa | Fn-45 kDa | Laminin | BSA |
| 104±19 | 152±27 | 244±15 | 132±30 | 359±68 | 408±94 | 460±70 | 410±81 | NS |
Estimated as nanomolar concentration at half-maximal binding by OmpL37. NS, not saturating.
Abbreviations: Fg-D, fibrinogen fragment D; Fn, plasma fibronectin; Fn-30 kDa, plasma fibronectin 30 kDa fragment (heparin-binding domain); Fn-45 kDa, plasma fibronectin 45 kDa fragment (gelatin-binding domain).
In addition, we investigated whether OmpL37 immobilized on microtiter wells bound freely soluble host proteins (Fig. 3). Immobilized OmpL37 exhibited significant binding to free laminin (P<0.001 compared to collagen IV), human skin elastin (P<0.001), and plasma fibronectin (P<0.05) (Fig. 3). It is evident that immobilized OmpL37 binds to virtually the same host proteins as freely soluble OmpL37, with the exception of fibrinogen and fibrinogen fragment D (Fig. 1 and 3). Although immobilized OmpL37 can bind to freely soluble ligands, the interaction appears to be weaker when compared to that of free OmpL37 binding to immobilized ligands (Fig. 1 and 3). This could be due to an impairment of the active conformation of OmpL37 caused by its immobilization to the microtiter wells, to the differences between antibodies utilized for detection or to the intrinsic property of less efficient binding of soluble versus immobilized host proteins, which has been described for pathogenic bacteria and their surface proteins [41]–[43].
Figure 3. Binding of immobilized OmpL37 to freely soluble host proteins.
Microtiter plates were coated with 1 µg of recombinant OmpL37 or OmpL36. One µg of plasma fibronectin (Fn), plasma fibrinogen (Fg), plasma fibrinogen fragment D (Fg-D), laminin (Lm), collagen type I (Col I), collagen type IV (Col IV), or skin elastin was added per well and binding was measured by ELISA. Data represent the mean absorbance at 450 nm ± the standard deviation from triplicate wells. The binding of recombinant OmpL37 to tissue components was compared to its binding to collagen type IV, which was chosen as a host protein control for non-specific binding based on our data in Fig. 1, by Student's two-tailed t test (** P<0.001, * P<0.05).
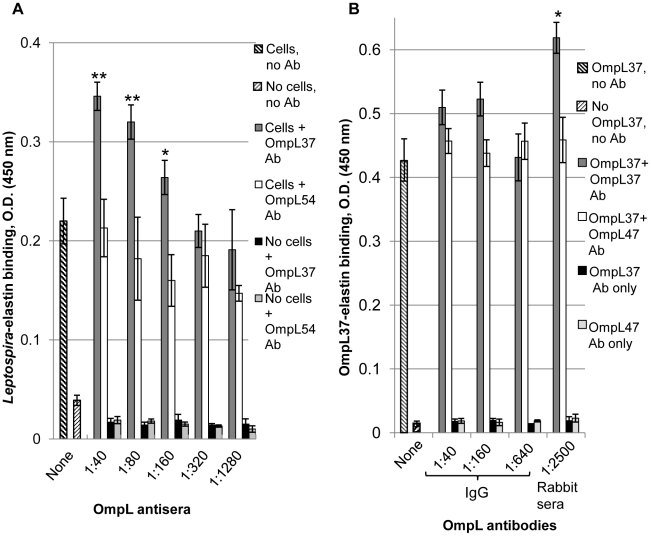
OmpL37 antiserum enhances leptospiral binding to skin elastin
We examined whether OmpL37 mediates the attachment of leptospires to human skin elastin by testing for OmpL37 antiserum inhibition of live Leptospira binding to immobilized skin elastin in an ELISA. Surprisingly, the OmpL37 antiserum enhanced adhesion in a dose-dependent manner, whereas antiserum for another surface-exposed OMP, OmpL54, had no effect (Fig. 4A). Leptospiral binding to skin elastin was enhanced by the OmpL37 antiserum at dilutions of 1∶40 (1.6-fold compared to no antibody, P<0.001), 1∶80 (1.5-fold, P<0.001), and 1∶160 (1.3-fold, P<0.05); the effect was not evident at higher dilutions (Fig. 4A). The increase in adhesion was not due to leptospiral agglutination as determined with a systematic examination for the presence of leptospiral aggregates or decrease in leptospiral numbers in multiple fields by dark-field microscopy (data not shown). We also investigated the effect of OmpL37 antiserum on the binding to free skin elastin by Leptospira immobilized on microtiter wells and found that a 1∶40 dilution of antiserum increased binding 1.4-fold (data not shown). Further, we assessed the effect of the OmpL37 antibodies on the binding of recombinant OmpL37 to skin elastin (Fig. 4B). The OmpL37 antiserum significantly enhanced recombinant OmpL37 binding to skin elastin at a dilution of 1∶2500 (1.4-fold, P<0.05), a >10-fold lower antiserum concentration compared to that required for an effect on the adhesion of leptospires (Fig. 4A and 4B). IgG purified from the OmpL37 antiserum slightly enhanced (1.2-fold) OmpL37 binding to skin elastin at dilutions of 1∶40 to 1∶160 (Fig. 4B); however the effect was not statistically significant (P>0.05), indicating the possibility that along with OmpL37-specific antibody, another serum component removed in the purification of IgG could be required for binding enhancement. The antiserum and purified IgG for another surface-exposed OMP, OmpL47, was used as a negative control and did not have any effect on OmpL37 binding (Fig. 4B). The OmpL37 antiserum did not exhibit any statistically significant effect on recombinant OmpL37 binding to fibronectin, fibrinogen, and laminin (data not shown). Further, we investigated whether convalescent leptospirosis patient sera enhance leptospiral binding to skin elastin. Dark-field microscopy revealed leptospiral agglutination by patient sera (data not shown), which prevented the assessment of leptospiral adhesion. We also investigated the effects of convalescent leptospirosis patient sera and Leptospira-infected hamster sera on recombinant OmpL37 binding to skin elastin but no statistically significant enhancement was observed (Fig. S1 and S2). We also tested for the inhibition of leptospiral binding to skin elastin by pre-treating the wells with a saturating concentration (0.5 µM) of recombinant OmpL37 alone or pre-incubated with the OmpL37 antiserum and found no effect (Fig. S3), indicating that additional leptospiral surface proteins could mediate elastin binding or that the avidities of recombinant and native cellular OmpL37 are significantly different.
