Abstract
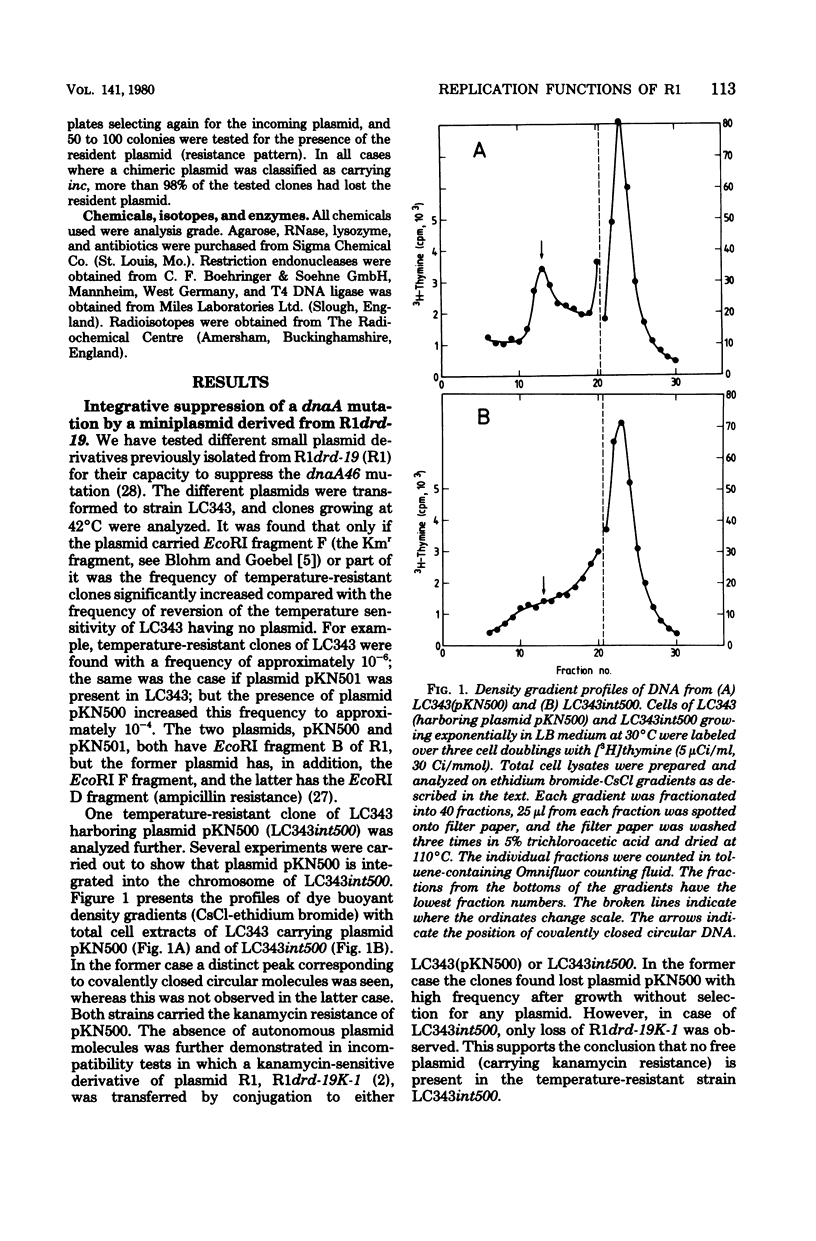
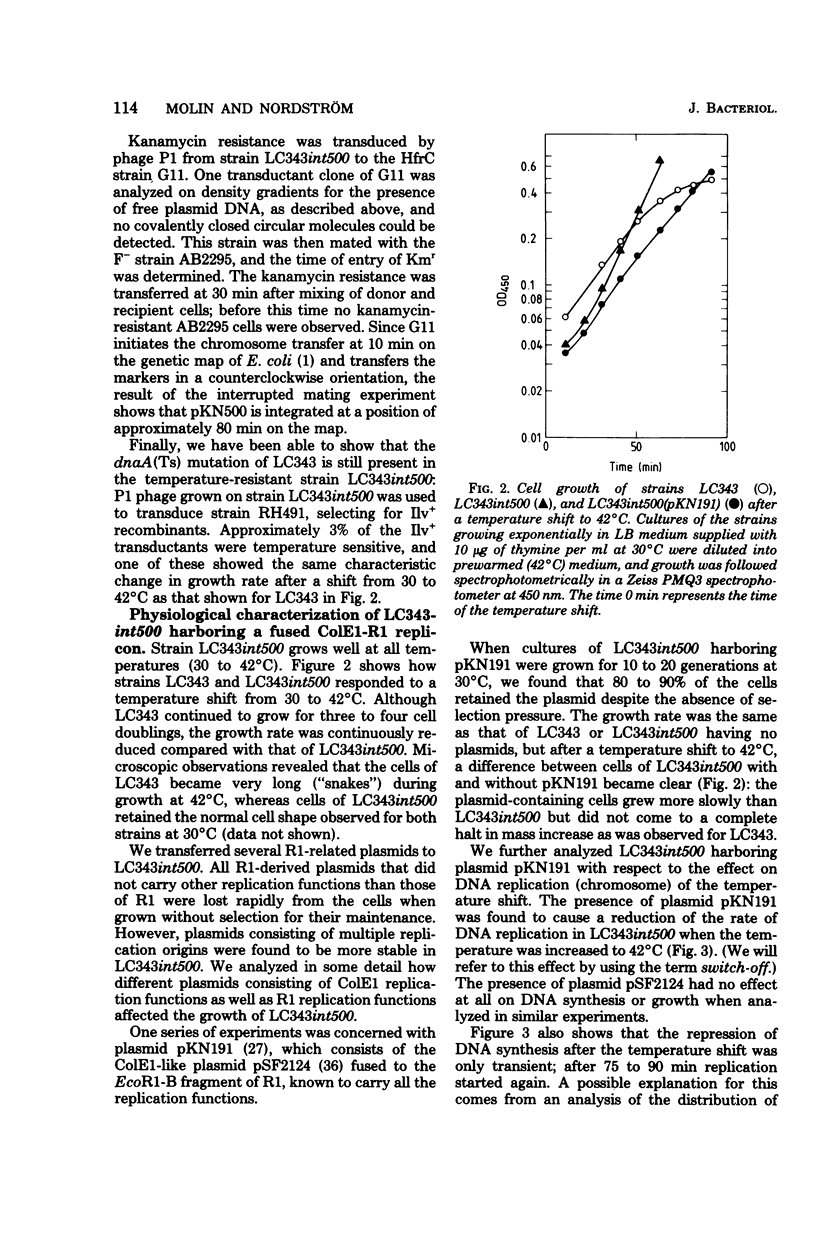
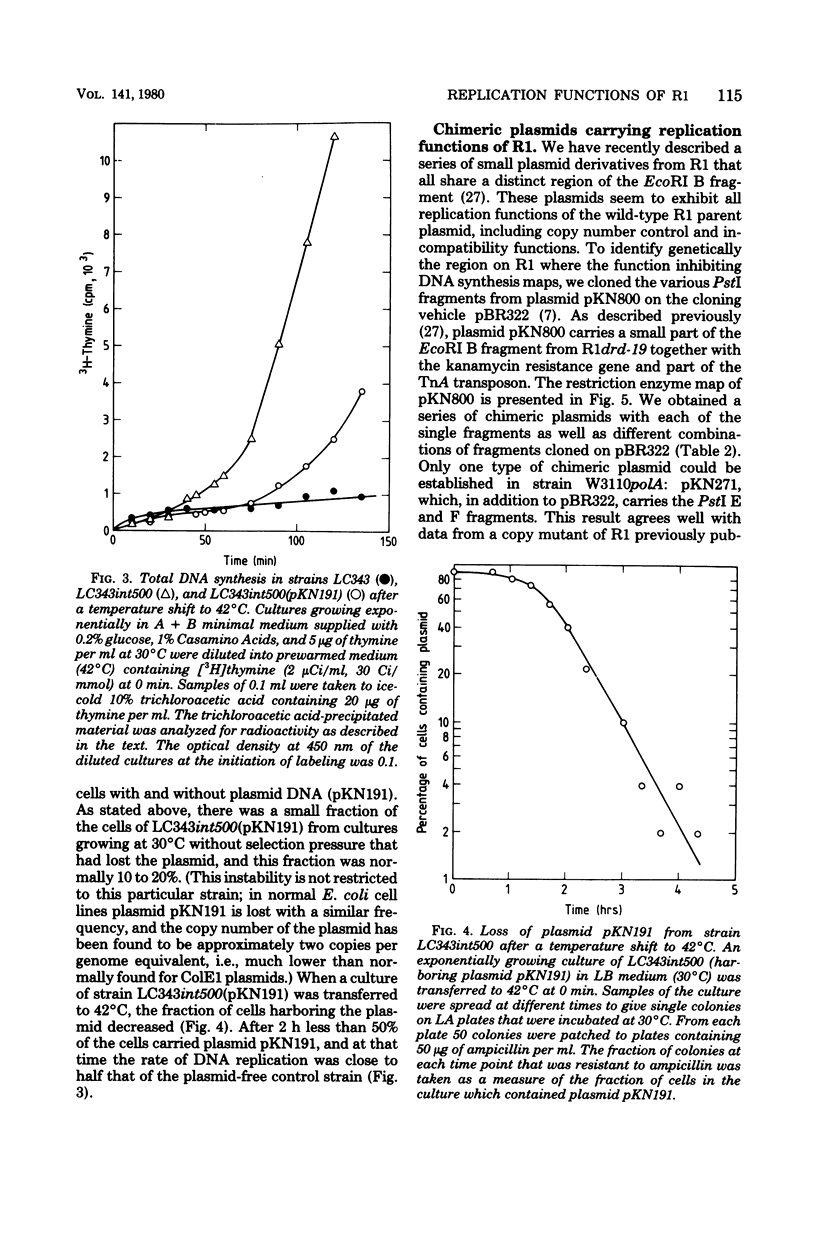
A small derivative of plasmid R1 was used to integratively suppress a chromosomal dnaA(Ts) mutation. The strain obtained grew normally at 42°C. The integratively suppressed strain was used as recipient for various plasmid R1 derivatives. Plasmid R1 and miniplasmid derivatives of R1 could be established in the strain that carried an integrated R1 replicon, but they were rapidly lost during growth. However, plasmids also carrying ColE1 replication functions were almost completely stably inherited. The integratively suppressed strain therefore allows the establishment of bacteria diploid with respect to plasmid R1 and forms a useful and sensitive system for studies of interaction between plasmid R1 replication functions. Several of the chimeric plasmids caused inhibition of growth at high temperatures. All plasmids that inhibited growth carried one particular PstI fragment from plasmid R1 (the PstI F fragment), and in all cases the growth inhibition could be ascribed to repression of initiation of chromosome replication at 42°C, i.e., they carry a trans-acting switch-off function. Furthermore, the analogous PstI fragments from different copy mutants of plasmid R1 were analyzed similarly, and one mutant was found to lack the switch-off function. The different chimeric plasmids were also tested for their incompatibility properties. All plasmids that carried the switch-off function (and no other plasmids) also carried R1 incompatibility gene(s). Since the PstI F fragment, which is present on all these plasmids, is very small (0.35 × 106), it is suggested that the switch-off regulation of replication (by an inhibitor), incompatibility, and copy number control are governed by the same gene.