Abstract
Alum is used as a vaccine adjuvant and induces Th2 responses and Th2-driven antibody isotype production against co-injected antigens. Alum also promotes the appearance in the spleen of Gr1+, IL-4+ innate cells that, via IL-4 production, induce MHC II mediated signaling in B cells. To investigate whether these Gr1+ cells accumulate in the spleen in response to other Th2 inducing stimuli and to understand some of their functions, the effects of injection of alum and eggs from the helminth, Schistosoma mansoni, were compared. Like alum, schistosome eggs induced the appearance of Gr1+IL-4+ cells in spleen and promoted MHC II-mediated signaling in B cells. Unlike alum, however, schistosome eggs did not promote CD4 T cell responses against co-injected antigens, suggesting that the effects of alum or schistosome eggs on splenic B cells cannot by themselves explain the T cell adjuvant properties of alum. Accordingly, depletion of IL-4 or Gr1+ cells in alum injected mice had no effect on the ability of alum to improve expansion of primary CD4 T cells. However, Gr1+ cells and IL-4 played some role in the effects of alum, since depletion of either resulted in antibody responses to antigen that included not only the normal Th2-driven isotypes, like IgG1, but also a Th1-driven isotype, IgG2c. These data suggest that alum affects the immune response in at least two ways, one, independent of Gr1+ cells and IL-4, that promotes CD4 T cell proliferation and another, via Gr1+IL-4+ cells that participate in the polarization of the response.
Keywords: Antibodies, vaccine adjuvant, T cells, Th2, helminth
Introduction
Recently there has been a tremendous increase in the understanding of the innate immune system and its impact on specific immunity. Amongst the subjects that have received a great deal of attention are adjuvants, the means by which they activate innate immunity and the consequences of this activation for antigen specific T and B cells. Adjuvants greatly improve specific immune responses by increasing the numbers of antigen specific T and B cells, by increasing the titers of antibody made, and by increasing the numbers of T and B cells that convert to memory cells (1,2). Understanding how adjuvants work will enhance our understanding for the development of effective vaccines.
Some vaccines contain endogenous adjuvants in the form of microbial molecules. The innate immune system uses pattern recognition receptors such as toll like receptors (TLRs) to broadly recognize molecules produced by invading organisms (3-5). Once engaged, directly or indirectly, by ligands, TLRs promote responses in innate cells that have the potential to affect developing adaptive immune responses. TLR ligands potently activate dendritic cells (DC) to process antigen and upregulate costimulatory molecules and cytokines to provide the required signals to activate naïve T cells (6). Different adjuvants stimulate different responses from DC and innate cells, and consequently the nature of the specific immune response to antigen is affected by the adjuvant present at the time (7).
Many vaccines contain alum, a TLR independent adjuvant that induces Th2 polarized responses (8-10). Probably because of the bias in the type of T cell induced, alum markedly improves IgG1 and IgE response against coinjected antigens and suppresses production of antibody isotypes associated with Th1 responses such as IgG2a/c (10,11). Like alum, some infectious agents make T cells differentiate into Th2 cells. Most notable amongst these are the helminths, including the eggs of the trematode worm, Schistosoma mansoni (Sm). Sm eggs induce very powerful Th2 responses and high titers of antibody of the isotypes which are stimulated by Th2 cells, including IgG1 and IgE (12,13).
Surprisingly little is known about how alum and other Th2 driving substances are recognized by the body or how they act as adjuvants on the adaptive immune response in vivo. Unlike adjuvants that promote Th1 responses, Th2-promoting adjuvants do not induce DC to produce Th1 polarizing cytokines such as IL-12 (7,14-16). Moreover, alum does not fully activate DC in vitro, although it does induce caspase 1 activation and release of IL-1β and IL-18 cytokines by these cells, a property that may contribute to the effects of alum on CD4 cells in vivo (17-19). Alum may also directly enhance antigen presentation as DC pulsed with alum-adsorbed antigen prime CD4 cells in vitro more effectively than DC pulsed with soluble antigen do (20).
In vivo, Th2 cells can develop in the absence of IL-4 and IL-4−/− mice injected with alum containing adjuvants are able to mount robust Th2 responses (21-24). However in the absence of IL-4 or IL-4 receptor signaling, Th1 responses and Th1 dependent antibody isotypes also occur (23,24). Thus IL-4 plays an important role suppressing Th1 development and for ultimately biasing the immune response against antigens with which alum is coinjected. In the primary response to alum adsorbed antigen, IL-4 suppresses Tbet expression and Th1 responses as early as day 3 following injection, yet the means by which alum makes T cells differentiate into Th2 cells remains unknown (25). Although large numbers of CD4 cells themselves produce IL-4 during the primary response against alum adsorbed antigen (26), it is clear that other innate cells in the spleen also express IL-4 in an antigen independent manner (27). The relative importance of innate- versus CD4 cell-derived IL-4 in the suppression of type 1 responses to alum adsorbed antigen has not been dissected in vivo.
We have shown that increased numbers of innate, IL-4 expressing cells are detected in the spleen after exposure to alum (27). Two types of cells fall into this category, one expressing intermediate levels of Gr1 (Gr+) and another population that is Gr1 negative (Gr1−). After alum injection, Gr1+ cells are found in the red pulp of the spleen and near B cell follicles and IL-4 expressing cells in this population “prime” B cells to respond to cross linking of their MHC II proteins by fluxing calcium (Ca2+) rather than dying, the fate of a non primed B cell on which MHC II has been crosslinked (27,28). To find out whether the appearance of these cells is unique to alum exposure, we tested whether Sm eggs, a natural Th2 stimulus, would also cause the appearance of innate IL-4 producing cells and consequent effects on MHC II mediated signaling in splenic B cells. Here we show that, like alum, Sm eggs cause Gr1− and Gr1+ IL-4 producing cells to appear in spleen. Like the cells induced by alum, the egg-induced Gr1 expressing cells promote enhanced Ca2+ fluxes in splenic B cells stimulated through their MHC II molecules. Alum greatly enhances expansion of antigen specific CD4 cells to coinjected antigen while Sm eggs do not. Thus, alum has several adjuvant effects, some of which occur by separate mechanisms. Enhanced expansion of antigen specific CD4 cells to antigen coinjected with alum and the development of Th2 cells was independent of IL-4 and Gr1 expressing cells, but Th1 responses were enhanced when mice were treated with Gr1 or IL-4 depleting antibodies. This suggests that IL-4 expressing Gr1+ cells play a role in suppressing type 1 immune responses potentiated by alum.
