SUMMARY
Background
Cardiac rehabilitation (CR) is a proven means of reducing mortality but it is grossly underutilized owing to factors involving both the health system and patients. These issues have not been investigated concurrently; to this end, we employed a hierarchical design to examine physician and patient factors affecting verified CR referral.
Methods
This study was prospective with a multilevel design. We assessed 1,490 coronary artery disease outpatients nested within 97 cardiology practices. Cardiologists completed a survey about CR referral attitudes. Outpatients were surveyed prospectively to assess sociodemographic, clinical, behavioral, psychosocial and health system factors affecting CR referral. Responses were analyzed by mixed logistic regression analyses. After 9 months, CR referral was verified at 40 centers.
Results
Health-care providers referred 550 (43.4%) outpatients to CR. Factors affecting verified referral included positive physician perceptions of CR (P = 0.03), short distance to the closest CR site (P = 0.003), fewer perceived barriers to CR (P <0.001) and a sense of personal control over their condition by the patient (P = 0.001).
Conclusions
Physician-related and patient-related factors both contribute to CR referral. The most relevant physician perceptions of such programs are program quality and perceived benefit. For patients, the most relevant factors are perceived barriers to CR, which might be conveyed during prereferral discussions. Work to improve physicians’ perceptions and patients’ understanding might improve use of rehabilitation services.
Keywords: cardiac rehabilitation, coronary artery disease, physician factors, referral
INTRODUCTION
Secondary preventive measures against cardiovascular disease, such as cardiac rehabilitation (CR), can greatly reduce associated burden on health systems. CR is an evidence-based outpatient program consisting of structured exercise, education, psychosocial support and risk reduction. Among other benefits, evidence indicates that CR can reduce mortality by approximately 25%.1–3
Participation in CR programs is increasing in North America, mainly due to the publication of guidelines and targets4,5. Despite these efforts, however, CR remains grossly underutilized with rates of participation of 15–20% in North America, Europe and Australia. 6–9 CR is under-used even in those clinical situations where referral is indicated in guidelines, and subsequent participation would improve prognosis, and perhaps delay or prevent the use of expensive procedures.3,10 Because underuse of CR represents inferior quality of care, it is essential to examine why all patients do not receive CR when it is indicated.
Moving patients from acute care to CR requires referral by health-care providers. In Canada, the referral process is dependent upon completion of a referral form, and signature by a physician before being sent to a CR program. The CR service contacts the patient directly for enrollment. Only three studies have surveyed physicians to determine what factors affect their CR referral practices.11–13 No data are readily available on the contributions of both physician and patient factors to suboptimum referral to CR. Herein we attempted to investigate this issue.
METHODS
Design and procedure
This prospective study used a multilevel design of outpatients nested within cardiologists’ practices. Ethics approval was obtained from all participating institutions. The sample of cardiologists was generated through a national Canadian medical physician directory (www.mdselect.com). Basic sociodemographic data were extracted. All cardiologists meeting inclusion criteria were mailed an invitation to participate in a study regarding secondary prevention, a consent form and a survey assessing their CR referral attitudes.
All consenting cardiologists were visited by a research assistant to obtain a consecutive sample of approximately 25 recent coronary artery disease (CAD) outpatients eligible for CR per physician. Patients were invited by mail to participate; cardiologists were not aware which patients were invited. Written informed consent was obtained from patients who wished to participate. Basic clinical data and information on cardiovascular risk were recorded from their charts and they were mailed a self-report survey on factors affecting health-care use. Nine months later, participants were mailed a follow-up survey to assess self-reported CR referral. Distances and total journey duration (driving) between participants’ homes and the closest CR sites were computed based on postal codes using Geographical Information Systems software. Forty CR sites, to which participants reported referral, were contacted to verify referral.
Participants
Inclusion criteria for physicians were having a nonpediatric practice located in a major center in the Windsor to Ottawa corridor of Ontario, Canada, to ensure they were actively treating coronary artery disease (CAD) and were located in proximity to a CR service.
