Abstract
Background
Subjective and objective measures of poor sleep in alcoholic insomniacs predict relapse to drinking. Non-alcoholic insomniacs underestimate their total sleep time (TST), and overestimate their sleep onset latency (SOL) and wake time after sleep onset (WASO) compared to polysomnography (PSG). This study evaluated three hypotheses: (1) subjective SOL would predict frequency of drinking during and after treatment; (2) participants would overestimate SOL and WASO and underestimate TST; and (3) higher amounts of over- and underestimates of sleep at baseline would predict worse drinking outcomes during and after treatment.
Methods
Participants (N=18), mean age 44.6 years (±13.2) underwent an adaptation night and two nights of PSG. They provided morning estimates of SOL, WASO, TST, and sleep efficiency (SE). Following PSG, participants were randomized to 6 weeks of placebo or gabapentin as part of a separate study. After 6 weeks, participants discontinued medication and were followed to week 12. A two-way ANOVA (night x method of measuring sleep) compared results and regression analyses predicted drinking. Drinking outcomes were defined as number of days drinking (DD) and number of heavy drinking days (HDD) during two consecutive 6-week periods.
Results
Most participants (72%) overestimated SOL by a mean of 21.3 (±36) minutes compared to PSG, F (1, 14) =7.1, p<.03. Unexpectedly, 89% underestimated WASO by a mean difference of 48.7 (±49) minutes, F (1, 14) =15.6, p<.01. Drinking during the 6-week study period was predicted by both subjective estimates of WASO and their accuracy. Post-treatment drinking was also predicted by subjective estimations of sleep and REM sleep latency.
Conclusion
Greater subjective accuracy of wakefulness at night provided by the patient predicted drinking during treatment. Unlike non-alcoholic insomniacs, this alcoholic sample significantly underestimated WASO compared to PSG values. The predictive ability of sleep parameters depended on the selected measure of drinking outcomes, and when outcomes were measured. Subjective sleep measures were better predictors of future drinking than corresponding PSG measures.
Keywords: Insomnia, Alcohol dependence, Sleep perception, Polysomnography, Sleep
INTRODUCTION
Alcohol has soporific effects in non-alcoholics, but after chronic use, the effects on sleep become deleterious. Within a night, alcohol has a biphasic effect on sleep. Initially, sleep onset latency (SOL), or the time it takes to fall asleep, is shortened and there is an increase in slow-wave sleep (SWS) in the first half of the night. In the second half of the night, sleep quality deteriorates and there are more awakenings (Rundell et al., 1977). As alcohol use becomes more chronic, tolerance to the sedating effects develops and individuals require greater amounts of alcohol more frequently to derive the same sleep-promoting effects. As the neurochemical systems of the brain adapt, sleep-promoting systems adapt their responses to the chronic alcohol administration and sleep propensity is dampened (Brower, 2003).
Sleep continues to be severely disturbed in early recovery from alcohol dependence. A review of studies estimated that 36–72% of participants in early recovery from alcohol dependence complained of insomnia (Brower, 2001). Cohn, Foster, and Peters (2003) found that 91% of alcoholic participants suffered from sleep disturbance as measured by a well validated measure of sleep disturbance, the Pittsburgh Sleep Quality Index (Buysse et al., 1989). These findings have been supported by earlier experimental studies documenting complaints of sleep disturbance during early recovery (Baekeland et al., 1974; Brower et al., 2001; Caetano, 1998; Foster et al., 2000; Mello and Mendelson, 1970). Both subjective complaints of poor sleep and disrupted sleep measured by polysomnography (PSG) have been shown in naturalistic treatment outcome studies to predict relapse to drinking among alcohol-dependent participants. For example, Brower et al. (1998) found that sleep onset latency (SOL), whether measured by PSG or self-report, predicted return to any drinking within 5 months of starting treatment.
Understanding how a patient perceives his or her sleep is important because it may have an impact on the patient’s future behaviors. It is well established that non-alcoholic participants with insomnia are subjectively inaccurate: they underestimate their total sleep time (TST), overestimate SOL and the time they are awake in the night after they fall asleep, i.e. wake time after sleep onset (WASO) as compared to same-night objective results of PSG (Baekeland and Hoy, 1971; Carskadon et al., 1976; Frankel et al., 1976; Kales and Kales, 1984; Monroe, 1967) This misperception may contribute to sleep-disruptive behaviors and dysfunctional beliefs about one’s sleep that ultimately perpetuate factors in the course of the insomnia. Misperception of sleep in a recovering alcohol-dependent individual with insomnia may be additionally detrimental. If a recovering alcoholic perceives his or her sleep to be poor, then this perception may contribute to a relapse to drinking.
Only one study has looked at the relationship between subjective and objective assessments of sleep in a population of alcoholic insomniacs (Currie et al., 2004). The authors directly compared subjective reports of insomnia (including sleep logs, questionnaires, and reports from the patient’s partner and clinician) to wrist actigraphy, which served as the objective measure of insomnia. Results from one week of simultaneous sleep log and actigraphy data revealed that participants overestimated SOL by a mean of 16 minutes, but accurately perceived SE and TST. In contrast to non-alcoholic insomniacs, however, alcoholic participants underestimated WASO by a mean of one hour. In other words, after falling asleep, they perceived they slept better than they actually did. This finding was unexplained.