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J. P., Connolly J. C. Detection of a protein, similar to the sex pilus subunit, in the outer membrane of Escherichia coli cells carrying a derepressed F-like R factor. J Bacteriol. 1975 Apr;122(1):59–65. doi: 10.1128/jb.122.1.59-65.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Chandler M., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R.100.1: Change of chromosome replication origin in synchronized cultures. J Bacteriol. 1976 Jun;126(3):1215–1223. doi: 10.1128/jb.126.3.1215-1223.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blohm D., Goebel W. Restriction map of the antibiotic resistance plasmid R1drd-19 and its derivatives pKN102 (R1drd-19B2) and R1drd-16 for the enzymes BamHI, HindIII, EcoRI and SalI. Mol Gen Genet. 1978 Nov 29;167(2):119–127. doi: 10.1007/BF00266905. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Betlach M. C., Heyneker H. L., Shine J., Rodriguez R. L., Boyer H. W. Origin of replication of pBR345 plasmid DNA. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5265–5269. doi: 10.1073/pnas.74.12.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chandler M., Silver L., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R100.1: origin of chromosome replication during exponential growth. J Bacteriol. 1977 Aug;131(2):421–430. doi: 10.1128/jb.131.2.421-430.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Silver L., Frey J., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R100.1: generation of small plasmids after integration. J Bacteriol. 1977 Apr;130(1):303–311. doi: 10.1128/jb.130.1.303-311.