Patients with a confirmed diagnosis of CAD were eligible for inclusion. Diagnosis was confirmed by the indication in patients’ charts of a detailed history, focused physical examination, diagnostic electrocardiographic changes (i.e. Q waves, and/or ST–T segment changes), and/or troponin levels above the 99th percentile of normal. Patients who had undergone percutaneous coronary interventions or coronary artery bypass grafting were also eligible.
Measured variables and survey questions
Physician referral to CR was assessed via patient-report in the follow-up survey, and verified with the CR site to which they reported referral (i.e., receipt of referral form from physician: yes/no).
We assessed physician-level and patient-level factors that affected CR referral. Physician level data obtained from the physician directory related to sex, graduation year, location of medical school (Ontario, Canada, international), whether the physician held a university appointment, and subspecialty. The physician survey comprised a question on weekly patient volume and 19 investigator-generated, Likert-style items with a scale of 1 (strongly agree) to 5 (strongly disagree) to rate attitude to CR referral, including perception of barriers (health system and other barriers); all questions had been previously validated.12
We gathered information on patients’ Canadian Cardiovascular Society (CCS) class and cardiac risk factors (diabetes mellitus, BMI, smoking, family history and hypertension) from their charts. If information on these variables was not available, patients were asked to self-report the data.
The initial patient survey addressed factors affecting CR referral. Some questions were generated by investigators, based on studies of CR use,14,15 and had been previously validated by us.16,17 These items address sociodemographic factors (age, sex, ethnocultural background open-ended and forced choice), marital status, work status, level of education and gross annual family income. Questions were also incorporated from several established questionnaires.
The Duke Activity Status Index (DASI)18 is a brief, 12-item, self-administered survey to determine functional capacity, and includes details of personal care, ambulation, household tasks, sexual function and recreational activities. We accompanied these questions with a yes/no response item: “Do you have any other medical conditions that would prevent you from exercising?”
To assess physical activity we used the Physical Activity Scale for the Elderly (PASE),19 which is short and has proven reliability in people aged 65 years and older. PASE addresses occupational, household and leisure activities assessed during a 1-week period. We created an accompanying yes/no response item to assess participants’ past exercise habits: “Did you exercise to the point of getting short of breath on a regular basis (as an adult) prior to your cardiac event?”.
The Exercise Benefits/Barriers Scale (EBBS)20 was used to determine respondent’s health beliefs concerning participation in exercise. The EBBS is a 43-item instrument that uses a four-point Likert scale for each item, with responses ranging from 4 (strongly agree) to 1 (strongly disagree). Mean benefit and barrier scores were computed.
The Beck Depression Inventory-II (BDI-II)21,22 was used to assess depressive symptoms. It is a reliable and well-validated 21-item scale that uses a forced-choice format. Each item has four possible responses. This questionnaire has been widely used in the general population and in populations with long-term illness, including cardiac problems.23 Higher scores reflect greater depressive symptomatology, with scores above 10 reflecting mild to severe symptoms.
The ENRICHD Social Support Inventory (ESSI) is a seven-item measure developed and validated in a cardiac randomized controlled trial.24 It includes items on structural, tangible and emotional features of social support found to be predictive of outcome in cardiac patients.
Finally, we incorporated the Illness Perception Questionnaire (IPQ-R)25 to assess cognitive representations of cardiovascular disease. The following IPQ-R subscales were included: personal control, timeline (acute/chronic), timeline cyclical or episodic, consequences, and treatment cure/controllability. All items are scored on a five-point Likert-type scale, which ranges from ‘strongly disagree’ to ‘strongly agree’. Mean subscale scores were computed with higher scores denoting greater endorsement of the given construct.
To assess the driving distances and times between participants’ homes and CR sites we mapped all buildings by postal code. The list of CR sites was based on the Canadian Association of CR, Canadian CR Foundation, and CR Network of Ontario directories, as well as any additional sites identified by participants in the survey. CR sites were cross-referenced with the postal codes of patients’ homes by geographic information systems.