Figure 4. Antiserum enhancement of binding by Leptospira and OmpL37 to skin elastin.
Microtiter wells were coated with 1 µg of human skin elastin and binding was measured by ELISA. (A) PBS-5 mM MgCl2 (no cells) or 1×108 L. interrogans L1-130 in PBS-5 mM MgCl2 (cells) without antiserum (no Ab) or in the presence of OmpL37 or OmpL54 antiserum (Ab) diluted 1∶40, 1∶80, 1∶160, 1∶320, and 1∶1280 were added to the elastin-coated wells. (B) Recombinant OmpL37 (0.5 µg) was preincubated with or without the IgG purified from 10 times diluted OmpL37 or OmpL47 antiserum at 1∶40, 1∶160, and 1∶640 dilution and the OmpL37 or OmpL47 antiserum at 1∶2500 dilution prior to addition to elastin-coated wells. Mean absorbance at 450 nm ± the standard deviation of a representative experiment performed in triplicate is shown. Statistical significance was evaluated by one-way ANOVA comparing leptospiral or recombinant OmpL37 binding in the presence of antibodies compared with binding in the absence of antibodies (** P<0.001, * P<0.05).
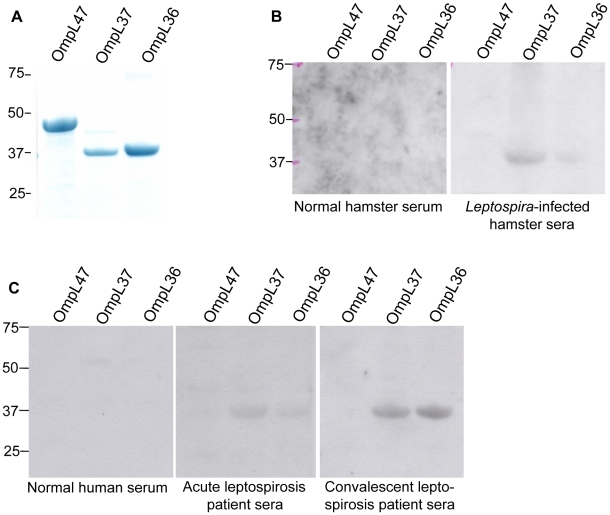
OmpL37 is recognized by the immune system of infected hosts
To investigate whether OmpL37 can elicit an immune response from an infected host, we examined the reactivity of sera from L. interrogans L1-130-infected hamsters and leptospirosis patients to recombinant OmpL37 by immunoblot (Fig. 5). The results show that sera pooled from two L1-130-infected hamsters recognized OmpL37 and OmpL36 but not OmpL47 (Fig. 5B). Control hamster serum obtained after sham infection with sterile EMJH medium did not react to any of the proteins tested (Fig. 5B). Similar results were obtained when sera from acute and convalescent leptospirosis patients were tested (Fig. 5C). Pooled sera from 13 individuals diagnosed with either acute leptospirosis or recovering from the disease recognized OmpL37 and OmpL36, while no significant reactivity was found for OmpL47 and pooled normal human serum did not react to any of these proteins (Fig. 5C).
Figure 5. Host immune response to OmpL proteins.
Recombinant OmpL47, OmpL37, and OmpL36 were separated by gel electrophoresis (2.5 µg per lane), stained with Coomassie G-250 (A) or blotted onto PVDF membrane, and analyzed for serum reactivity. (B) Membranes probed with a pool of two L. interrogans L1-130-infected hamster sera (1∶1000) or non-infected control hamster serum (1∶1000). (C) Membranes probed with normal, acute, or convalescent pooled human sera (1∶300). The positions of molecular mass standards (in kilodaltons) are indicated on the left.