Methods
Mice
Female BALB/c and C57Bl/6 WT mice were obtained from The Jackson Laboratories. IL-4−/− mice on a B6 background were kindly provided by Dr. Erwin Gelfand at NJRC. GFP IL-4 reporter (4Get) mice on BALB/c and C57Bl/6 backgrounds were a gift from Dr. Richard Locksley at UCSF Medical Center. IL-4 reporter mice and IL-4−/− were bred and maintained in a specific-pathogen-free environment at the Animal Care Facility at National Jewish Biological Resource Center. All animals were housed and maintained in accordance with the research guidelines of the Institutional Animal Care & Use Committee.
Antibodies and reagents
Monoclonal Abs against Gr1 (RB6-8C5), IL-4 (11B11), IAb/d (MKD6 and D3.137), IAb (Y3P) and FcγRIII/II (2.4G2) were purified from hybridoma supernatants in our laboratory. Alexa Fluor (AF) 408 was conjugated to Y3P antibodies using the AF408 protein conjugation kit (Molecular Probes). The following fluorochrome labeled mAbs were purchased from BD Pharmingen: APC-CY7 anti-CD4 (GK1.5), APC anti-CD44 (IM7), PE anti-B220 (RA3-6B2), PE conjugated anti-IAb(AF6-120.1), PE and PerCP labeled anti-Gr1 (RB6-8C5). Pacific Blue conjugated anti-Gr1 (RB6-8C5), F480 (BM9), B220 (RA3-6B2) and PE-Cy5 labeled anti-CD3 mAb (145-2C11) were purchased from eBiosciences. A647 labeled F480 (C1:A3-1) was from Serotec, PE labeled anti-CCR3 (83101) was from R&D Systems and Indo-1AM dye was purchased from Calbiochem. Alkaline phosphatase conjugated antibodies against mouse IgG1 and IgG2c heavy chains were purchased from Southern Biotech. Chicken egg ovalbumin protein for antibody experiments was purchased from Sigma or was from the Imject Maleimide Activated OVA kit (Pierce Biotechnology, Inc.) and conjugated to a cysteine linked 3K peptide (FEAQKAKANKAVDGGGC) (prepared at The Molecular Resources Center at the National Jewish Medical and Research Center) to form the 3K-Ova conjugate. PE-labeled IAb/3K tetramer was produced as described in our laboratory (29). E. coli LPS was purchased from Difco. Alum preparations were performed as previously described (27). S. mansoni eggs were provided by Dr. Fred Lewis (Biomedical Research Institute, Rockville, MD).
Injections
To follow Gr1+IL-4+ cells and to assay B cell Ca2+ responses to alum or helminth products in both BALB/c and B6 strains WT or 4get mice of both backgrounds were used. Age matched females between 6 and 10wk of age were injected with either 40mg alum, Sm eggs at indicated doses, or with 200μl PBS i.p.. In experiments designed to follow antigen specific CD4 and antibody responses, 10μg 3K-ova or ova protein either prepared in PBS, in combination with Sm eggs, or precipitated with alum was injected i.p. into WT or IL-4−/− B6 mice. For depletion of Gr1+ cells or neutralization of IL-4 in vivo, mice were given 1mg anti-Gr1 mAb, 500ug anti-IL-4 mAb, or purified rat IgG (Jackson ImmunoResearch Laboratories, Inc.) by i.v. injection 1, 3, and 5 days after immunization.
Cell preparation
Spleens and peritoneal cells were harvested into cold BSS at indicated times after injection. In some experiments, peritoneal cells were collected by peritoneal lavage in BSS. Blood was collected in some experiments and sera were stored at −20 C until analysis by ELISA. Splenocytes were processed into single cell suspensions using nylon mesh and red cells lysed with ammonium chloride. Nucleated cells were enumerated using a Coulter Counter.
Flow Cytometry
For analysis of Gr1+ IL-4 expressing cells, peritoneal cells from challenged IL-4 reporter mice were stained with reagents against F480, cKit, and Gr1 and splenocytes were stained with reagents against CD4, CD8, B220 and Gr1. For analysis of B cell Ca2+ responses in response to MHC II ligation, splenocytes (107/ml in (Ca2+ free) SMEM + 2.5% FBS) were incubated with 10% 2.4G2 hybridoma supernatant, biotinylated anti-MHC II antibody, 5μM Indo-1AM dye and fluorochrome labeled mAb against B220 for 45 min (37°C in 5% CO2). Cells were washed in SMEM/2.5%FBS, resuspended to 107 cells/ml, and allowed to rest 1hr at RT prior to data acquisition on an LSR flow cytometer using Cell Quest software (Beckton Dickenson). To crosslink MHC II molecules, 5μg/ml extravadin (Sigma) was added to each sample. Kinetic and population analysis of intracellular Ca2+ responses was performed using Flow-Jo Software (Tree-Star Inc). To follow IAb/3K specific CD4 cells, spleens were harvested 9 days after immunization. 1.25 × 107 splenocytes were added to the wells of 96 round well plates in 25μl complete tumor medium (CTM) and stained with 3K/IAb tetramers as described (29). APC-Cy7 anti-CD4, PB anti-B220, PB anti-F480, Alexa405 anti-IAb, and APC anti-CD44 were added at 4°C for 20min and cells were wash and analyzed immediately. Flow cytometry data was acquired on a Cyan cytometer using Summit software (Cytomation). Data were analyzed using Flow-Jo software.