In the follow-up patient survey investigator-generated items were used to gather information on the number of visits to a cardiac specialist and primary care physician in the 9 months since the baseline survey was completed, and perceived facilitators and barriers to CR use. We included 19 previously validated items, each with a five-point Likert scale ranging from strongly disagree (1) to strongly agree (5)16 The internal consistency was α=0.92, and nearly all of these variables were significantly related to referral. Therefore, a total score was computed.
Statistical analyses
The following analyses were conducted using SPSS 15.0 software.26 A descriptive examination of self-reported and verified CR referral was performed.
Bivariate screening based on CR referral was performed on variables at the physician level and patient level, using χ2 and t-tests, as appropriate. This analysis was performed to enable variable selection for an adjusted model based on theoretical and empirical (i.e. P <0.1) criteria. Significant variables were screened for multicollinearity, and in some cases decisions were made to exclude variables from the model. For instance, distance and travel time to a CR site were highly correlated; given the greater t value for distance, we chose to include this variable over time in the model. Similarly, exercise barriers and benefits were highly correlated, thus we chose benefits for inclusion. With regard to illness perceptions, the timeline cyclical, consequences (trend only) and cure/controllability subscales were highly correlated, and the latter subscales were excluded. Finally, with regard to physician items assessing CR referral, items 16, 17 and 18, which refer to physicians’ evaluative perceptions of CR were highly correlated. A principal components analysis was undertaken of all 19 variables, and these three items loaded highly (<0.8) on one factor, which explained the greatest degree of variance. A variable was computed based on the mean score on these three items.
Overall checks of statistical assumptions revealed the ‘distance to CR’ variable to be highly skewed, and, therefore rank of distance was entered into the models. Finally, mixed logistic regression analysis predicting verified CR referral was conducted using R,27,28 which takes into account the clustering of patients within physicians.
RESULTS
Respondent characteristics
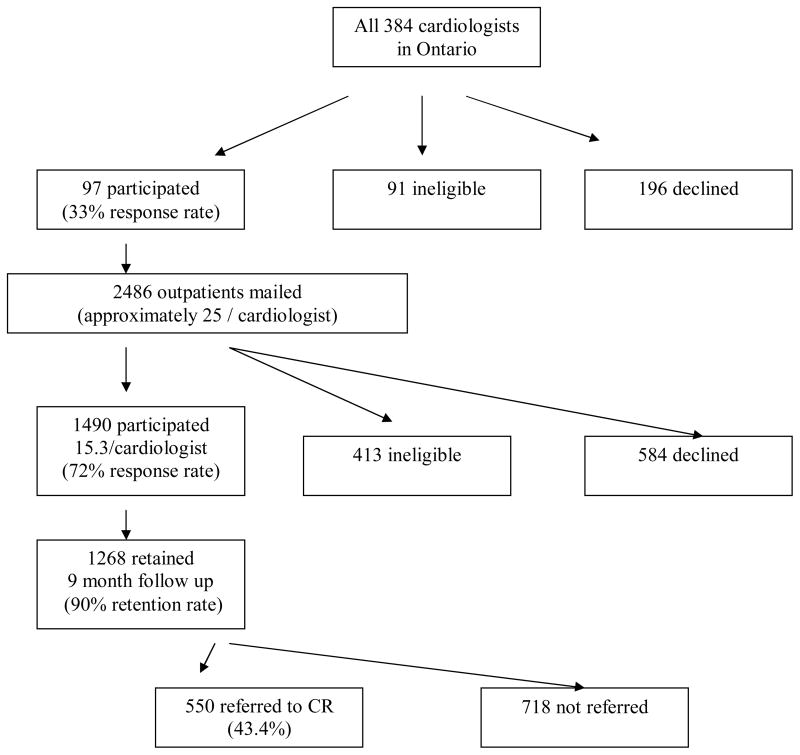
From the 384 cardiologists contacted, 97 consented (33% response rate, 91 were deemed ineligible and 196 declined to participate (Figure 1). Reasons for ineligibility were, not having CAD patients (n = 57, 63%), not being an outpatient practice (n = 12, 13%), incorrect physician address or had stopped practicing for reasons other than retirement (n = 9, 10%), retired from clinical practice (n = 2, 2%) or other reasons, such as the physician was on sabbatical or maternity leave, had left the country, illness, or had an independent practice and was not covered by hospital ethics approval (n = 11, 12%; Figure 1). Table 1 displays the characteristics of participating cardiologists.