OmpL37 is detected in pathogenic but not saprophytic Leptospira serovars
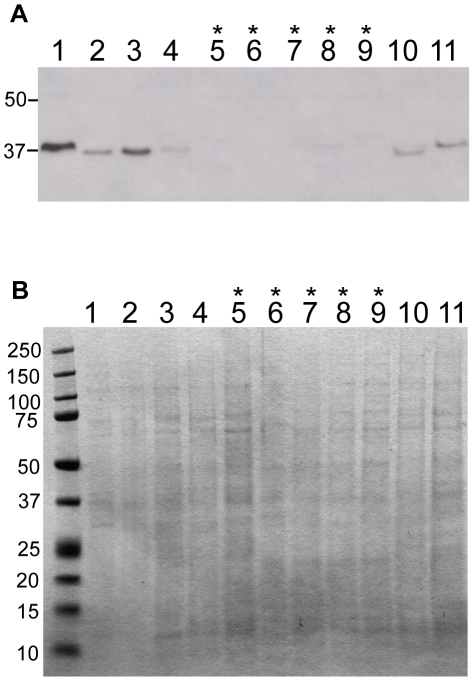
The expression of OmpL37 by pathogenic and saprophytic Leptospira serovars was investigated by immunoblot using OmpL37 antiserum (Fig. 6A). Pathogenic Leptospira included L. interrogans serovars Copenhageni strain Fiocruz L1-130 and Pomona strain PO-01, L. kirschneri serovars Mazdok strain 5621 and Grippotyphosa strain RM 52, and L. borgpetersenii serovars Tarrasovi strain Perepelicin and Javanica strain Veldrat Bataviae 46. Saprophytic Leptospira and Leptonema included Leptonema illini strain 3055, Leptospira weilii serovar Celledoni strain Celledoni, L. wolbachii serovar Biflexa strain codice, L. inadai serovar Lyme strain 10, and L. biflexa serovar Patoc strain Patoc 1. Loading of equal amounts of proteins from whole cell lysates was confirmed by Coomassie Brilliant G-250 staining (6B). As expected, the immunoblot revealed the highest reactivity of OmpL37 antibodies against L. interrogans L1-130 (Fig. 6A). OmpL37 was detected in all other pathogenic Leptospira strains investigated with various levels of band intensity reflecting variations in either expression or antiserum reactivity (Fig. 6A). Interestingly, the OmpL37 homolog in L. interrogans serovar Pomona (LIP_1392, 99% identity) is expressed in considerably lower amounts (Fig. 6A). OmpL37 was not detected in any saprophytic strains investigated, with the exception of Leptonema illini, where a very weak band could be detected (Fig. 6A). In L. biflexa a homolog of OmpL37 has been annotated (LBF_0995), but the amino acid sequence reveals only 47% identity (Fig. S4), and is either not recognized by the OmpL37 antibodies or is not expressed in L. biflexa under the in vitro conditions used (Fig. 6A). The genomes of the other Leptospira serovars we tested have not been sequenced precluding identification of OmpL37 homologues in those organisms. Taken together, our results show that OmpL37 was detectable only in pathogenic Leptospira spp.
Figure 6. OmpL37 expression in pathogenic and saprophytic isolates of Leptospira serovars.
Extracts of 5×107 leptospires per lane were separated by gel electrophoresis, blotted onto PVDF membrane, and probed with OmpL37 antiserum (1∶1000) (A) or stained with Coomassie G-250 (B). L. interrogans serovar Copenhageni strain Fiocruz L1-130 (Lane 1), L. interrogans serovar Pomona strain PO-01 (Lane 2), L. kirscheneri serovar Mazdok strain 5621 (Lane 3), L. kirscheneri serovar Grippotyphosa strain RM 52 (Lane 4), L. weilii serovar Celledoni strain Celledoni (Lane 5), L. biflexa serovar Patoc strain Patoc 1 (Lane 6), L. wolbachii serovar Biflexa strain codice (Lane 7), Leptonema illini strain 3055 (Lane 8), Leptospira inadai serovar Lyme strain 10 (Lane 9), L. borgpetersenii serovar Tarrasovi strain Perepelicin (Lane 10), L. borgpetersenii serovar Javanica strain Veldrat Bataviae 46 (Lane 11). The saprophytic species in lanes 5 to 9 are indicated by asterisks. The positions of molecular mass standards (in kilodaltons) are indicated on the left.
Discussion
Bacterial adhesins are surface-exposed OMPs, often playing roles as determinants of pathogenicity that allow bacteria to colonize host tissues by attaching to host molecules, such as ECM proteins [14], [15]. L. interrogans is an extracellular pathogen that enters the susceptible host through skin abrasions and mucous membranes, disseminates through the bloodstream, and colonizes kidneys, lungs, and other organs. The capacity of L. interrogans to adhere to tissues of various organs requires surface-exposed proteins with high affinity for host cell-surface and ECM components. The abundance of elastin in the inner layers of skin (reticular region of the dermis), blood vessels, and lungs implies that Leptospira have the ability to recognize and attach to elastin. Whereas various leptospiral proteins have been shown to have the capacity to bind multiple ECM components, such as laminin, fibronectin, fibrinogen, and collagens [18], [19], [22]–[30], [32], only the LigB repeated domains have been shown to exhibit elastin-binding activity [21]. Thus, it is of interest to identify any additional elastin-binding proteins. We previously identified four novel surface-exposed transmembrane OMPs, OmpL36, OmpL37, OmpL47, and OmpL54 [33] and have been investigating whether these proteins bind to host proteins. The recombinant OmpL36, OmpL37, and OmpL47 proteins are soluble and suitable for functional analysis by ELISA.