To further characterize an individual’s estimate of sleep in this study, we used a sleep parameter that measures the relationship between subjective estimates of sleep parameters (obtained via sleep logs) and corresponding objective sleep measures (obtained via PSG) for the same night of sleep. This measure was termed the objective sleep time estimated (OSE) score by Edinger and Fins (1995). The score, expressed as a percentage, provides a quantitative measure of accuracy that can be used as a standard method to compare the accuracy of perceptions among different sleep variables and subject groups. In particular, it has been used to distinguish primary insomnia from secondary insomnia and to compare insomnia subgroups (Means et al., 2003). It has also been used to compare subjective estimates of sleep to actigraphy data in recovering alcoholics post-withdrawal (Currie et al., 2004).
The present study extends the existing literature in several ways. First, no study has conducted a quantitative comparison of subjective sleep measures to PSG, the gold standard of objective sleep measurement, in alcohol-dependent insomnia participants. Actigraphy is only able to detect the presence or absence of movement and therefore is an indirect measure of sleep. By contrast, PSG provides direct quantitative measures of sleep architecture (sleep stages) and continuity (e.g., SOL, TST, WASO). In general, the relationship between objective and subjective sleep measures is poorly understood, but studies are particularly sparse among individuals with a history of alcohol dependence because few insomnia trials include alcohol-dependent participants.
This study includes only alcoholic participants with a clinical diagnosis of insomnia whereas previous sleep studies of alcoholic-dependent participants selected participants meeting criteria for alcohol dependence only (cf. Brower et al., 1998). No previous study has examined either the subjective accuracy of sleep or the relationship between the accuracy of these perceptions and future drinking behavior in patients with alcohol dependence and comorbid insomnia. Additionally, this study adds to the existing literature on impaired sleep and future drinking because, instead of utilizing only a single dichotomous measure of relapse (i.e., return to any drinking) over one time interval, we utilized two drinking frequency measures (number of drinking days and number of heavy drinking days) and assessed when drinking occurred (during both a six-week treatment period and a six-week post-treatment period). We hypothesized that subjective SOL in alcohol-dependent participants with insomnia would predict drinking during and after treatment. Secondly, we hypothesized that participants would overestimate SOL and WASO, and underestimate TST, and that the greater the subjective/objective discrepancies at baseline (i.e., less accurate OSE scores) the worse drinking outcomes would be during and after treatment.
MATERIALS AND METHODS
Participants
Twenty-one (10 women) alcoholic-insomnia participants in early recovery from alcohol dependence were recruited from a local outpatient alcohol treatment facility, or the community, by way of advertising. All of these individuals were taking part as paid volunteers in a randomized controlled trial of the effects of gabapentin on relapse to alcohol use.
Procedures
Participants responded to flyers advertising a study for individuals who “have problems sleeping” and who “use alcohol to help them sleep.” Interested participants telephoned the study’s research coordinator and underwent an initial screening interview. The interview consisted of questions relating to quantity and frequency of drinking, date of last alcohol use, sleeping habits, medications, and psychiatric history. Participants needed to express a desire to stop drinking, or a willingness to abstain from use of alcohol and other drugs of abuse (except nicotine) throughout participation in the study. A study investigator (SS) evaluated data collected from a telephone screening interview, and determined whether or not an interested participant was appropriate for further screening. At that time, a date and time were set for a more comprehensive screening appointment. At the time of appointment, participants verbally agreed to have a breath alcohol content measurement taken. Written informed consent for additional screening and research participation was obtained only if blood alcohol levels were less than .05%.
Participants were excluded if they met the following criteria: were <18 years of age; met DSM-IV criteria for panic disorder, social phobia, generalized anxiety disorder, post-traumatic stress disorder, major depression, bipolar disorder, anorexia nervosa, bulimia nervosa in the past month; met criteria for dependence on any psychoactive substance other than alcohol (except nicotine) in the past 1 month; had a medical condition or chronic pain syndrome that caused insomnia, had insomnia that was associated with sleep apnea (Respiratory Disturbance Index >10) or periodic limb movements (periodic limb movement index with arousals >15) (determined via PSG); required treatment with medications known to affect sleep such as mirtazepine, trazodone, tricyclic antidepressants, neuroleptics, sedative hypnotics, stimulants, centrally acting antihistamines or antihypertensives, oral corticosteroids, or theophylline. Patients were excluded if they were taking mood stabilizers or other antidepressants only if there had been a recent change in the past 2 months in the medications or a change was anticipated during the course of the study or if the study investigator determined the medication or its underlying disorder was contributing to their insomnia. Of the 35 participants who qualified for insomnia and who underwent in-person screening, 14 were excluded.
Participants reported persistent insomnia for at least one week post-abstinence with a score on the revised version of the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-A) of <8 (Sullivan et al., 1989) to insure that persisting insomnia was not due to acute alcohol withdrawal. They were excluded if they rated their insomnia as “much improved” or “very much improved” on the Clinical Global Impression Scale after one week of abstinence (Guy, 1976). Insomnia was assessed and diagnosed by the authors (KB or SS) using the Sleep Disorders Questionnaire (Douglass et al., 1994), the Sleep Problems Questionnaire (Jenkins et al., 1988), and the Insomnia Interview Schedule (Morin, 1993). Participants meeting the above criteria were scheduled to return for a baseline sleep assessment.