ELISAs
96-well Immulon plates (Thermo) were coated with ovalbumin protein at 100μg/ml in PBS. Plates were washed using ELx405 autoplate washer (BioTek Instruments) and then blocked with 10%FCS/PBS for 2hr RT. Plates were washed and antibody serum samples diluted in 10%FCS/PBS were added to the plates. To determine RU we used a positive control pooled serum sample from B6 and Balb/c mice that contained ova specific Th2 and Th1 isotypes. The samples were incubated overnight at 4°C. Plates were washed and alkaline phosphatase conjugated anti-IgG1 (X56) or anti-IgG2a/c (R19-15) or biotinylated anti-IgE (R35-119) (Pharmingen) detection antibodies were added for 2hr RT. For IgE alkaline phosphatase conjugated streptavadin was added for 1hr RT. Substrate (p-nitrophenyl phosphate) diluted in glycine buffer was added to each well and 405nm absorbance values were collected on Elx808 microplate reader.
Statistics
Data are presented as indicated in the figure legends and statistical significance determined using Student's two-tailed T test with GraphPad Prism software version 4.
Results
A natural Th2 driving stimulus induces accumulation of similar IL-4 expressing cells as alum
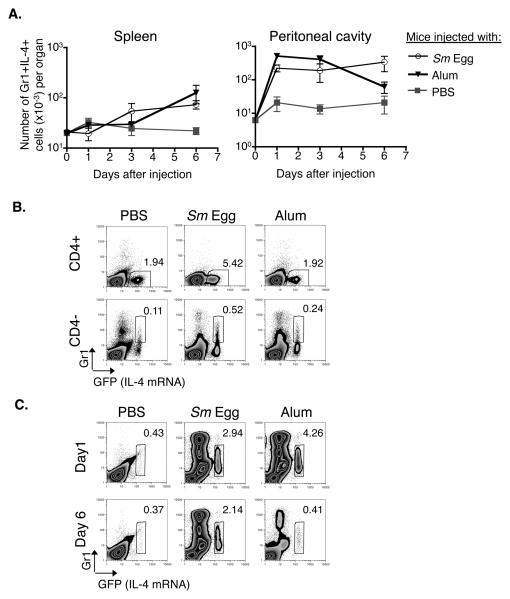
In mice injected with the adjuvant, alum, a population of innate cells expressing intermediate levels of Gr1 (Gr1+) and IL-4 accumulate in the spleen, reaching a peak 6 days after alum administration (27). To determine whether a similar population of cells accumulates in the spleen following exposure to a “natural” material that is known to induce Th2 cells, IL-4 transcriptional reporter (4Get) mice (30) were injected with Sm eggs, alum or PBS. CD4 positive and CD4 negative cells in their spleens and the site of injection, the peritoneal cavities, were analyzed for GFP and Gr1 expression at various times thereafter. 4Get animals were used because they express GFP under the control of the IL-4 promoter and thus GFP expression by cells from these animals is an indication that their IL-4 locus is open (30).
Injection of either Sm eggs or alum induced the appearance of increased numbers of GFP+Gr1+ cells in both locations over the time analyzed. The kinetics of accumulation of GFP+Gr1+ cells in the spleen was much later than the accumulation of GFP+Gr1+ cells in the peritoneal cavity. In the peritoneal cavity, the peak accumulation of GFP+Gr1+ cells occurred on day 1, while in the spleen accumulation of these cell peaked on day 6 (Fig. 1A and (27)). In the spleens 6 days after injection, both alum and Sm eggs induced accumulation of CD4 negative cells that coexpressed GFP (Fig. 1B, lower 3 panels). These cells were found to be negative for B220, CD4 or CD8, and in the spleen consisted of two different populations, a Gr1 negative population and a Gr1+ population (Fig. 1B and data not shown).
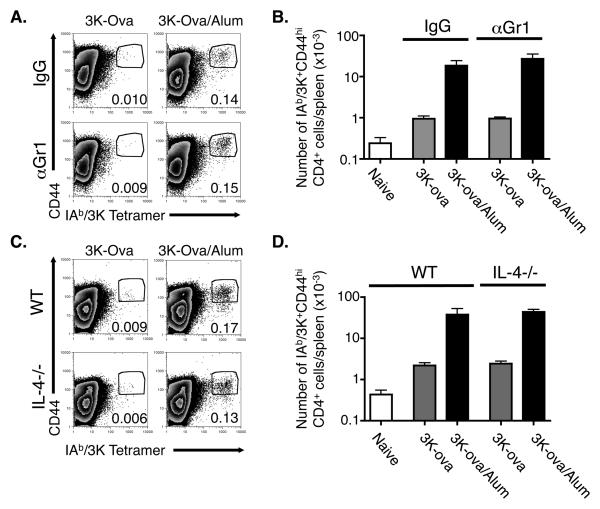
Figure 1. Sm eggs, like alum, induce the appearance of Gr1+IL-4+ innate cells.
A. 4Get, IL-4 indicator mice were injected i.p. with alum, Sm eggs or PBS. At the indicated times, cells were isolated from the spleens and peritoneal cavities of the animals and stained with antibodies against CD4, CD8, B220 and Gr1 (splenocytes) or F480, cKit and Gr1 (peritoneal cells). Shown are the means and standard errors of numbers of B220, CD4 and CD8 negative splenocytes or F480 and cKit negative peritoneal cells that were Gr1+ IL-4+ (GFP+) isolated from 3 identically treated animals per group. B. Expression of Gr1 and GFP (IL-4 mRNA) on CD4 negative or CD4 positive spleen cells 6 days after i.p. injection with alum, Sm eggs or PBS. Numbers on graphs indicate the percent of gated CD4− or CD4+ cells that fall within the gates on each individual FACS plot. C. FACS plots show Gr1 and GFP (IL-4 mRNA) expression on F4/80- cKit- peritoneal cells from mice injected 1 or 6 days previously with PBS, Sm eggs, or alum. Numbers on graphs indicate the percent of F4/80- cKit- cells that fell within the gate on each plot for the sample shown. For B and C, data are representative FACS plots from 3 individual mice per group. 40mg alum or 104 Sm eggs were used for injections in this experiment. Data are representative of 3 independent experiments.
In mice injected with Sm eggs, an increased percentage of splenic CD4+ cells coexpressed GFP compared to mice injected with PBS or alum particles alone (Fig.1B, top 3 panels). This is consistent with the known ability of Sm egg components to drive Th2 responses against intrinsic schistosome antigens. Although alum also is known to drive Th2 responses, no antigen was included in these experiments and so the alum was not expected to affect T cells. In addition GFP+ CD4+ cells did not coexpress Gr1 (Fig. 1B).