The binding activity of OmpL37 for the host components tested varied widely, with the most significant binding to human skin elastin, followed by human aorta elastin, laminin, fibrinogen fragment D, fibrinogen, and plasma fibronectin (Fig. 1). The weakest binding was observed for chondroitin sulfate, heparan sulfate, and collagen type IV (Fig. 1). Although we used BSA as a widely accepted negative control for host-ligand binding, it is important to note that albumin occurs in blood and the OmpL37 binding observed with BSA and fetuin may be important in host-pathogen interactions that require further investigation. This is the first report of a leptospiral protein showing pronounced specificity for human skin elastin. The strong dose-dependent, saturating binding to skin elastin gave an estimated apparent Kd of 104±19 nM (Fig. 2 and Table 1), which is one of the highest affinities for all leptospiral ligand-binding proteins investigated to date, comparable to that of LigB U1 binding to fibronectin and fibrinogen with Kd of 72.6±11.7 nM and 87.1±2.4 nM, respectively [18], LenB binding to fibronectin and laminin with Kd of 106±8 nM and 118±39 nM, respectively [30], and LigB Cen binding to lung elastin with Kd of 101±11 nM [21]. It is apparent that LigB exhibits strongest affinity for fibronectin and fibrinogen when compared to its affinity for lung elastin and other host proteins tested [18], [21]. It is noteworthy that OmpL37 showed much stronger affinity for human skin and aorta elastin (Fig. 2 and Table 1) compared to the binding of lung elastin by multiple parts of LigB, with the exception of LigB Cen [21].
Dose-dependent, saturating binding by OmpL37 was also observed for fibrinogen and its fragment D, with considerably weaker activity for plasma fibronectin and its 30-kDa and 45-kDa fragments (Fig. 2 and Table 1). Note that whereas OmpL37 seemed to interact very efficiently with laminin (Fig. 1), the reaction kinetics revealed barely saturating binding with Kd greater than 410±81 nM (Fig. 2 and Table 1). Our results show that OmpL37 interacts with multiple host proteins, a property that is common with previously characterized ligand-binding proteins of Leptospira [18], [19], [22]–[30], [32].
L. interrogans was recently shown to bind to elastin in vitro and LigB has been attributed a partial role in this binding, and it was proposed that there are additional elastin-binding proteins [21]. This observation and our results showing strong elastin binding by recombinant OmpL37 prompted us to investigate the contribution of OmpL37 to leptospiral adherence to elastin. The addition of saturating amounts of recombinant OmpL37 did not affect L. interrogans binding to immobilized human skin elastin, raising the possibility that the avidity of recombinant OmpL37 for elastin is different from that of the native OmpL37 on the cell surface, affecting the ability of the recombinant protein to compete with native OmpL37. However, we observed significant increases in leptospiral elastin binding in the presence of rabbit antiserum for OmpL37, with the effect being concentration dependent (Fig. 4A). In contrast, antiserum for the surface-exposed OmpL54 did not affect elastin binding by the spirochetes (Fig. 4A), suggesting that antiserum specific for OmpL37 can promote the interaction of OmpL37 on the cell surface with elastin. Convalescent leptospirosis patient sera were also tested for effects on leptospiral binding to elastin, but the strong agglutinating effect prevented assessment of leptospiral adhesion.
Further, the OmpL37 antiserum but neither leptospirosis patient sera nor Leptospira-infected hamster sera enhanced the binding of recombinant OmpL37 to immobilized skin elastin (Fig. 4B and Fig. S1–S2). The discrepancy might be due to the different nature of the antibodies for OmpL37, with antibodies in rabbit serum capable of recognizing additional epitopes not recognized by antibodies generated in the infected hosts. As with the enhancement of leptospiral adhesion, the effect on recombinant OmpL37 was also specific for the OmpL37 antiserum, since rabbit antiserum for OmpL47 did not produce an effect (Fig. 4B). Interestingly, purified IgG specific for OmpL37 did not exhibit statistically significant enhancement compared to that of antiserum for OmpL37 (Fig. 4B). This discrepancy did not appear to be due to inactivation of IgG molecules as we confirmed their recognition of OmpL37 by immunoblot (data not shown). However, we cannot exclude the possibility that some level of degradation or partial loss of IgG activity could have occurred. The possibility that the enhancement effect is due to another Ig type is not likely as the IgG purification process also yields up to 20% of IgA and IgM molecules. Since antisera for other surface-exposed leptospiral proteins did not exhibit enhancement (Fig. 4A and B), we hypothesize that another serum component in addition to OmpL37 antibody might be required to enhance OmpL37 binding to elastin. Further, the OmpL37 antiserum did not enhance recombinant OmpL37 binding to fibronectin, fibrinogen, and laminin (data not shown), suggesting that this effect is specific to elastin. Finally, the OmpL37 antiserum enhanced immobilized Leptospira binding to freely soluble skin elastin (data not shown).