After completing the study instruments and measures in the screening visit, all participants were placed on a single-blind placebo washout period for 7 to 14 days (Phase 1). The purpose of this period was to promote and assess for abstinence and to document persisting insomnia prior to randomization. During this first phase of the study, participants were provided with a 300 mg placebo capsule and were asked to take it every evening approximately 30–60 minutes before bedtime. Participants were also provided with a sleep log and asked to record their sleep schedule.
At the conclusion of one week of abstinence, each eligible participant returned to the University of Michigan General Clinical Research Center (GCRC) sleep laboratory, and underwent nocturnal PSG recordings on two consecutive nights. Upon arrival for each study night, they were given a test for breath alcohol content, and were either excluded or rescheduled for any positive result. The first night was a baseline laboratory adaptation night to additionally rule out occult sleep disorders. Participants took their placebo medication prior to bedtime on that night. On the second night, participants again took their placebo medications in the evening as scheduled and slept overnight. This PSG is referred to as the “pre-treatment night.” Participants generally arrived in the laboratory at approximately 9:00 pm and left between 7:00–8:00 am the next day. Participants completed sleep logs prior to leaving the laboratory each morning.
Following the second night of PSG, qualified participants were randomized to either placebo (n=11) or gabapentin (n=10) for 6 weeks as part of a separate double-blind randomized controlled trial. Although that study was designed to investigate the effects of gabapentin on relapse (Brower et al., 2003), only the effects of pre-treatment sleep parameters on drinking outcomes (while controlling for treatment group) are examined in this secondary analysis. During the treatment phase, gabapentin dosage was titrated to a fixed dosage of 5 capsules (1500 mg) at bedtime over a two-week period as tolerated, resulting in a mean dose of 1388 (±152.8)mg.
After three weeks of double-blind treatment, participants returned for a third night of PSG. This PSG is referred to as the “post-treatment study” night. Participants took either placebo or gabapentin in the laboratory on that night. After an additional three weeks, the medication was tapered over 4 days. Participants were followed for six more weeks after medication treatment until the end of week 12. Participants also received manualized behavioral therapy to enhance adherence to the medication (Carroll and O’Malley, 1996). Upon morning awakening after each night, participants provided estimates of their SOL, WASO, and TST in their sleep log.
Of the 21 subjects that qualified, data for two participants were not included because they did not report their subjective assessment of sleep on the pre-treatment night. Data for another subject could not be made available, leaving a total of 18 subjects for analysis.
Primary Outcome Measures
Objective sleep
Objective sleep measurement consisted of an electroencephalogram (C3/A2 EEG), referential electro-oculogram (EOG) with electrodes placed at the outer canthus of each eye, one immediately above the other just below the horizontal plane, to record both horizontal and vertical slow and rapid eye movements, submental electoromyogram (EMG), respiratory monitoring (nasal-oral airflow monitor, abdominal and chest monitors of respiratory effort, and ear or finger oximetry), electrocardiogram, and EMG of the anterior tibialis muscle (to document periodic leg movements). Data were recorded using a paperless system (Telefactor Corporation). The primary PSG dependent variables were TST, SOL, WASO, and SE. In addition, the percentage of time spent in Stages 1, 2, 3, 4 and rapid eye movement (REM) sleep were determined, as was REM onset latency. PSG studies were scored by standard criteria (Rechtschaffen and Kales, 1968).
Subjective sleep estimates
Subjective sleep was measured via sleep logs that were completed upon morning awakening from the second (pre-treatment) and third (post-treatment) nights in the laboratory. Estimates used in for this study included SOL, WASO, and TST. Participants did not provide estimates of SE. Rather, subjective sleep efficiency (SE) for this study was calculated by a research assistant, based on the ratio of subjectively estimated TST divided by reported time in bed, and multiplied by 100. Thus, SE is expressed as a percentage, and lower numbers are indicative of greater sleep discontinuity.
Accuracy measurements
To calculate the subjective accuracy of each sleep parameter, a variation of the Objective Sleep-time Estimated (OSE) (Edinger and Fins, 1995) was used. This measure is derived by using the following formula: OSE = (subjective parameter/objective parameter) × 100%. In this formula, the subjective parameter is the sleep log estimate and the objective measure is the PSG for the parameter being examined. The OSE score for SE reflects the ratio of subjective SE that was calculated by a research assistant divided by the SE value determined polysomnographically. An OSE score of 100% indicates perfect agreement between objective and subjective estimates; OSE <100% indicates subjective underestimation relative to PSG; >100% indicates subjective overestimation.
Alcohol consumption
Daily alcohol consumption was obtained using the Timeline Follow-Back Interview (TLFB) (Sobell et al., 1988). Frequency of any drinking was defined by the number of drinking days (DD) during a six-week period. Frequency of heavy drinking (which combines frequency and quantity) was defined as the number of heavy drinking days (HDD) during a six-week period. Heavy drinking was defined as >4 standard drinks in a day for women and >5 standard drinks in a day for men. A standard drink is equivalent to 12 oz of beer, 5 oz of table wine, or 1–1.5 oz of liquor (NIAAA, 2005).