Examination of cells at the site of injection after i.p. injection of alum or parasite eggs revealed that both stimuli induced accumulation of IL-4 expressing cells in the peritoneal cavity, as they did in the spleen. In the peritoneum the cells were relatively homogeneous since >95% of these cells coexpressed Gr1 (Fig. 1C) and when sorted were found to be >95% eosinophils (data not shown). In contrast only 50% of the Gr1+IL-4+ population in the spleen were eosinophils while the remaining 50% have a mononuclear morphology (27).
Exposure to Th2 inducing stimuli increased MHC II mediated signaling in a large percentage of splenic B cells
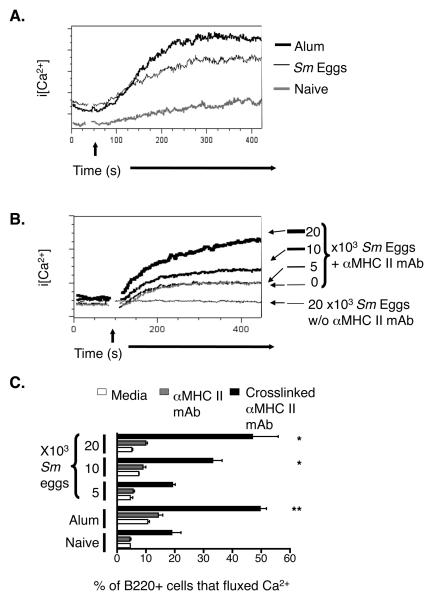
Aggregation of antigen receptors on B cells, or exposure of B cells to IL-4, causes IgαIgβ signaling chains to associate with MHC II and thus enables MHC II, after crosslinking, to transduce signals leading to mobilization of intracellular calcium (Ca2+) and proliferation of the cells involved (28,31). Recently we discovered that injection of alum into mice causes upregulation of MHC II expression and enhanced Ca2+ signals after crosslinking of these receptors on B cells in their spleens, a phenomenon which improves early IgM responses to coinjected antigens (27). Since anti-Gr1 depletion experiments implicated the Gr1+IL-4+ cells in this phenomenon, we wondered whether the Gr1+IL-4+ cells induced by Sm eggs would have similar effects. Thus, we injected C57Bl/6 (B6) mice i.p. with Sm eggs or alum and, 6 days later, tested the responses of the B cells in their spleens to crosslinking of the MHC II proteins on their surfaces. Like the B cells from alum-injected mice, B cells from Sm egg-injected mice contained elevated concentrations of cytoplasmic Ca2+ in response to MHC II crosslinking when compared to B cells from untreated mice (Fig. 2A). In each mouse we also compared the percent of B220+ cells that fluxed Ca2+ at the peak of the response to MHC II crosslinking to the percent that fluxed Ca2+ in the absence of stimulus. In mice that had been injected 6 days prior with alum or Sm eggs there was an up to 2.5 fold increase in the percent of B cells fluxing Ca2+ than in untreated control mice (Fig. 2C). We did a dose response and found at least 104 Sm eggs had to be injected to observe the effects on MHC II mediated signaling (Fig. 2B). Thus the polyclonal effects on B cells that we have previously found to accompany the presence of enhanced numbers of IL-4 expressing cells in mice exposed to alum, also occurred in mice exposed to helminth products.
Figure 2. MHC II mediated Ca2+ responses in B cells are enhanced after exposure to Th2 stimuli.
A. Spleen cells were isolated from mice injected 6 days previously with nothing (grey histogram), 104 Sm eggs (bold black histogram), or alum (fine black line histogram), and stained as described in Materials and Methods. MHC II molecules on the surface of the cells were crosslinked with biotinylated anti-MHC II followed by avidin. The time of addition of avidin is indicated on the Figure by the arrow. Shown are the changes in intracellular Ca2+ levels in B220+ cells. B. Mice were given the indicated numbers of Sm eggs. Six days later, spleen cells from the mice were stained and their MHC II molecules crosslinked as described in Figure 1A. Shown are the changes in intracellular Ca2+ induced by the crosslinking. Data in A and B are representative of 3 mice per group in a single experiment. C. Shown are the percent of B220+ cells undergoing Ca2+ flux in the absence of any stimulation (white bars), after addition of anti-MHC II mAb but before avidin mediated crosslinking (grey bars) and during the peak of the response after addition of avidin (solid bars) in mice injected 6 days previously with Sm egg, alum or PBS. Means +/− standard errors for values from 3 mice are shown and asterisks indicate results that differed from those in naïve mice with p<0.05 (*) or p<0.01 (**). Data in this Figure are from a representative experiment of 3.
Depletion of Gr1 or IL-4 expressing cells eliminated the in vivo effects of Sm eggs or alum exposure on splenic B cell signaling
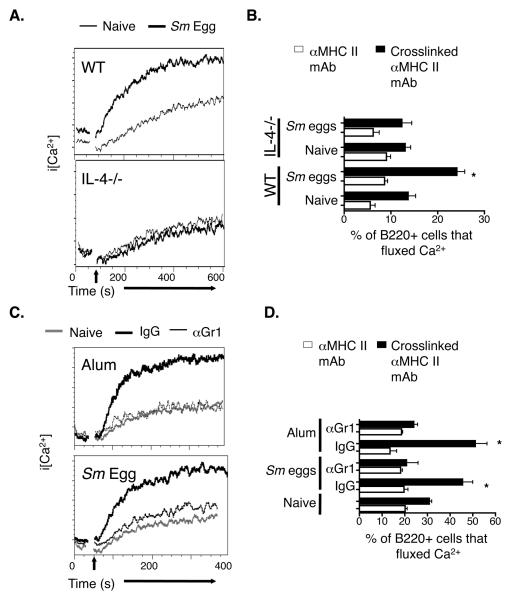
We have previously shown that IL-4 neutralization blocks the ability of alum to promote enhanced MHC II signaling in splenic B cells. To find out whether IL-4 is required for these polyclonal effects on B cells in response to Sm eggs, IL-4−/− and WT B6 mice were injected with Sm eggs i.p. and MHC II-mediated Ca2+ responses in splenic B cells were analyzed 6 days later. In contrast to the high MHC II-induced Ca2+ flux that occurred in B cells from Sm egg injected WT mice, intracellular Ca2+ levels increased only slightly following MHC II aggregation on B cells from egg injected IL-4−/− mice (Fig. 3A) and Sm egg injection did not increase the percentage of B cells that fluxed Ca2+ in IL-4−/− mice as it did in WT mice (Fig. 3B). These data suggest that helminth products, like alum, mediate polyclonal effects on B cells via an IL-4 dependent mechanism.