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Silver L., Roth Y., Caro L. Chromosome replication in an Hfr strain of Escherichia coli. J Mol Biol. 1976 Jun 25;104(2):517–523. doi: 10.1016/0022-2836(76)90285-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Dreisig H., Molin S., Nordström K., Uhlin B. E. DNA replication control: studies of plasmid R1. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):419–425. doi: 10.1101/sqb.1979.043.01.048. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K. Control of plasmid R1 replication: kinetics of replication in shifts between different copy number levels. J Bacteriol. 1980 Jan;141(1):106–110. doi: 10.1128/jb.141.1.106-110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess R. H., Herman R. K. Segregation of Induced Frameshift Mutations and the Sequence of Gene Replication in ESCHERICHIA COLI K12. Genetics. 1973 Jun;74(2):227–242. doi: 10.1093/genetics/74.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. Restriction endonuclease mapping and mutagenesis of the F sex factor replication region. Mol Gen Genet. 1977 Apr 29;152(3):175–182. doi: 10.1007/BF00268815. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Takeda Y. Role of the tof gene in the production and perpetuation of the lambdadv plasmid. Mol Gen Genet. 1975 Dec 30;142(3):225–230. doi: 10.1007/BF00425647. [DOI] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Molin S., Stougaard P., Uhlin B. E., Gustafsson P., Nordström K. Clustering of genes involved in replication, copy number control, incompatibility, and stable maintenance of the resistance plasmid R1drd-19. J Bacteriol. 1979 Apr;138(1):70–79. doi: 10.1128/jb.138.1.70-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A., Nishimura Y., Caro L. Isolation of Hfr strains from R+ and ColV2+ strains of Escherichia coli and derivation of an R'lac factor by transduction. J Bacteriol. 