Data Analyses
A two-way ANOVA of the sleep assessment method (sleep log versus PSG) versus night (pre-treatment versus post-treatment) was computed for each of the dependent variables SOL, WASO, TST, and SE, to examine within night and between night differences in sleep parameters. Paired t-tests were also computed. Regression analyses were computed to examine the relationships between objective and subjective measures of baseline sleep and subsequent alcohol consumption during and after treatment. Statistical analyses were performed by using SPSS (Version 13.0).
RESULTS
1. Sample Demographics
Eighteen participants (9 females) completed the pre-treatment night only. Fifteen participants (7 females) completed both the pre-treatment and the post-treatment night. Prior to entering the study, participants reported having a mean (S.D.) of 31.2(±13) drinking days and 21.2(±16) heavy drinking days in the last 90 days. Participants were abstinent in the week prior to the randomization trial, except for one subject that had two drinks four days before the trial began. Independent sample t-tests revealed no differences between the gabapentin group and placebo group on any of the subjective or objective sleep measures for the pre-treatment night or post-treatment night, therefore these groups were collapsed.
2. Objective Sleep by PSG from Pre-treatment to Post-treatment
Mean sleep parameters for the pre-treatment versus post-treatment nights are displayed in Table 1. A paired sample t-test was conducted to evaluate the difference between study nights. There were no differences between pre-treatment sleep and post-treatment sleep variables.
Table 1.
Objective Sleep Measures
| Sleep measure | Pre-treatment (n=15) | Post-treatment (n=15) | p |
|---|---|---|---|
| Total Sleep Time (min) | 317.5 (±47.4) | 303.5 (±68.8) | .27 |
| Sleep Onset Latency (min) | 28.4 (±32.0) | 35.3 (±45.9) | .39 |
| Sleep Efficiency % | 77.4 (±7.1) | 75.7 (±13.9) | .63 |
| Stage 1 % | 14.1 (±10.4) | 13.0 (±9.6) | .47 |
| Stage 2 % | 55.7 (±10.6) | 59.2 (±8.8) | .27 |
| Stage ¾ % | 11.2 (±7.6) | 12.8 (±9.3) | .45 |
| REM % | 19.0 (±6.5) | 14.9 (±5.8) | .11 |
| REM Latency (min) | 97.8 (±46) | 110.9 (±56.7) | .43 |
| Wake After Sleep Onset (min) | 92.4 (±32.3) | 96.9 (±57.5) | .77 |
3. Subjective vs. Objective Accuracy
Using paired sample t-tests and correlation coefficients, results comparing subjective estimates (log) and objective (PSG) sleep parameters within each night are shown in Table 2. A two-way within groups analysis of variance was conducted to explore the impact of night (pre-versus post-treatment) and assessment (objective PSG data versus subjective sleep log) on subjective estimations only.
Table 2.
Comparison of Subjective and Objective Measures of Sleep
| Variables | Sleep Log (±SD) | PSG (±SD) | Mean Discrepancy (±SD) | Paired Samples t-tests |
|---|---|---|---|---|
| Pre-treatment (n=18) | ||||
| SOL (min) | 48.1 (±49) | 26.8 (±30) | −21.3 (±36) | −2.5* |
| WASO (min) | 38.2 (±41) | 86.9 (±35) | 48.7 (±49) | 4.2** |
| SE (%) | 76.09 (±15) | 79.0 (±8) | 2.9 (±14) | 0.9 |
| TST (min) | 324.0 (±92) | 328.94 (±51) | 4.97 (±75) | 0.3 |
| Post-treatment (n=15) | ||||
| SOL (min) | 62.5 (±58) | 35.3 (±46) | −17.1 (±36) | −2.0 |
| WASO (min) | 48.3 (±58) | 96.91 (±58) | 42.5 (±78) | 3.1* |
| SE (%) | 70.7 (±24) | 75.7 (±14) | 2.8 (±20) | 0.5 |
| TST (min) | 286.9 (±103) | 303.5 (±68) | 5.1 (±73) | 0.3 |
Sleep onset latency (SOL), Wake time after sleep onset (WASO), Sleep Efficiency (SE), Total Sleep Time (TST).
p<.05,
p<.001.
On the pre-treatment night, there was a statistically significant main effect for SOL assessment F (1, 14) =7.1, p<.03. Post-hoc comparison using a paired sample t-test indicated that the mean score for the pre-treatment subjective assessment of SOL, (48.1±49.2) minutes, was significantly higher than the objective data, 26.8 (±29.9) minutes. Most (72%) participants overestimated SOL compared to PSG (p<.05), with a group mean of 21.3 (±36) minutes and a mean of 30.5 (±36.7) minutes among the overestimators.
There was a statistically significant main effect for WASO assessment F (1, 14) =15.6, p<.01. The mean subjective assessment of WASO was 38.2 (±40.7) minutes, which was unexpectedly lower than the objective data, which was 86.9 (±34.5) minutes. The majority (89%) of the participants underestimated WASO by a mean difference of 48.7 (±49.14) minutes for the overall group and 58.8(±41.5) minutes among the underestimators. Assessments of TST or SE by log estimates did not differ significantly from PSG values.