Figure 1. Participant Recruitment and Verified CR Referral.
Participant recruitment and outcomes of cardiac rehabilitation referral. Abbreviation: CR, cardiac rehabilitation.
Table 1.
Characteristics of participating cardiologists.
| Characteristic | Participants’ data |
|---|---|
| Female | 14 (14.4%) |
| Mean (SD) graduation year for medical degree | 1982 ± 8.48 |
| Medical school in Ontario | 55 (57.0%) |
| University appointed | 43 (44.0%) |
| Subspecialty internal cardiology | 62 (25.6%) |
| Mean (SD) weekly patient volume | 46.34 ± 33.48 |
Research assistants identified retrospective data of suitable consective CAD patients of whom 2,486 consecutive CAD outpatients were sent invitations to participant. From these, 1,490 consented to participate, 413 were ineligible and 583 declined to participate (72% response rate). Thus, a mean of 15.3 patients were recruited per cardiologist. Reasons for ineligibility included a lack of English language proficiency (n = 145, 35%), inability to locate the patient (n = 86, 21%), no CAD diagnosis (n = 41, 10%), orthopedic, neuromuscular, cognitive or vision impairment (n = 37, 9%), the patient had died (n = 34, 8%), index event or treatment prior to 2004 (n = 18, 4%), ineligibility for CR based on Canadian guidelines (n = 10, 2%),31 previous attendance at CR (n = 5, 1%), nondysphoric psychiatric conditions (n = 3, 1%), and other reasons, such as left the country (n = 34, 9%).
Of the 1,490 consenting participants, 1,268 completed the 9 month follow-up survey but 86 were ineligible (retention rate = 1,268/(1490–86) = 90.3%). Reasons for ineligibility included incorrect contact information/inability to locate the patient (n = 37, 43%), the patient had died (n = 24, 28%), new onset of an orthopedic, neuromuscular, cognitive, psychiatric or vision impairment (n = 6, 7%), and other reasons, such as being too ill to participate or left the province/country (n = 19, 22%; Figure 1). Characteristics of participants and those who refused or were ineligible at follow-up are summarized in Table 2.
Table 2.
Baseline sociodemographic and clinical characteristics of patient sample at 9-month follow-up
| Characteristic | Retained Participants (n = 1268) | Ineligible (n = 86) | Declined (n = 138) |
|---|---|---|---|
| Mean (SD) age (years) | 67.28 ± 11.16 | 66.56 ± 13.60 | 64.46 ± 11.44a |
| Female | 358 (28.2%) | 23 (26.7%) | 43 (31.2%) |
| Mean (SD) BMI (kg/m2)d | 27.53 ± 5.39 | 27.43 ± 5.58 | 27.61 ± 5.69 |
| Marriedd | 910 (72.3%)b | 52 (60.5%) | 81 (60.0%) |
| Ethnocultural minority | 174 (13.7%)c | 21 (24.4%) | 37 (26.8%) |
| Educated to higher than high schoold | 670 (86.1%) | 38 (44.7%) | 70 (52.2%) |
| Family income ≥Can$50,000 per yeard | 560 (48.5%)b | 26 (32.1%) | 43 (37.4%) |
| Employed (full or part time)d | 406 (32.3%) | 27 (31.8%) | 53 (39.6%) |
| Mean (SD) systolic blood pressure (mmHg) | 131.15 ± 19.15 | 136.06 ± 20.82 | 131.35 ± 19.31 |
| Mean (SD) diastolic blood pressure (mmHg) | 74.50 ± 10.21 | 77.51 ± 13.19a | 73.23 ± 10.42 |
| Total cholesterol/HDL ratio | 4.24 ± 1.21 | 4.07 ± 1.09 | 4.15 ±1.18 |
| Mean (SD) HDL level (mmol/l) | 1.22 ± 0.42 | 1.09 ± 0.29 | 1.18 ± 0.30 |