The enhancement of adhesin binding to host ligands by antibodies has been observed previously for fibronectin-binding protein FnbA of Streptococcus dysgalactiae [44], fibronectin-binding protein FnBPA of Staphylococcus aureus [45], and Lewis X (Lex) expressed by both human gastric mucosa and Helicobacter pylori [46]. Antibodies recognizing the fibronectin-binding site, Au, of FnbA bound to Au only in the presence of fibronectin [44]. Moreover, the Au antibodies enhanced ligand binding by recombinant proteins or synthetic peptides containing the Au sequence, suggesting that the antibodies can stabilize the ligand-adhesin complex, resulting in enhanced fibronectin binding. This would provide an advantage to the pathogen, where antibodies could enhance adherence to host tissue rather than inhibit this critical step in tissue colonization [44]. Interestingly, although the fibronectin-binding repeats of FnBPA are recognized weakly by antibodies in the absence of fibronectin, epitopes induced in FnBPA by its interaction with fibronectin greatly enhance its recognition by both monoclonal antibodies and sera from patients with staphylococcal infections [45]. In H. pylori it has been shown that Lex antibodies can specifically increase bacterial adhesion [46]. This potentially positive effect on colonization could be due to an increase in bacterial aggregation or to the antibodies mediating a bivalent H. pylori Lex – antibody– human gastric mucosa Lex interaction that forms a bridge between bacteria and host cells [46]. Our interesting but somewhat surprising finding that OmpL37 antiserum enhances leptospiral binding suggests that OmpL37 contributes to the adhesion of leptospires to skin elastin. Since the OmpL37 antibodies do not promote leptospiral agglutination, we hypothesize that they in conjunction with another serum component alter the conformation of OmpL37 to promote more efficient binding to elastin. Alternatively, the antibodies may stabilize the elastin-OmpL37 complex as described for FnbA [44], resulting in enhanced elastin binding and leptospiral adhesion. The exact mechanism and functional role of this enhancement needs further investigation, which is currently under way in our laboratory.
To support our hypothesis that antibodies against OmpL37 may aid Leptospira during the infection process, we wanted to verify that there is a host immune response towards OmpL37. We found that an antibody response to OmpL37 occurs in both the hamster model and leptospirosis patients (acute and convalescent) (Fig. 5). Further, we investigated whether OmpL37 is expressed in pathogenic leptospiral isolates and found that OmpL37 could be detected only in pathogenic Leptospira serovars (Fig. 6), albeit a homologue is present in the L. biflexa genome (Fig. S4). Given that the amino acid sequences of OmpL37 in serovars Copenhageni and Pomona are 99% identical, the lower immunoblot reactivity indicates that OmpL37 is expressed at much lower levels in serovar Pomona (Fig. 6).
It has been reported that OmpL36 (LIC13166) and OmpL47 (LIC13050) are potential virulence factors of L. interrogans that are recognized by leptospirosis patient sera [47]. However, we observed the recognition of OmpL36 but not OmpL47 by the sera from infected hosts (Fig. 5). This could be due to the different techniques (ELISA versus immunoblot) employed in the studies. In addition, proteomic analysis of total protein extracts separated by 2D-gel electrophoresis identified OmpL36 and OmpL47 along with other well described leptospiral OMPs in virulent L. interrogans serovar Pomona cultured from infected hamsters [48]. The failure of these proteomic studies to detect OmpL37 may be explained by another mass spectrometry study focusing on cellular protein concentrations in L. interrogans serovar Copenhageni, which has revealed that OmpL36 is the most abundant transmembrane OMP (13th highest of all cell proteins), OmpL47 is very abundant (29th highest), and OmpL37 is less abundant (204th highest) [49]. The lower abundance of OmpL37 in serovar Copenhageni compared to OmpL36 and OmpL47 and our data showing lower expression of OmpL37 by serovar Pomona compared to serovar Copenhageni suggest that there could be insufficient OmpL37 for detection by proteomic analysis of total proteins separated by 2D-gel electrophoresis. Although OmpL47 had very little ligand-binding activity and OmpL36 did not show significant ligand-binding activity (Fig. 1), the involvement of these proteins in virulence awaits further investigation.
Based on the results presented here, we hypothesize that OmpL37 might be involved in the pathogenesis of leptospirosis. During the initial stage of infection, the strong binding affinity of OmpL37 for human skin elastin would facilitate the attachment of leptospires to an inner elastin-rich layer of the skin exposed by abrasion. Later during dissemination, the ability of OmpL37 to efficiently bind human aorta elastin suggests that OmpL37 could promote leptospiral attachment to elastin-rich vascular structures, including the walls of the pulmonary, cardiac, and other blood vessels, possibly explaining the propensity of leptospirosis to result in hemorrhagic complications [1], [50], [51]. The enhancement of leptospiral binding to elastin by OmpL37 antiserum supports the hypothesis that the host immune response to OmpL37 could promote rather than inhibit the adherence of infecting spirochetes to elastin-rich tissues, possibly also aiding in evading immunological clearance. Future studies are planned to map the elastin-binding sites in OmpL37 to further understand the antiserum enhancement effect. We also plan to identify the serum component that is apparently required in addition to OmpL37 antibodies in the enhancement of elastin-binding. Finally, we plan to study the contribution of OmpL37 during the initial and subsequent stages of leptospirosis.