On the post-treatment night, 11 of 15 participants (73%) overestimated SOL by a mean of 17.09(±35.5) minutes compared to PSG, although the effect was not significant (p<.07). Fourteen out of fifteen participants (93%) underestimated WASO 42.54(±78.3) minutes (p<.02). Assessments of TST or SE by log estimates did not differ significantly from PSG values.
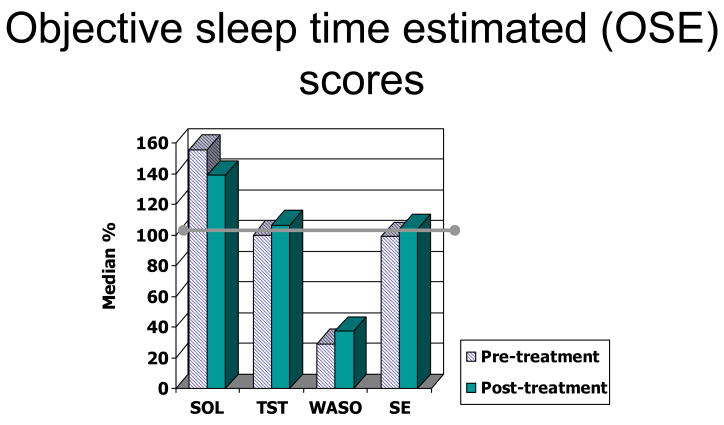
Median OSE scores were used to describe OSE scores. Kolmogorov-Smirnov tests of normality revealed that six of the eight OSE scores (except for night two TST and SE) were not normally distributed. These scores across the two study nights are displayed in Figure 1. Participants consistently underestimated WASO, with a median OSE score of 29% on the pre-treatment night and 38% on post-treatment nights. Median OSE scores for SOL were 156% and 138%, indicating overestimation on both nights. Participants were generally accurate in their estimation of the number of hours they slept (98% and 106%) and how well they slept, as indicated by sleep efficiency (97% and 104%). The degree of over- or under-estimation within each variable did not differ from pre-treatment to post-treatment night. A split plot analysis of variance revealed that there was no medication effect on OSE scores for SOL, TST, SE, or WASO on pre- or post-treatment nights.
Figure 1.
OSE scores across following two nights of study for each sleep variable. Greater than 100% represent overestimation of the respective variable and less than 100% represent underestimation of the respective variable. The horizontal bar at 100% represents perfect accuracy. OSE scores demonstrate a consistent underestimation of WASO both across subjects and across the two study nights. Sleep onset latency (SOL), Wake time after sleep onset (WASO), Sleep Efficiency (SE), Total sleep time (TST).
4. Relationship to Drinking
A series of multiple regressions were conducted to examine the relationship between pre-treatment objective sleep and subjective estimates of sleep and drinking variables after controlling for previous drinking (number of drinking days or heavy drinking days) and treatment group (i.e., either gabapentin or placebo). Baseline drinking frequencies and medication group were entered as control variables into the regression analyses to predict subsequent alcohol consumption from baseline sleep parameters. Initial analyses using logistic regression to predict drinking versus no drinking revealed no significant relationships between sleep variables and drinking and therefore HDD and DD were analyzed as outcome measures.
The more accurate that participants were at detecting periods of wakefulness in their sleep during the study, the more alcohol they consumed, and the more frequently they drank. Frequency of heavy drinking (HDD) during the 6-week medication phase was predicted by subjective-log estimates and OSE scores of WASO (R2=.69 p<.02 and R2=.49, p<.02, respectively). Similarly, frequency, expressed as drinking days (DD) during this phase was predicted by subjective-log estimates of WASO and accuracy (R2=.53, p <.03 and R2=.55, p<.02, respectively). Overestimation of SOL was not predictive of drinking during the treatment phase.
The single objective variable that significantly predicted relapse was REM sleep latency (R2=.59, p<.05). After controlling for treatment group and previous days drinking, baseline REM latency predicted HDD during treatment, such that the longer the REM latency, the more HDD. PSG measures of SOL, TST, SE, WASO, REM sleep percentage and sleep stages 1–4 did not predict any drinking outcomes.
In the six weeks following treatment, the following variables were predictive of HDD: OSE scores of SOL (R2=.67, p<.03), subjective estimates of SOL (R2=.58, p<=.05), OSE scores of WASO (R2=.53, p<.05), and OSE scores of SE (R2=.70, p<.02). DD after treatment was predicted by OSE scores of SE (R2=.51, p<.05), subjective estimations of TST (R2=.79, p<.02), and the accuracy (OSE scores) of TST estimations (R2=.49, p=.05).