Figure 3. The effects of Sm egg or alum on B cells are dependent upon IL-4 and Gr1 expressing cells.
A. Wild type (WT) and IL-4−/− mice were injected i.p. with nothing (fine black line) or Sm eggs (bold black lines). Six days later, spleen cells were isolated and stained and MHC II crosslinked as described in Figure 2. Shown are the changes in intracellular Ca2+ levels in B220+ cells after MHC II crosslinking. B. Wild type or IL-4−/− mice were injected with nothing or Sm eggs and analyzed six days later as described in Figure 3A. Graphs show the percentage of splenic B220+ cells from either naïve, or Sm egg injected WT or IL-4−/− mice that that fluxed Ca2+ before (white bars) and during the peak (black bars) of the response to avidin mediated crosslinking of their MHC II molecules. C. Mice were injected i.p. nothing (grey lines), alum or Sm eggs (black lines). Alum or Sm egg injected mice were treated with either control rat IgG (bold black lines) or anti-Gr1 mAb (fine black lines). Six days after alum or Sm egg injection, spleen cells were isolated and their MHC II molecules crosslinked as described in Figure 2. Shown are the changes in intracellular Ca2+ levels in B220+ cells after MHC II crosslinking. D. Mice were injected with nothing, Sm eggs or alum, later with control rat IgG or anti-Gr1 and their spleen cells analyzed six days after injection of Sm eggs or alum as described in Figure 3C. Shown are the means +/− standard errors of the percentages of B220+ cells that had increased levels of intracellular Ca2+ before (white bars) or after (black bars) crosslinking of their MHC II proteins. Asterisks indicate a group with a percentage of cells fluxing Ca2+ after MHC II crosslinking that was significantly different from that observed in cells from naïve mice (p<0.05). Results in A and C are representative of two individual experiments and data in B and D are from a representative experiment of three.
Alum cannot promote MHC II mediated signaling in B cells if Gr1+ cells are removed with a Gr1 depleting antibody (27). To determine whether a Gr1 expressing cell type is involved in Sm egg-induced effects on MHC II mediated Ca2+ responses in B cells, we injected Sm eggs into B6 mice that had been treated either with anti-Gr1 antibodies or with control rat IgG. We observed that after treatment with anti-Gr1 antibodies, MHC II crosslinking neither induced Ca2+ fluxes nor increased the percentage of B cells that increased their levels of intracellular Ca2+ (Fig. 3C and D). Thus, Gr1+IL-4+ cells probably play a role in polyclonal B cell effects induced by Sm eggs, just as they do during responses to alum.
Alum promotes expansion of CD4 cells to coinjected antigen but Sm eggs do not
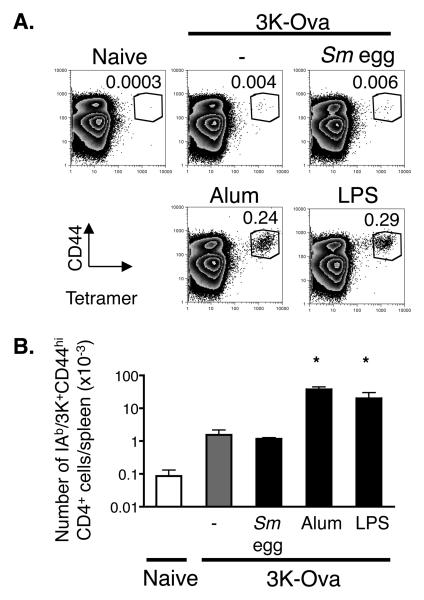
The enhanced ability of B cells to signal downstream of MHC II engagement could improve their ability to present antigen, thereby affecting the CD4 T cell response. To test this, we decided to compare the effects of alum and Sm eggs on T cell priming. In the past such experiments have been done by following the consequences of T cell activation, such as helper function or cytokine release. However, here we wanted to measure T cell numbers. Such experiments can be performed by transfer of T cells expressing transgenic T cell receptors, but artifacts have been associated with the use of fairly large populations of these cells, bearing a single TCR. In fact we have noticed that such cells may not be good representatives of endogenous host T cells ourselves. For example, if too many TCR transgenic T cells were transferred, they did not all increase their surface CD44 and differentiate into Th2 cells, in mice given antigen + alum. To circumvent these problems, we decided to follow endogenous T cells specific for IAb bound to the 3K peptide, a combination for which excellent staining tetramers can be made. To allow us to follow both antibody and CD4 T cell responses, the 3K peptide was conjugated to ovalbumin (3K-Ova). This combination still allowed presentation of 3K to T cells and ova to B cells, since 3K-Ova induced the rapid expression of CD69 on IAb/3K specific T cells in vitro, induced anti-Ova antibodies, detected by ELISA, in immunized mice and could be used to detect anti-Ova antibodies in capture ELISA reactions (data not shown).
We have estimated that naïve B6 mice contain 50-100 T cells specific for IAb/3K (M. MacLeod, unpublished data). The numbers of IAb/3K specific CD4 T cells peak 9 days after injection of 3K-Ova when either alum or LPS were used as adjuvants (unpublished data). Administration of 3K-Ova bound to alum, rather than free 3K-Ova alone greatly increased the numbers of 3K specific T cells in the spleen at this time (Fig. 4). We were surprised to find that alum promoted priming of CD4 cells as well as LPS, a TLR agonist often used as an adjuvant (32,33). In contrast there was no difference in the percent or total number of IAb/3K specific CD4 cells detected in the spleens of mice injected with 3K-Ova in the presence or absence of Sm eggs (Fig 4). Thus the bystander enhancement of MHC II mediated signaling in B cells, which occurs in the Sm egg injected animals, is not sufficient for enhanced CD4 priming to coinjected antigen. This implies that alum greatly promotes CD4 priming through another mechanism.