Supporting Information
Effects of convalescent leptospirosis patient sera on recombinant OmpL37 binding to skin elastin. Microtiter wells were coated with 1 µg of human skin elastin and binding was measured by ELISA. Recombinant OmpL37 (0.5 µg) was preincubated for 1 h at room temperature with convalescent leptospirosis patient sera or normal human sera at 1∶640, 1∶2560, and 1∶10,240 dilution prior addition to elastin-coated wells. Mean absorbance at 450 nm ± the standard deviation of a representative experiment performed in triplicate is shown.
(0.20 MB PPT)
Effects of Leptospira-infected hamster sera on recombinant OmpL37 binding to skin elastin. Microtiter wells were coated with 1 µg of human skin elastin and binding was measured by ELISA. Recombinant OmpL37 (0.5 µg) was preincubated for 1 h at room temperature with Leptospira-infected hamster sera or normal hamster sera at 1∶640 and 1∶2560 dilution prior addition to elastin-coated wells. Mean absorbance at 450 nm ± the standard deviation of a representative experiment performed in triplicate is shown.
(0.19 MB PPT)
Leptospiral binding to elastin after recombinant OmpL37 has been pre-bound to elastin in presence of OmpL37 antiserum. Microtiter wells were coated with 1 µg of human skin elastin and binding was measured by ELISA. Prior the addition of leptospires (1.4×108 per well), 0.5 µM of recombinant OmpL37 was either added directly to elastin-coated microtiter wells or pre-incubated with anti-OmpL37 or anti-OmpL47 (negative control) at a 1∶500 dilution and added to the microtiter wells. Mean absorbance at 450 nm ± the standard deviation of a representative experiment performed in triplicate is shown. Statistical significance was evaluated by one-way ANOVA comparing leptospiral binding without recombinant OmpL37 compared with binding after addition of recombinant OmpL37 (P>0.05).
(0.17 MB PPT)
Amino acid sequence homology of L. interrogans OmpL37 (LIC12263) and L. biflexa LBF 0995. BLAST analysis was performed between L. interrogans LIC12263 (OmpL37) and L. biflexa genome. The highest scored homolog, LBF 0995 had 154/326 identities (47%), 219/326 positives (67%) and 14/326 gaps (4%).
(0.11 MB PPT)
Acknowledgments
We thank Drs. Jim Matsunaga and Jane T. Babbitt for valuable discussions and assistance. We also thank Dr. Albert I. Ko for providing leptospirosis patient serum samples, Dr. José Antonio Guimarães Aleixo for providing LipL32 monoclonal antibody 1D9, and Mariana Loner Coutinho for assistance in obtaining hamster sera samples.
Footnotes
D.H. and H.C. are coauthors on a manuscript in preparation with Associate Editor Mathieu Picardeau. D.A.H. is a consultant for Merial, which markets vaccines for prevention of leptospirosis.
This work was supported by Public Health Service grant AI-34431 (to D.A.H.) from the National Institute of Allergy and Infectious Diseases and by VA Medical Research Funds (to D.A.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.Ganoza CA, Matthias MA, Saito M, Cespedes M, Gotuzzo E, et al. Asymptomatic renal colonization of humans in the peruvian Amazon by leptospira. PLoS Negl Trop Dis. 2010;4:e612. doi: 10.1371/journal.pntd.0000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 5.Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2009;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Faine S, Adler B, Bolin C, Perolat P. Melbourne, Victoria, Australia: MedSci; 1999. Leptospira and leptospirosis. 2 ed. [Google Scholar]
- 7.Trevejo RT, Rigau-Perez JG, Ashford DA, McClure EM, Jarquin-Gonzalez C, et al. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J Infect Dis. 1998;178:1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- 8.Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 9.Zuerner R, Haake D, Adler B, Segers R. Technological advances in the molecular biology of Leptospira. J Mol Microbiol Biotechnol. 2000;2:455–462. [PubMed] [Google Scholar]
- 10.Cullen PA, Haake DA, Adler B. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol Rev. 2004;28:291–318. doi: 10.1016/j.femsre.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake DA. Spirochaetal lipoproteins and pathogenesis. Microbiology. 2000;146(Pt 7):1491–1504. doi: 10.1099/00221287-146-7-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonrier C, Branger C, Michel V, Ruvoen-Clouet N, Ganiere JP, et al. Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine. 2000;19:86–94. doi: 10.1016/s0264-410x(00)00129-8. [DOI] [PubMed] [Google Scholar]
- 13.Mulvey MA. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 2002;4:257–271. doi: 10.1046/j.1462-5822.2002.00193.x. [DOI] [PubMed] [Google Scholar]
- 14.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 15.Pizarro-Cerda J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Breiner DD, Fahey M, Salvador R, Novakova J, Coburn J. Leptospira interrogans binds to human cell surface receptors including proteoglycans. Infect Immun. 2009;77:5528–5536. doi: 10.1128/IAI.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirathaworn C, Patarakul K, Saksit V, Poovorawan Y. Binding of Leptospira to extracellular matrix proteins. J Med Assoc Thai. 2007;90:2136–2142. [PubMed] [Google Scholar]