DISCUSSION
The primary aim of the study was to evaluate the association between objectively measured sleep and the subjective estimates of sleep in a sample of alcohol-dependent participants with insomnia. The secondary aim was to examine objective and subjective indices of sleep as predictors of drinking outcomes during and after treatment. There were four main study findings. First, as expected and similar to non-alcoholic insomnia participants, most participants overestimated SOL. However unlike non-alcoholic insomnia participants, this alcoholic sample significantly underestimated WASO compared to actual PSG values. Second, subjective sleep measures (SOL and WASO) were better predictors of future drinking than corresponding PSG measures. Third, the predictive ability of baseline sleep parameters depended on the selected measure of drinking outcomes and when such an outcome was measured (i.e., during treatment vs. after treatment). Finally, there were no changes in objective or subjective sleep measures from pre-treatment to post-treatment.
Subjective Estimates
Subjective sleep estimates, collected from sleep logs, and their accuracy, reported as objective sleep estimation (OSE) percentages, revealed a consistent underestimation of WASO among participants and across the study nights (Figure 1). These findings, although counter to our original hypothesis, are consistent with Currie et al. (2004), who found that alcohol-dependent insomnia participants similarly underestimated WASO. In that study, median WASO OSE scores were 70.7%, which was higher than our findings of 29% and 38% on pre-treatment and post-treatment nights, respectively. Two factors may account for these discrepancies. First, Currie et al. (2004) used wrist actigraphy, which measures activity level, an indirect measure of sleep, rather than PSG and, therefore, may have detected less wakefulness during the night. Actigraphs do underestimate awakenings if no motor activity accompanies the arousal (Hoffmann et al., 2004). Second, participants in the Currie study were abstinent for an average of 15.7 (±20.2) months, whereas the mean duration of abstinence in the present study was only 20 days. Thus, changes in objective sleep over prolonged abstinence may be associated with changes in subjective sleep, but little is known about this relationship. It is also possible that participants become more aware of wakefulness in the night with prolonged abstinence, a well-documented characteristic of chronic insomnia participants (Rosa and Bonnet, 2000). Overall, these results suggest that alcohol-dependent participants in early recovery may not initially report wakefulness in the night, even though sleep disruption is evident on the PSG. Additionally, they may be more likely to report difficulty falling asleep than waking during the night.
The mechanisms underlying the evolution of the insomnia complaint in alcohol-dependent participants are not known. Since alcohol affects many of the same areas of the brain that are involved in the initiation and regulation of sleep (for review, see Brower, 2001 and Brower, 2003), it is tempting to speculate that neurotoxicity from chronic alcohol use may be affecting the memory for, and perceptual distinction of, wakefulness and sleep upon morning awakening. The particular pattern of WASO underestimation observed in this study may be a characteristic of insomnia in early recovery among this sub-population.
Predicting Relapse
Overestimation of SOL on the sleep logs and accuracy by OSE percentages of SOL were better predictors of drinking only after medical treatment for alcoholism. Although it has been well-documented that subjectively reported SOL predicts relapse in alcohol-dependent participants (Brower et al., 2001; Brower et al., 1998; Drummond et al., 1998; Foster et al., 1998; Foster and Peters, 1999; Skoloda et al., 1979) the current study focused on additional variables and their relationship between drinking behaviors during and after treatment. Our findings suggest that difficulty falling asleep may present the greatest risk for relapse once the patient finishes an episode of treatment for alcohol dependence.
Subjective estimation of WASO on sleep logs as well as OSE WASO percentages were better predictors of drinking during the treatment period. WASO has not been shown to predict relapse in previous studies of alcohol-dependent participants (Brower et al., 1998; Foster and Peters, 1999). However, results from the present study may differ from previous studies because participants enrolled in this study were selected because of an insomnia complaint, not simply because they were recovering alcohol-dependent patients. These results suggest that different rates and degrees of relapse may be associated with the particular type of insomnia complaint in recovering alcoholics.
Overall, this study suggests that subjective impressions of sleep were better predictors of relapse than PSG measures. Unlike previous studies (Brower et al., 1998; Drummond et al., 1998), objectively determined SOL by PSG did not predict relapse. The percent of slow-wave sleep also did not predict relapse, contrary to some previous findings (Aldrich et al., 1999; Allen et al., 1977), but consistent with others (Brower et al., 1998; Gann et al., 2004; Gillin et al., 1994).
The only objective measure that predicted relapse was REM sleep latency. However, these findings indicate an association between a long REM sleep latency on the pre-treatment night and the quantity of drinking days after the study period. This finding is inconsistent with previous studies (Aldrich et al., 1994; Allen and Wagman, 1975; Brower et al., 1998; Clark et al., 1998; Freemon, 1982; Gann et al., 2001; Gillin et al., 1994) which showed that a short REM latency predicted relapse.
There were limitations to this study. First, there were no direct comparisons to age- and sex-matched non-alcoholic insomniac controls. Second, the sample size was small. Finally, because it has been suggested that cognitive profile (Bastien et al 2003), personality (Dorsey and Bootzin, 1997), and sleep-related beliefs (Edinger et al 2000) impact subjective assessments of sleep, additional sleep-related questionnaires may have provided more information about a given participant’s sleep-related thoughts and behaviors.
