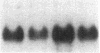
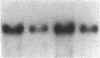
Abstract
The mechanisms by which hypoxia causes vasoconstriction in vivo are not known. Accumulating evidence implicates the endothelium as a key regulator of vascular tone. Hypoxia induces the expression and secretion of endothelin-1 (ET-1), a potent vasoconstrictor in cultured human endothelial cells. We report here that nitric oxide (NO), an endothelial-derived relaxing factor, modifies this induction of ET-1. Whereas low oxygen tension (PO2 = 20-30 Torr) increases ET-1 expression four- to eightfold above that seen at normal oxygen tension (PO2 = 150 Torr), sodium nitroprusside, which releases NO, suppresses this effect. This inhibition of hypoxia-induced ET-1 expression occurs within the first hour of exposure of cells to sodium nitroprusside. Moreover, when the endogenous constitutive levels of NO made by endothelial cells are suppressed using N-omega-nitro-L-arginine, a potent competitive inhibitor of NO synthase, the baseline levels of ET-1 produced in normoxic environments are increased three- to fourfold. The effects of hypoxia and the NO synthase inhibitor on ET-1 expression are additive. The regulation of ET-1 production by NO appears to be at the level of transcription. Similar effects of NO were observed on the expression of the PDGF-B chain gene. PDGF-B expression was suppressed by NO in a hypoxic environment and induced by N-omega-nitro-L-arginine in both normoxic and hypoxic environments. These findings suggest that in addition to its role as a vasodilator, NO may also influence vascular tone via the regulated reciprocal production of ET-1 and PDGF-B in the vasculature.
Full text
PDF

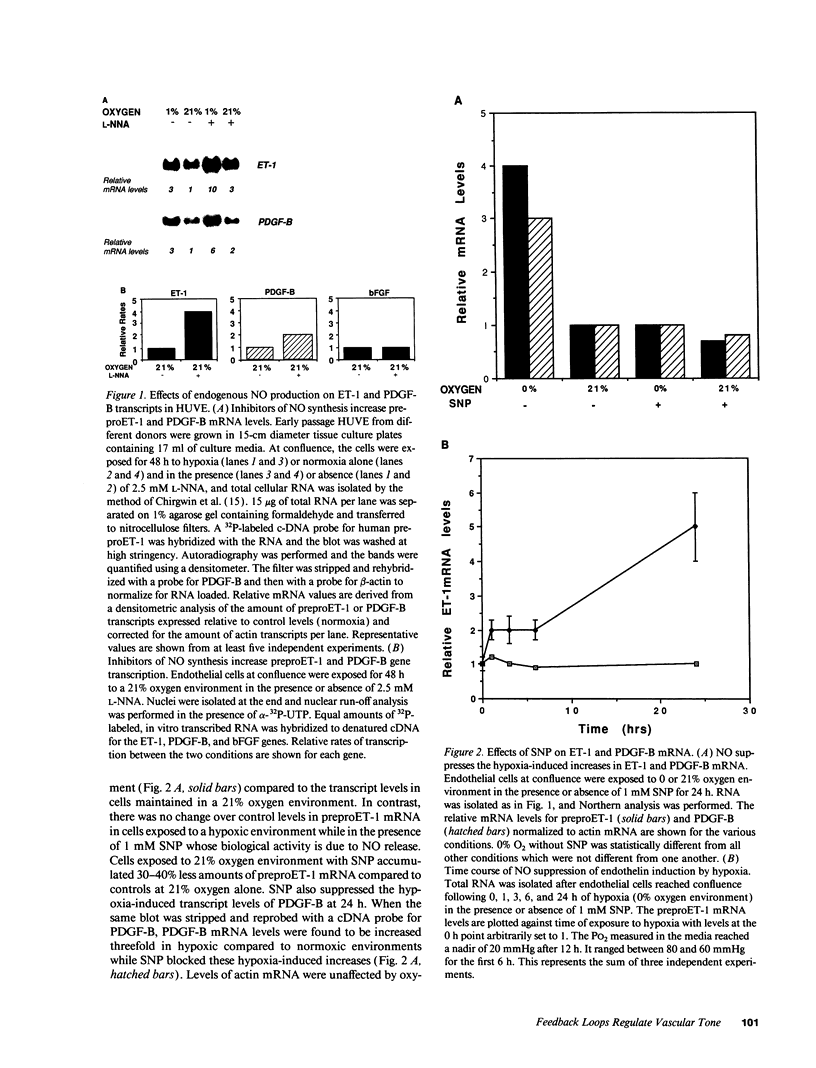
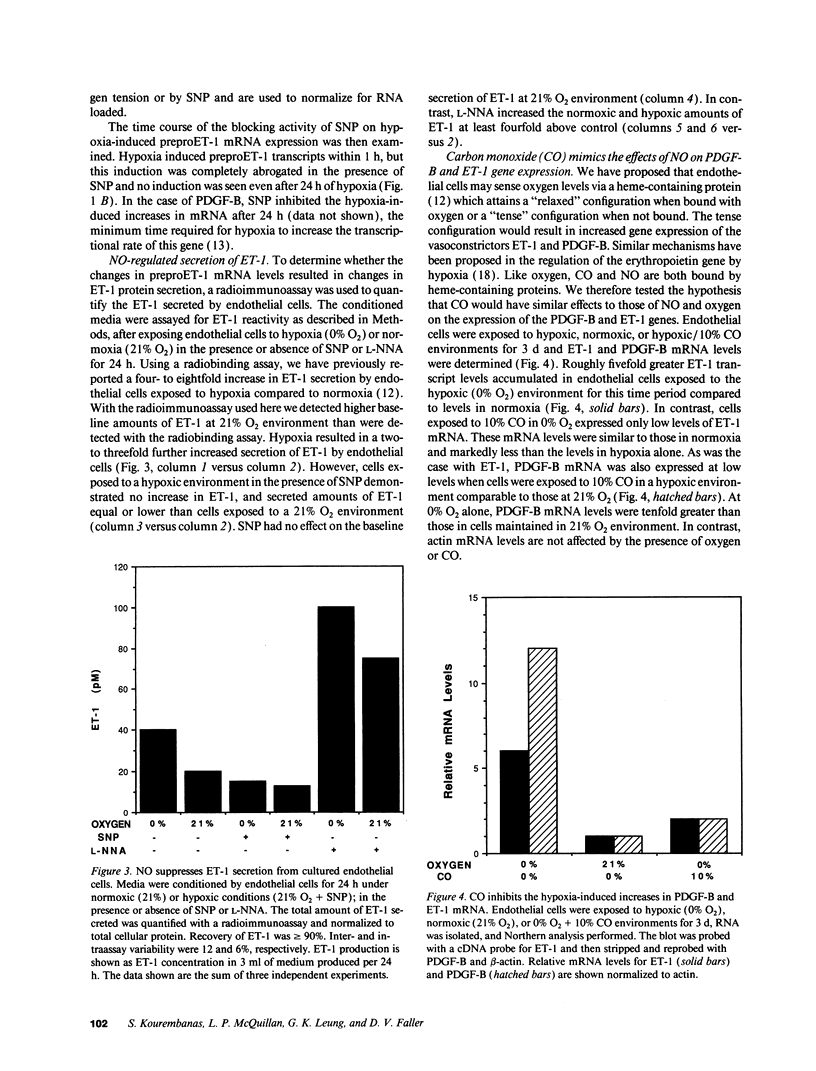