- 18.Choy HA, Kelley MM, Chen TL, Moller AK, Matsunaga J, et al. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect Immun. 2007;75:2441–2450. doi: 10.1128/IAI.01635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoke DE, Egan S, Cullen PA, Adler B. LipL32 is an extracellular matrix-interacting protein of Leptospira spp. and Pseudoalteromonas tunicata. Infect Immun. 2008;76:2063–2069. doi: 10.1128/IAI.01643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T, Yanagawa R. Leptospiral attachment to four structural components of extracellular matrix. Nippon Juigaku Zasshi. 1987;49:875–882. doi: 10.1292/jvms1939.49.875. [DOI] [PubMed] [Google Scholar]
- 21.Lin YP, Lee DW, McDonough SP, Nicholson LK, Sharma Y, et al. Repeated domains of Leptospira immunoglobulin-like proteins interact with elastin and tropoelastin. J Biol Chem. 2009;284:19380–19391. doi: 10.1074/jbc.M109.004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atzingen MV, Barbosa AS, De Brito T, Vasconcellos SA, de Morais ZM, et al. Lsa21, a novel leptospiral protein binding adhesive matrix molecules and present during human infection. BMC Microbiol. 2008;8:70. doi: 10.1186/1471-2180-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atzingen MV, Gomez RM, Schattner M, Pretre G, Goncales AP, et al. Lp95, a novel leptospiral protein that binds extracellular matrix components and activates e-selectin on endothelial cells. J Infect. 2009;59:264–276. doi: 10.1016/j.jinf.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Barbosa AS, Abreu PA, Neves FO, Atzingen MV, Watanabe MM, et al. A newly identified leptospiral adhesin mediates attachment to laminin. Infect Immun. 2006;74:6356–6364. doi: 10.1128/IAI.00460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho E, Barbosa AS, Gomez RM, Cianciarullo AM, Hauk P, et al. Leptospiral TlyC is an extracellular matrix-binding protein and does not present hemolysin activity. FEBS Lett. 2009;583:1381–1385. doi: 10.1016/j.febslet.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Hauk P, Macedo F, Romero EC, Vasconcellos SA, de Morais ZM, et al. In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infect Immun. 2008;76:2642–2650. doi: 10.1128/IAI.01639-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YP, Chang YF. A domain of the Leptospira LigB contributes to high affinity binding of fibronectin. Biochem Biophys Res Commun. 2007;362:443–448. doi: 10.1016/j.bbrc.2007.07.196. [DOI] [PubMed] [Google Scholar]
- 28.Longhi MT, Oliveira TR, Romero EC, Goncales AP, de Morais ZM, et al. A newly identified protein of Leptospira interrogans mediates binding to laminin. J Med Microbiol. 2009;58:1275–1282. doi: 10.1099/jmm.0.011916-0. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira TR, Longhi MT, Goncales AP, de Morais ZM, Vasconcellos SA, et al. LipL53, a temperature regulated protein from Leptospira interrogans that binds to extracellular matrix molecules. Microbes Infect. 2009 doi: 10.1016/j.micinf.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson B, Choy HA, Pinne M, Rotondi ML, Miller MC, et al. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS ONE. 2007;2:e1188. doi: 10.1371/journal.pone.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma A, Hellwage J, Artiushin S, Zipfel PF, Kraiczy P, et al. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect Immun. 2006;74:2659–2666. doi: 10.1128/IAI.74.5.2659-2666.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieira ML, de Morais ZM, Goncales AP, Romero EC, Vasconcellos SA, et al. Lsa63, a newly identified surface protein of Leptospira interrogans binds laminin and collagen IV. J Infect. 2009;60:52–64. doi: 10.1016/j.jinf.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 33.Pinne M, Haake DA. A comprehensive approach to identification of surface-exposed, outer membrane-spanning proteins of Leptospira interrogans. PLoS One. 2009;4:e6071. doi: 10.1371/journal.pone.0006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graf R, Neudeck H, Gossrau R, Vetter K. Elastic fibres are an essential component of human placental stem villous stroma and an integrated part of the perivascular contractile sheath. Cell Tissue Res. 1996;283:133–141. doi: 10.1007/s004410050521. [DOI] [PubMed] [Google Scholar]
- 35.Mithieux SM, Weiss AS. Elastin. Adv Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- 36.Starcher BC. Elastin and the lung. Thorax. 1986;41:577–585. doi: 10.1136/thx.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RC, Rogers P. Metabolism of leptospires. II. The action of 8-azaguanine. Can J Microbiol. 1967;13:1621–1629. doi: 10.1139/m67-212. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes CP, Seixas FK, Coutinho ML, Vasconcellos FA, Seyffert N, et al. Monoclonal antibodies against LipL32, the major outer membrane protein of pathogenic Leptospira: production, characterization, and testing in diagnostic applications. Hybridoma (Larchmt) 2007;26:35–41. doi: 10.1089/hyb.2006.033. [DOI] [PubMed] [Google Scholar]