Clinical Implications
The results of the present study have important implications for understanding and managing alcoholic participants with insomnia. First, such patients may be prone to overestimate initial insomnia and underestimate middle insomnia. When targeting insomnia with treatment, therefore, both initial and middle insomnia should be considered even though one type of insomnia may generate the complaint. Second, both subjective estimates of sleep and their accuracy compared to objective sleep measures may predict future drinking better than objective measures alone. Therefore, the use of sleep logs and subjective sleep assessments may be a useful tool in the clinic. Moreover, normalization of PSG in the presence of persisting sleep complaints may be an insufficient goal of treatment. Nevertheless, health care professionals working with recovering alcohol-dependent participants with insomnia should consider referring their participants for a more extensive sleep evaluation if initial treatment efforts despite continuing abstinence fail to rule out sleep apnea and periodic limb movement disorder. Finally, gabapentin which has been reported to improve subjective sleep in open-label studies with alcohol-dependent patients (Chouinard et al., 1998; Karam-Hage and Brower, 2003a; Karam-Hage and Brower, 2000; Karam-Hage and Brower, 2003b; Rosenberg, 2003) was not predictive of sleep outcomes in this study. Therefore, further trials are necessary before gabapentin can be recommended to aid sleep in alcohol-dependent patients.
In conclusion, we found that alcohol-dependent insomnia participants early in recovery overestimate sleep onset latency and underestimate the amount of wakefulness in sleep. In addition, the study highlights a potential distinction between insomnia complaints in recovering alcoholic patents versus non-alcoholic participants. This pattern of sleep perception may be a signature characteristic of this insomnia sub-population and directly contribute to relapse, but this requires further study.
Acknowledgments
This research was supported by Grants T32AA07477, K24AA00304 and MO1 RR00042.
Abbreviations and Formulas
- TST
Total Sleep Time
- SOL
Sleep Onset Latency
- WASO
Wake Time After sleep Onset
- SE (%)
Sleep Efficiency=Total Sleep Time/Time in Bed*100
- REM
Rapid Eye Movement sleep
- OSE (%)
-
Objective Sleep time Estimated=subjective parameter/objective parameter *100
Overestimation= >100%
Underestimation= <100%
Perfect accuracy= 100%
- HDD
Heavy Drinking Days
- DD
Drinking Days
- PSG
Polysomnography
References
- Aldrich MS, Brower KJ, Hall JM. Sleep-disordered breathing in alcoholics. Alcohol Clin Exp Res. 1999;23:134–140. [PubMed] [Google Scholar]
- Aldrich MS, O’Neal E, Eiser A, Kroll P, Brower KJ, Shipley J. Slow wave sleep decrement and relapse tendency in alcoholics in treatment. Sleep Research. 1994;23(185) [Google Scholar]
- Allen R, Wagman A. Do sleep patterns relate to the desire for alcohol? Adv Exp Med Biol. 1975;59:495–508. doi: 10.1007/978-1-4757-0632-1_35. [DOI] [PubMed] [Google Scholar]
- Allen R, Wagman A, Funderburk F. Slow wave sleep changes: alcohol tolerance and treatment implications. Adv Exp Med Biol. 1977;85A:629–640. doi: 10.1007/978-1-4899-5181-6_40. [DOI] [PubMed] [Google Scholar]
- Baekeland F, Hoy P. Reported vs recorded sleep characteristics. Arch Gen Psychiatry. 1971;24:548. doi: 10.1001/archpsyc.1971.01750120064011. [DOI] [PubMed] [Google Scholar]
- Baekeland F, Lundwall L, Shanahan TJ, Kissin B. Clinical correlates of reported sleep disturbance in alcoholics. Q J Stud Alcohol. 1974;35:1230–41. [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Medicine Reviews. 2003;7(6):523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich M, Robinson EAR, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. American Journal of Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcoholism: Clinical & Experimental Research. 1998;22(8):1864–1871. [PubMed] [Google Scholar]
- Brower KJ, Kim HM, Karam-Hage M, Zucker RA, Greden JF. A Double-blind randomized clinical trial of gabapentin vs. placebo for treating alcohol dependence. Biol Psychiatry. 2003;53:845–855. [Google Scholar]
- Buysse D, Reynolds Cr, Monk T, SRB, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caetano R, Clark CL, Greenfield TK. Prevalence, trends, and incidence of alcohol withdrawal symptoms: Analysis of general population and clinical samples. Alcohol Health and Research World. 1998;22:73–79. [PMC free article] [PubMed] [Google Scholar]
- Carroll K, O’Malley S. Compliance Enhancement: A Manual for the Psychopharmacotherapy of Alcohol Dependence. Yale University; New Haven, CT: 1996. [Google Scholar]
- Carskadon M, Dement W, MMM, CG, VPZ, RS Self reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. American Journal of Psychiatry. 1976;12:1382–1388. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Beauclair L, Belanger MC. Gabapentin: long-term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders [letter] Canadian Journal of Psychiatry - Revue Canadienne de Psychiatrie. 1998;43(3):305. [PubMed] [Google Scholar]
- Clark C, Gillin J, Golshan S, Demodena A, Smith T, Danowski S, Irwin MMS. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol Psychiatry. 1998;(43):601–607. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Currie S, Malhotra S, Clark S. Agreement among subjective, objective, and collateral measures of insomnia in postwithdrawal recovering alcoholics. Behavioral Sleep Medicine. 2004;2(3):148–161. doi: 10.1207/s15402010bsm0203_4. [DOI] [PubMed] [Google Scholar]