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adnot S., Raffestin B., Eddahibi S., Braquet P., Chabrier P. E. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest. 1991 Jan;87(1):155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C., Lüscher T. F. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990 Feb;85(2):587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Chang J. K., Roman C., Heymann M. A. Effect of endothelium-derived relaxing factor inhibition on the umbilical-placental circulation in fetal lambs in utero. Am J Obstet Gynecol. 1992 Feb;166(2):727–734. doi: 10.1016/0002-9378(92)91704-e. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Frostell C., Fratacci M. D., Wain J. C., Jones R., Zapol W. M. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991 Jun;83(6):2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gelmann E. P., Petri E., Cetta A., Wong-Staal F. Deletions of specific regions of the simian sarcoma-associated virus genome are found in defective viruses and in the simian sarcoma virus. J Virol. 1982 Feb;41(2):593–604. doi: 10.1128/jvi.41.2.593-604.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. A., Dunning S. P., Bunn H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988 Dec 9;242(4884):1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Yanagisawa M., Ohkubo S., Kimura C., Kosaka T., Inoue A., Ishida N., Mitsui Y., Onda H., Fujino M. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett. 1988 Apr 25;231(2):440–444. doi: 10.1016/0014-5793(88)80867-6. [DOI] [PubMed] [Google Scholar]
- Kourembanas S., Hannan R. L., Faller D. V. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990 Aug;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991 Sep;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman A., Hildebrand F. L., Jr, Margulies K. B., O'Murchu B., Perrella M. A., Heublein D. M., Schwab T. R., Burnett J. C., Jr Endothelin: a new cardiovascular regulatory peptide. Mayo Clin Proc. 1990 Nov;65(11):1441–1455. doi: 10.1016/s0025-6196(12)62168-5. [DOI] [PubMed] [Google Scholar]
- Miyauchi T., Yanagisawa M., Tomizawa T., Sugishita Y., Suzuki N., Fujino M., Ajisaka R., Goto K., Masaki T. Increased plasma concentrations of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet. 1989 Jul 1;2(8653):53–54. doi: 10.1016/s0140-6736(89)90303-6. [DOI] [PubMed] [Google Scholar]