| Mean (SD) LDL level (mmol/l) | 2.33 ± 0.93 | 2.08 ±1.00 | 2.49 ± 0.91 |
| CCS angina class II–IV | 262 (20.7%) | 7 (8.1%) | 26 (18.8%) |
| Multivessel disease | 365 (28.7%) | 24 (27.9%) | 34 (24.6%) |
| Means (SD) Duke Activity Status Index scored | 37.23 ± 15.79c | 29.12 ± 18.64 | 34.28 ± 16.16 |
| Current or previous MI | 929 (73.2%) | 62 (72.1%) | 105 (76.1%) |
| Current or previous PCI | 558 (44.0%) | 37 (43.0%) | 56 (40.6%) |
| Current or previous ACB | 360 (28.4%) | 18 (21.9%) | 31 (22.5%) |
| Current or previous HF | 177 (14.0%) | 18 (20.9%) | 24 (17.4%) |
| Current or previous valve repair/replacement | 194 (15.3%) | 13 (15.1%) | 27 (19.6%) |
Abbreviation: CCS, Canadian Cardiovascular Society; MI, myocardial infarction; PCI, percutaneous coronary intervention; ACB, acute coronary bypass; HF, heart failure.
p< 0.05,
p<.01,
p<.0001
denotes data from patient report
Self-reported and verified cardiac rehabilitation referral
After 9 months 673 (53.1%) participants self-reported referral to CR at one of 40 sites. A further 102 (19.2%) were provided a reason why they were not referred by a health-care provider. Patients were most often referred to CR by their cardiac specialist (n = 461, 69%), followed by their family physician (n = 113, 17%) or an allied health professional (n = 61, 9%). Other types of referral, such as self-referral, were reported by 16 (2%) patients; 22 (3%) participants did not respond, which might have meant they were uncertain as to whom referred them. Geographic data revealed a mean CR travel time of 27.6 (SD 64.6) min from home to the closest site and a mean distance of 23.5 (SD 71.1) km for all patients regardless of CR referral.
Forty CR centers in Ontario were contacted to verify self-reported referral. Verification was received for 657 (98%) patients. Self-reporting was congruent with site-report for 550 (82%) of the 673 patients who self-reported. Owing to the high degree of congruence, we relied on selfreported data where CR referral could not be verified. Overall, 43.4% of patients were referred to CR.
Multilevel factors related to cardiac rehabilitation referral
In bivariate analyses of physician-level attitudes to CR referral, standard departmental referral practices, intention to refer, availability of standard referral forms for local sites, referral convenience (trend) and the composite mean of items 16, 17 and 18, which represent positive perceptions of CR, were the most relevant to physicians making a verified referral (Table 3). In bivariate analyses the most important patient-level factors for a verified referral were younger age, being employed, education above high school level, family income higher than Can$50,000 per year, an absence of comorbid conditions that affect ability to exercise, having good functional status, previous exercise history (trend) and perceiving benefits and rather than barriers from exercise, perceiving few CR barriers and depressive symptoms, a perception of being able to maintain personal control of their heart condition and that the disease is curable or controllable without a cyclical nature and few illness consequences. Distance and travel time to the CR site were also significant (Table 4).
Table 3.