- Douglass AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone V, Jr, Guilleminault C, Dement WC. The Sleep Disorders Quesetionnaire I: Creation and Multivariate Structure of SDQ. Sleep. 1994;17(2):160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- Drummond S, Gillin J, Smith T, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18(4):232–239. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- Foster J, Marshall E, Peters T. Predictors of relapse to heavy drinking in alcohol dependent subjects following alcohol detoxification: The role of quality of life measures, ethnicity, social class, cigarette and drug use. Addiction Biology. 1998;3:333–343. doi: 10.1080/13556219872146. [DOI] [PubMed] [Google Scholar]
- Foster J, Marshall E, Peters T. Application of a quality of life measure, the Life Situation Survey (LSS), to alcohol dependent subjects in relapse and remission. Alcohol Clin Exp Res. 2000;24:1687–1692. [PubMed] [Google Scholar]
- Foster J, Peters T. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23:1044–1051. [PubMed] [Google Scholar]
- Frankel B, Coursey R, Buchbinder R, Snyder F. Recorded and reported sleep in chronic primary insomnia. Archives of General Psychiatry. 1976;33:615–623. doi: 10.1001/archpsyc.1976.01770050067011. [DOI] [PubMed] [Google Scholar]
- Freemon F. The effect of chronically administered delta-9-tetrahydrocannabinol upon the polygraphically monitored sleep of normal volunteers. Drug Alcohol Dependence. 1982;10(4):345–353. doi: 10.1016/0376-8716(82)90036-9. [DOI] [PubMed] [Google Scholar]
- Gann H, Feige B, Hohagen F, van Calker D, Geiss D, Dieter R. Sleep and the cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biological Psychiatry. 2001;50:383–390. doi: 10.1016/s0006-3223(01)01172-6. [DOI] [PubMed] [Google Scholar]
- Gann H, van Calker D, Feige B, Cloot O, Bruck R, Berger M, Riemann D. Polysomnographic comparison between patients with primary alcohol dependency during subacute withdrawal and patients with a major depression. Eur Arch Psychiatry Clin Neurosci. 2004;254(4):263–71. doi: 10.1007/s00406-004-0494-1. [DOI] [PubMed] [Google Scholar]
- Gillin J, Smith T, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3 month follow up. Arch Gen psychiatry. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Guy W. Clinical Global Impressions. National Institute of Mental Health; Rockville, MD: 1976. [Google Scholar]
- Hoffmann R, Emslie G, Thompson J, Rintelmann J, Moore J, Armitage R. The Relationship Between Actigraphy and Polysomnography in Healthy Children and Adolescents. Sleep. 2004;27(A816) [Google Scholar]
- Jenkins C, Stanton B, Niemcryk S, Rose R. A scale for the estimation of sleep problems in clinical research. J Clinical Epidemiology. 1988;41:313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- Kales A, Kales J. Evaluation and treatment of insomnia. Oxford; New York: 1984. [Google Scholar]
- Karam-Hage M, Brower K. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry & Clinical Neurosciences. 2003a;57:542–544. doi: 10.1046/j.1440-1819.2003.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence [letter] Am J Psychiatry. 2000;157:151. doi: 10.1176/ajp.157.1.151. [DOI] [PubMed] [Google Scholar]
- Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry & Clinical Neurosciences. 2003b;57(5):542–544. doi: 10.1046/j.1440-1819.2003.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means M, Edinger J, Glenn M, Fins A. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Medicine. 2003;4:285–296. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Behavioral studies of sleep patterns in alcoholics during intoxication and withdrawal. J Pharmacol Exp Ther. 1970;175:94–112. [PubMed] [Google Scholar]
- Monroe L. Psychological and physiological differences between good and poor sleepers. Journal of Abnormal Psychology. 1967;72:255–264. doi: 10.1037/h0024563. [DOI] [PubMed] [Google Scholar]
- Morin CM. Insomnia Psychological Assessment and Management. The Guilford Press; New York: 1993. [Google Scholar]
- NIAAA. Helping Patients Who Drink Too Much: A Clinician’s Guide. 2005. NIAAA Publications Distribution Center; Rockville, MD: 2005. A Pocket Guide For Alcohol Screening and Brief Intervention. [Google Scholar]
- Rechtschaffen A, Kales AA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Government Printing Office; Washington, DC: 1968. [DOI] [PubMed] [Google Scholar]
- Rosa R, Bonnet M. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosomatic Medicine. 2000;62:474–482. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Rosenberg KP. Gabapentin for Chronic Insomnia. The American Journal on Addictions. 2003;12:273–274. [PubMed] [Google Scholar]
- Rundell OH, Williams HL, Lester BK. Sleep in alcoholic patients: longitudinal findings. Adv Exp Med Biol. 1977;85B:389–402. doi: 10.1007/978-1-4615-9038-5_25. [DOI] [PubMed] [Google Scholar]
- Skoloda T, Alterman A, Gottheil E. Sleep quality reported by drinking and non-drinking alcoholics. Pergamon Press; Elmsford, NY: 1979. [Google Scholar]
- Sobell L, Sobell M, Leo G, Cancilla A. Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addictions. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]