- Moon D. G., Horgan M. J., Andersen T. T., Krystek S. R., Jr, Fenton J. W., 2nd, Malik A. B. Endothelin-like pulmonary vasoconstrictor peptide release by alpha-thrombin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9529–9533. doi: 10.1073/pnas.86.23.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Ohe M., Katayose D., Takishima T. Modulatory role of EDRF in hypoxic contraction of isolated porcine pulmonary arteries. Am J Physiol. 1992 Mar;262(3 Pt 2):H691–H697. doi: 10.1152/ajpheart.1992.262.3.H691. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Sachinidis A., Locher R., Hoppe J., Vetter W. The platelet-derived growth factor isomers, PDGF-AA, PDGF-AB and PDGF-BB, induce contraction of vascular smooth muscle cells by different intracellular mechanisms. FEBS Lett. 1990 Nov 26;275(1-2):95–98. doi: 10.1016/0014-5793(90)81447-v. [DOI] [PubMed] [Google Scholar]
- Saijonmaa O., Ristimäki A., Fyhrquist F. Atrial natriuretic peptide, nitroglycerine, and nitroprusside reduce basal and stimulated endothelin production from cultured endothelial cells. Biochem Biophys Res Commun. 1990 Dec 14;173(2):514–520. doi: 10.1016/s0006-291x(05)80064-6. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Levy R. D., Cernacek P., Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991 Mar 15;114(6):464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Vender R. L., Clemmons D. R., Kwock L., Friedman M. Reduced oxygen tension induces pulmonary endothelium to release a pulmonary smooth muscle cell mitogen(s). Am Rev Respir Dis. 1987 Mar;135(3):622–627. doi: 10.1164/arrd.1987.135.3.622. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yokokawa K., Kohno M., Yasunari K., Murakawa K., Takeda T. Endothelin-3 regulates endothelin-1 production in cultured human endothelial cells. Hypertension. 1991 Sep;18(3):304–315. doi: 10.1161/01.hyp.18.3.304. [DOI] [PubMed] [Google Scholar]