Figure 4. Alum but not Sm eggs promotes enhanced CD4 responses to coinjected antigen.
A. B6 mice were injected with 3K-Ova protein combined with either nothing (−), Sm eggs, alum, or 7μg LPS and splenocytes were stained with antibodies against B220, F480, CD44, CD4 and IAb/3K tetramers nine days later. Plots show staining with anti-CD44 and IAb/3K tetramers on CD4 positive, F4/80- B220- spleen cells. Numbers on graphs indicate the mean percentages of CD4 cells that were CD44hi IAb/3K tetramer positive for the sample shown. Data shown are representative of three identically treated mice in each group. Cells that stained as CD44hi and with IAb/3K tetramer in naïve mice illustrate the level of background staining in these experiments. B. Shown are the mean +/− standard errors of the numbers of IAb/3K specific CD44hi CD4 T cells detected per spleen for three mice per group. Asterisks indicate samples in which the numbers of IAb/3K specific T cells that were detected were significantly different from the numbers detected in mice injected with 3K-Ova alone (p<0.05). Data are from a representative experiment of three.
Enhanced CD4 priming by alum adjuvant is independent of IL-4 and Gr1 expressing cells
Both alum and helminth eggs promoted recruitment of IL-4 expressing innate cells in the spleen, but only alum enhanced CD4 priming to coinjected antigen. To test directly whether or not the presence of Gr1 expressing cells affects CD4 priming, we injected B6 mice with 3K-Ova in the presence or absence of alum, treated them with either a Gr1 depleting antibody or control rat IgG and determined the number of IAb/3K specific CD4 T cells in the spleen 9 days later. We found a similar number of activated IAb/3K specific CD4 T cells in the spleens of 3K-Ova/alum injected mice whether or not the mice were treated with the Gr1 depleting antibody (Figs 5A and B).
Figure 5. The ability of alum to enhance expansion of antigen specific CD4 cells is independent of Gr1 expressing cells and IL-4.
A,B. C57BL/6 mice were injected with 3K-Ova either alone or adsorbed to alum and treated with either rat IgG or anti-Gr1 as described in Materials and Methods. Nine days later splenocytes were stained as in Figure 4. A. The FACS plots show the staining of F4/80- B220- CD4+ cells with anti-CD44 and IAb/3K tetramer. Numbers indicate the percentage of F4/80- B220- CD4+ cells that were CD44hi and IAb/3K tetramer positive and are representative of three individual samples. B. Shown are the average +/− standard error of the numbers of CD44hi IAb/3K-staining CD4+ cells/spleen of mice immunized as indicated. Results are representative of three independent experiments. C.D. Wild type or IL-4−/− mice were injected with 3K-Ova with or without alum. Nine days later their spleen cells were isolated and analyzed as in A, B. Results are representative of two separate experiments.
This experiment did not test the need for IL-4 in T cell priming, since Gr1+ cells are not the only cells induced by alum that can produce the cytokine (Fig 1A). Thus, to examine this issue, WT or IL-4−/− mice were injected with 3K-Ova in the presence or absence of alum and the numbers of IAb/3K specific CD4 T cells counted 9 days later. The absence of IL-4 had no effect on the percent or number of IAb/3K specific cells detected in the spleens of mice injected 9 days previously with 3K-Ova/alum (Fig. 5C and D). Moreover, the fold increase of such cells in mice that had been injected with 3K-Ova/alum versus those in mice that received 3K-Ova alone was similar between the two types of mice. These results show that, as previously reported, the ability of alum to increase T cell primary responses does not involve IL-4.
Depletion of Gr1 cells results in increased levels of Th1 associated antibody isotypes
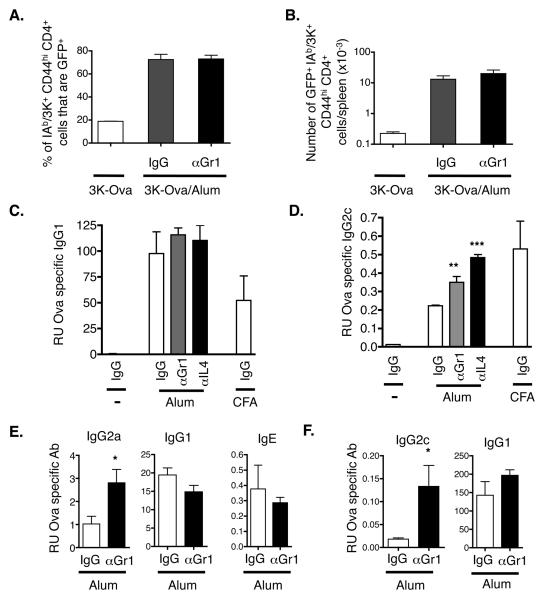
Alum increases the primary T cell response to antigen. However, even on the peak day, animals given alum plus 3K-Ova still contained only rare cells specific for IAb/3K. Because of this, it has been difficult to assess by intracellular staining whether the cells induced in such experiments are Th1 or Th2 in nature. Therefore, to examine the effects of Gr1 depletion on the phenotype of the primed CD4 cells, we tracked antigen specific CD4 cells in alum injected 4Get mice. We found that the percentage of IAb/3K specific cells that had the potential to make IL-4 was significantly larger in animals given antigen plus alum (Fig. 6A). This effect combined with the strong effect of alum on CD4 expansion (Fig. 4) resulted in greatly enhanced numbers of IAb/3K specific, IL-4 expressing CD4 cells in 3K-Ova/alum injected mice (Fig. 6B). Moreover, treatment of immunized mice with anti-Gr1 did not affect the percent or total number of IAb/3K specific cells capable of making IL-4 (Fig. 6A and B).
Figure 6. Gr1+ cells and IL-4 inhibit production of Th1-induced antibody isotypes.