1973 Dec;116(3):1107–1112. doi: 10.1128/jb.116.3.1107-1112.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Nordström K. Increased resistance to several antibiotics by one mutation in an R-factor, R 1a. J Gen Microbiol. 1971 May;66(2):205–214. doi: 10.1099/00221287-66-2-205. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Hoppensteadt F. C. On plasmid incompatibility. Plasmid. 1978 Sep;1(4):421–434. doi: 10.1016/0147-619x(78)90001-x. [DOI] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Two origins of replication in composite R plasmid DNA. Nature. 1976 Jan 29;259(5541):281–284. doi: 10.1038/259281a0. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N. Structural and functional analysis of cloned DNA segments containing the replication and incompatibility regions of a miniplasmid derived from a copy number mutant of NR1. J Bacteriol. 1979 Jan;137(1):92–104. doi: 10.1128/jb.137.1.92-104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Slocombe P. M. Plasmid incompatibility: cloning analysis of an incFII determinant of R6-5. Nature. 1978 May 4;273(5657):27–32. doi: 10.1038/273027a0. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. Plasmid incompatibility and control of replication: copy mutants of the R-factor R1 in Escherichia coli K-12. J Bacteriol. 1975 Nov;124(2):641–649. doi: 10.1128/jb.124.2.641-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr C. T., Waskell L., Glaser D. A. Characteristics of cold-sensitive mutants of Escherichia coli K-12 defective in deoxyribonucleic acid replication. J Bacteriol. 1975 Jan;121(1):99–107. doi: 10.1128/jb.121.1.99-107.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