- 39.Lüdtke CB, Coutinho ML, Jouglard SDD, Moreira CN, Fernandes CPH, et al. Monoclonal antibodies against an outer membrane protein from pathogenic leptospira Brazilian Journal of Microbiology. 2003;34:1–4. [Google Scholar]
- 40.Gamberini M, Gomez RM, Atzingen MV, Martins EA, Vasconcellos SA, et al. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol Lett. 2005;244:305–313. doi: 10.1016/j.femsle.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Courtney HS, Ofek I, Simpson WA, Hasty DL, Beachey EH. Binding of Streptococcus pyogenes to soluble and insoluble fibronectin. Infect Immun. 1986;53:454–459. doi: 10.1128/iai.53.3.454-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hull JR, Tamura GS, Castner DG. Interactions of the streptococcal C5a peptidase with human fibronectin. Acta Biomater. 2008;4:504–513. doi: 10.1016/j.actbio.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roche FM, Downer R, Keane F, Speziale P, Park PW, et al. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J Biol Chem. 2004;279:38433–38440. doi: 10.1074/jbc.M402122200. [DOI] [PubMed] [Google Scholar]
- 44.Speziale P, Joh D, Visai L, Bozzini S, House-Pompeo K, et al. A monoclonal antibody enhances ligand binding of fibronectin MSCRAMM (adhesin) from Streptococcus dysgalactiae. J Biol Chem. 1996;271:1371–1378. doi: 10.1074/jbc.271.3.1371. [DOI] [PubMed] [Google Scholar]
- 45.Meenan NA, Visai L, Valtulina V, Schwarz-Linek U, Norris NC, et al. The tandem beta-zipper model defines high affinity fibronectin-binding repeats within Staphylococcus aureus FnBPA. J Biol Chem. 2007;282:25893–25902. doi: 10.1074/jbc.M703063200. [DOI] [PubMed] [Google Scholar]
- 46.Sheu SM, Sheu BS, Yang HB, Lei HY, Wu JJ. Anti-Lewis X antibody promotes Helicobacter pylori adhesion to gastric epithelial cells. Infect Immun. 2007;75:2661–2667. doi: 10.1128/IAI.01689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eshghi A, Cullen PA, Cowen L, Zuerner RL, Cameron CE. Global proteome analysis of Leptospira interrogans. J Proteome Res. 2009;8:4564–4578. doi: 10.1021/pr9004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vieira ML, Pimenta DC, de Morais ZM, Vasconcellos SA, Nascimento AL. Proteome Analysis of Leptospira interrogans Virulent Strain. Open Microbiol J. 2009;3:69–74. doi: 10.2174/1874285800903010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malmstrom J, Beck M, Schmidt A, Lange V, Deutsch EW, et al. Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans. Nature. 2009;460:762–765. doi: 10.1038/nature08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dolhnikoff M, Mauad T, Bethlem EP, Carvalho CR. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz J Infect Dis. 2007;11:142–148. doi: 10.1590/s1413-86702007000100029. [DOI] [PubMed] [Google Scholar]
- 51.Seijo A, Coto H, San Juan J, Videla J, Deodato B, et al. Respiratory distress due to pulmonary hemorrhage in leptospirosis. Medicina (B Aires) 2002;62:135–140. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of convalescent leptospirosis patient sera on recombinant OmpL37 binding to skin elastin. Microtiter wells were coated with 1 µg of human skin elastin and binding was measured by ELISA. Recombinant OmpL37 (0.5 µg) was preincubated for 1 h at room temperature with convalescent leptospirosis patient sera or normal human sera at 1∶640, 1∶2560, and 1∶10,240 dilution prior addition to elastin-coated wells. Mean absorbance at 450 nm ± the standard deviation of a representative experiment performed in triplicate is shown.
(0.20 MB PPT)
Effects of Leptospira-infected hamster sera on recombinant OmpL37 binding to skin elastin. Microtiter wells were coated with 1 µg of human skin elastin and binding was measured by ELISA. Recombinant OmpL37 (0.5 µg) was preincubated for 1 h at room temperature with Leptospira-infected hamster sera or normal hamster sera at 1∶640 and 1∶2560 dilution prior addition to elastin-coated wells. Mean absorbance at 450 nm ± the standard deviation of a representative experiment performed in triplicate is shown.
(0.19 MB PPT)
Leptospiral binding to elastin after recombinant OmpL37 has been pre-bound to elastin in presence of OmpL37 antiserum. Microtiter wells were coated with 1 µg of human skin elastin and binding was measured by ELISA. Prior the addition of leptospires (1.4×108 per well), 0.5 µM of recombinant OmpL37 was either added directly to elastin-coated microtiter wells or pre-incubated with anti-OmpL37 or anti-OmpL47 (negative control) at a 1∶500 dilution and added to the microtiter wells. Mean absorbance at 450 nm ± the standard deviation of a representative experiment performed in triplicate is shown. Statistical significance was evaluated by one-way ANOVA comparing leptospiral binding without recombinant OmpL37 compared with binding after addition of recombinant OmpL37 (P>0.05).
(0.17 MB PPT)
Amino acid sequence homology of L. interrogans OmpL37 (LIC12263) and L. biflexa LBF 0995. BLAST analysis was performed between L. interrogans LIC12263 (OmpL37) and L. biflexa genome. The highest scored homolog, LBF 0995 had 154/326 identities (47%), 219/326 positives (67%) and 14/326 gaps (4%).
(0.11 MB PPT)