A, B. 4Get mice were injected with 3K-Ova with or without alum and were treated with either control rat IgG or anti-Gr1 mAb as described in Materials and Methods. Splenocytes were stained for the presence of IAb/3K specific CD4 T cells and gates were drawn around CD44hi IAb/3K+ CD4 cells as in Figs 4 and 5. A. Shown are the average +/−standard errors of the percentages of IAb/3K+ CD44hi CD4+ spleen cells that expressed GFP (IL-4 mRNA). The results are from three identically treated mice/group. B. Shown are the average numbers +/− standard errors of GFP+ IAb/3K+ CD44hi CD4+ cells/spleen from mice immunized as indicated. The results are from three identically treated mice/group. C, D. B6 mice were injected with Ova with or without alum or CFA. Mice were treated with IgG (white bars), anti-Gr1 (grey bars) or anti-IL-4 (black bars) on days one, three and five after primary immunization. On day nine mice were injected with Ova alone and on day twelve their sera were tested for Ova specific antibodies by ELISA as described in Materials and Methods. The results represent the mean relative units +/− standard error of Ova specific antibody detected from three individual mice per group. Asterisks indicate groups in which the amount of antibody detected was significantly different from that in similarly immunized mice that received control IgG. A single asterisk indicates p<0.05, double asterisk p<0.01, and triple asterisk indicates p<0.001. Results are representative of two independent experiments. E, F. Balb/c mice (E) or B6 mice (F) were injected with either alum (E) or Alhydrogel (F) adsorbed Ova and were treated with IgG (white bars) or αGr1 (black bars) antibody as above. On day 10, mice were boosted with alum adsorbed Ova and sera were tested for Ova specific isotypes 5 days later. Results are representative of 3 (E) and 2 (F) individual experiments.
The phenotype of T cells affects the isotypes of antigen specific antibodies produced during immune responses. Th2-driven responses are characterized by high levels of IgE and IgG1 while responses involving Th1 cells are characterized by elevated production of IgG2a/c isotypes. Mice injected with Ova/alum contained high levels of IgG1 anti-Ova antibodies compared with mice that received Ova alone (Fig. 6C). These titers were not affected by treatment with anti-IL-4 antibodies or removal of Gr1+ cells during the first week of the primary response. We did not observe ova specific IgE levels in these B6 mice at this timepoint.
As expected, Ova antigen given with complete Freund's adjuvant (CFA) induced large amounts of IgG2c anti-Ova (Fig. 6D). Not unexpectedly, given the Th2 phenotype of the T cell response, titers of IgG2c anti-Ova antibodies were only slightly elevated in mice given Ova/alum versus Ova alone (Fig. 6D). In this case, however, as has been shown in IL-4 deficient mice (23), anti-IL-4 dramatically increased the ability of Ova to induce IgG2c antibodies, and surprisingly anti-Gr1 had a similar, although not quite so dramatic effect (Fig. 6D). We found a similar effect of Gr1 depletion in mice that had been immunized with Ova adsorbed to a commercial form of alum, Alhydrogel™. In addition, we saw a similar effect of Gr1 depletion on antibody isotypes in Balb/c mice (Fig. 6E). While Ova specific IgG2a levels were elevated in mice treated with Gr1 depleting antibody, Ova specific IgG1 and IgE levels in these mice were unaffected by Gr1 depletion (Fig. 6E). In combination with the T cell results (above) these data suggest that the ability of alum to preclude Th1-like responses depends, to some extent, on Gr1+ cells and IL-4 production.
Discussion
It is known that helminth products and alum have overtly similar effects on the immune response. Both stimulate strong Th2 responses and elicit high levels of IgE and IgG1 against associated antigens (10,12,13). Others have therefore suggested that the two substances may promote Th2 responses in a similar way (34). The ability of these two quite different materials to have similar effects on the innate immune system suggests that they are somehow recognized via similar pathways early after exposure. Many adjuvants activate innate immune responses via engaging TLRs, however the effects of alum on antibody responses are independent of MyD88 and TRIF, essential signaling components downstream of TLRs (8,9). Likewise, other studies have indicated that Th2 responses in response to S. mansoni are largely TLR independent (35). Therefore, both alum and Sm eggs appear to act as Th2 driving adjuvants independently of TLRs, and must act via some other set(s) of receptors. Alum and Sm eggs may act via the same receptor or, alternatively, they may be first detected by different receptors that converge on the same Th2-inducing pathway. Other products that induce a similar type 2 inflammation, such as chitin (36) may converge on these same receptors and/or intracellular signaling pathways.
Here we show that Sm eggs and alum activate similar innate immune responses. When injected i.p., both cause the accumulation of Gr1+ IL-4 producing cells in the spleen and peritoneum. Via a process that requires these cells, both substances promote changes in B cells in the spleen to respond with intense Ca2+ fluxes to crosslinking of their MHC II molecules. These shared effects on the innate immune system are apparent within 24 hours of administration, therefore responses to the two products converge quickly and are not due to later downstream effects.
The two materials do not share all properties since alum, but not Sm eggs, acts as an adjuvant, improving T and B cell responses to co-injected antigen. At first sight this is an unexpected result, since others have shown that a Lewisx containing oligosaccharide on Sm eggs, lacto-N-fucopentaose III (LNFPIII), bound to proteins, helps B cell production of Th2-driven antibodies and Sm eggs induce powerful antibody responses against their own antigens (37). We believe that the contradictions are related to whether or not the co-administered antigen is physically associated with the intrinsic adjuvant within Sm eggs. Many proteins bind well to alum, and the reported adjuvant effects of LNFPIII are on responses to covalently linked antigens. In the experiments described here, in which Sm eggs failed to act as good adjuvants, the eggs and antigen were injected at the same site, but not bound to each other. Therefore, we predict that the abilities of Sm eggs or alum to improve immune responses probably depend on their being taken up together with the antigen by the same cell. The adjuvant effects of helminth products described elsewhere, and, by extension, alum, may then be mediated by direct effects of the materials on the properties of the antigen presenting cells. This idea is supported by the fact that alum and extracts from Sm eggs have similar effects on DC, modulating the outcome of TLR engagement in the cells, inducing dendritic cells to promote Th2 responses without elevating certain costimulatory proteins or promoting production of IL-12 (16-18,38,39).
Others have previously shown that IL-4 and IL-13 are not required for alum to prime good Th2 cell responses but do play a role in suppressing Th1-related cytokine production after exposure to antigen plus alum (23,24). Here we confirm those observations and extend them by showing that the Gr1+ cells induced by the adjuvant have parallel effects. Like IL-4, they are not required for alum to improve T cell priming and, also like IL-4, they do suppress the ability of alum to induce Th1-driven responses such as production of antibodies of the IgG2a/c isotype. These results suggest that the Gr1+ cells may be an important source of the IL-4 that suppresses the type 1 responses. Strategies that prevent production of such cells during vaccination with alum might actually improve the efficacy of the vaccine, broadening the classes of antibody produced without reducing the titer.
Previously, others have shown that IL-4−/− mice injected with alum have Th2 responses that are somewhat reduced by comparison with those of wild type mice (23). Here, we found that although depletion of IL-4 on days 1, 3, and 5 of the primary response was sufficient to prevent suppression of Th1 dependent antibodies, it had no effect on the amount of IgG1 produced, a result which appears to contradict the previous observations (23). The difference may lie in the fact that the T cells in the IL-4−/− mice had never been exposed to IL-4, and IL-4 may have some effect on T cells before they respond overtly to antigen, since naïve T cells can detect the cytokine (40,41). Thus prexposure to IL-4 may have some effect on the ability of T cells to differentiate into Th2 cells, whereas acute lack of IL-4, just during response to antigen, may affect only the ability to suppress type 1 responses.
In other systems, similar effects of Gr1+ cells have been observed, for example, Gr1+ cells accumulate in secondary lymphoid organs during sepsis and depletion of these cells resulted in elevated Th1 responses (42). In other studies Gr1+ cells were found to accumulated after exposure to parasites and have a suppressive effect on the proliferation of CD4 cells (43). The effects of Gr1+ cells seen here are unlikely to occur by suppressing proliferation of CD4 cell however, because total numbers of IAb/3K specific CD4 cells were unaffected in 3K-Ova-injected mice, depleted of Gr1+ cells.
In summary, here we show that alum and Sm eggs have similar effects on innate IL-4 producing cells. Via these cells alum, and probably also Sm eggs suppress Th1-related immune responses, leaving the host with only Th2-related immunity. Further understanding of the mechanisms by which this occurs could lead to development of vaccines that induce a less Th2 biased immune response.
Acknowledgements
We thank Drs. Michael Jordan, John Cambier, Raul Torres, Peter Henson and the members of the Kappler Marrack Laboratory for their intellectual contributions to this project.
Abbreviations used in this paper
- TLR
toll like receptor
- Ca2+
calcium
- BSS
balanced salt solution
- Tg
transgenic
- CTM
complete tumor medium
- Sm
Schistosoma mansoni
- LNFPIII
Lacto-N-fucopentaose III
Footnotes
This work was supported by USPHS grants AI-18785, and AI-52225.
The authors have no conflicting financial interests.
References
- 1.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Janeway CA., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–44. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–4. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 7.Kapsenberg ML, Kalinski P. The concept of type 1 and type 2 antigen-presenting cells. Immunol Lett. 1999;69:5–6. doi: 10.1016/s0165-2478(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 8.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 9.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–8. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–45. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 11.Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980;39:435–41. [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce EJ, Sher A. Functional dichotomy in the CD4+ T cell response to Schistosoma mansoni. Exp Parasitol. 1991;73:110–6. doi: 10.1016/0014-4894(91)90014-n. [DOI] [PubMed] [Google Scholar]
- 13.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–7. [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 15.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–8. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 17.Sun H, Pollock KG, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;21:849–55. doi: 10.1016/s0264-410x(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–6. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 19.Pollock KG, Conacher M, Wei XQ, Alexander J, Brewer JM. Interleukin-18 plays a role in both the alum-induced T helper 2 response and the T helper 1 response induced by alum-adsorbed interleukin-12. Immunology. 2003;108:137–43. doi: 10.1046/j.1365-2567.2003.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SL. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588–95. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 21.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr., Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci U S A. 1997;94:10838–43. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 23.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–6. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 24.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 25.Cunningham AF, Serre K, Toellner KM, Khan M, Alexander J, Brombacher F, MacLennan IC. Pinpointing IL-4-independent acquisition and IL-4-influenced maintenance of Th2 activity by CD4 T cells. Eur J Immunol. 2004;34:686–94. doi: 10.1002/eji.200324510. [DOI] [PubMed] [Google Scholar]
- 26.McDonald F, Mohrs M, Brewer J. Using bicistronic IL-4 reporter mice to identify IL-4 expressing cells following immunisation with aluminium adjuvant. Vaccine. 2006;24:5393–9. doi: 10.1016/j.vaccine.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 27.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–10. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 28.Cambier JC, Lehmann KR. Ia-mediated signal transduction leads to proliferation of primed B lymphocytes. J Exp Med. 1989;170:877–86. doi: 10.1084/jem.170.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–82. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 30.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 31.Lang P, Stolpa JC, Freiberg BA, Crawford F, Kappler J, Kupfer A, Cambier JC. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-alpha/beta dimers. Science. 2001;291:1537–40. doi: 10.1126/science.291.5508.1537. [DOI] [PubMed] [Google Scholar]
- 32.Alving CR. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–46. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 33.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–20. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Liu Q, Pesce J, Anthony RM, Lamb E, Whitmire J, Hamed H, Morimoto M, Urban JF, Jr., Gause WC. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol Rev. 2004;201:57–74. doi: 10.1111/j.0105-2896.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 35.Layland LE, Wagner H, da Costa CU. Lack of antigen-specific Th1 response alters granuloma formation and composition in Schistosoma mansoni-infected MyD88−/− mice. Eur J Immunol. 2005;35:3248–57. doi: 10.1002/eji.200526273. [DOI] [PubMed] [Google Scholar]
- 36.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okano M, Satoskar AR, Nishizaki K, Harn DA., Jr. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167:442–50. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- 38.Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, Hunter CA, Pearce EJ. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173:7454–61. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 39.Sokolovska A, Hem SL, HogenEsch H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25:4575–85. doi: 10.1016/j.vaccine.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 40.Brogdon JL, Leitenberg D, Bottomly K. The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naive CD4+ T cells. J Immunol. 2002;168:3825–32. doi: 10.4049/jimmunol.168.8.3825. [DOI] [PubMed] [Google Scholar]
- 41.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–30. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley K,A, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J Immunol. 2001;167:4293–302. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]