Physician-level factors associated with verified referral to a CR program.
| Factors | CR Referral |
t or χ2 value | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Sociodemographic | ||||
| Female | 86 (15.6%) | 102 (14.2%) | 0.50 | 0.52 |
| Mean (SD) graduation year for medical degree | 1983 ± 8.52 | 1982 ± 8.48 | 0.99 | 0.32 |
| Medical school in Ontario | 332 (60.4%) | 406 (56.5%) | 1.86 | 0.17 |
| University appointed | 251 (49.3) | 320 (49.3) | 0.92 | 0.34 |
| Subspecialty internal medicine |
349 (63.5) | 460 (64.1) | 0.05 | 0.82 |
| Mean (SD) self-reported weekly patient volume | 46.53 ± 32.93 | 46.68 ± 33.82 | −0.08 | 0.94 |
| Physician attitude (mean, SD) | ||||
| Clinical practice guidelines promote referral to CR | 1.92 ± 0.91 | 1.90 ± 0.80 | 0.29 | 0.77 |
| Colleagues generally refer patients to CR | 2.25 ± 0.96 | 2.24 ± 0.81 | 0.23 | 0.81 |
| Department/practice generally refers all eligible patients to CR as a standard of care | 2.25 ± 1.01 | 2.36 ± 0.94 | −2.02 | 0.04 |
| Reimbursement policies are a financial disincentive to CR referral | 2.93 ± 1.24 | 2.89 ± 1.16 | 0.59 | 0.55 |
| Follow-up care, including referral, is handled by another health-care professional | 3.46 ± 1.08 | 3.44 ± 1.06 | 0.33 | 0.74 |
| Intend to refer patients to CR | 1.61 ± 0.71 | 1.79 ± 0.76 | −4.43 | <0.001 |
| Not familiar with the CR programs in my area | 4.58 ± 0.70 | 4.55 ± 0.75 | 0.47 | 0.64 |
| Not familiar with CR sites outside geographic area | 3.19 ± 1.36 | 3.13 ± 1.31 | 0.87 | 0.39 |
| No standard referral form for CR, making it more effort to refer to sites closest to patients’ homes | 3.24 ± 1.48 | 3.07 ± 1.41 | 2.07 | 0.04 |
| Allied health professional fills out referral forms on physician’s behalf | 3.79 ± 1.12 | 3.72 ± 1.13 | 1.52 | 0.25 |
| Inconvenient to make a referral to CR | 3.77 ± 1.05 | 3.67 ± 1.05 | 1.89 | 0.06 |
| Prefer to manage patients’ secondary prevention | 3.29 ± 1.13 | 3.21 ± 1.15 | 1.37 | 0.17 |
| Patient education materials in office are sufficient for promoting behavioral change | 3.90 ± 0.97 | 3.81 ± 1.03 | 1.22 | 0.22 |
| Can prescribe an exercise regimen for my patients without referral | 3.77 ± 1.13 | 3.75 ± 1.06 | 0.30 | 0.76 |
| Female cardiac patients generally don’t like to exercise | 3.97 ± 1.02 | 3.91± 0.97 | 1.00 | 0.32 |
| Skeptical about the benefits of CR | 4.61 ± 0.55 | 4.53 ± 0.54 | 2.23 | 0.02 |
| Available CR program is of poor quality | 4.55 ± 0.68 | 4.38 ± 0.76 | 4.21 | <0.001 |
| Had a bad experience with a CR program | 4.64 ± 0.61 | 4.50 ± 0.70 | 3.87 | <0.001 |
| CR program does not provide patient discharge summaries | 4.56 ± 0.71 | 4.51 ± 0.73 | 1.14 | 0.26 |
Table 4.
Patient-level and health system factors associated with verified referral to a CR program.,
| CR Referral | t or χ2 value | P value | ||
|---|---|---|---|---|
| Yes 550 (43.4%) | No 718 (56.6%) | |||
| Sociodemographic | ||||
| Mean (SD) age (years)a | 65.62 ± 10.45 | 68.55 ± 11.52 | −4.67 | <0.001 |
| Femalea | 144 (26.2%) | 214 (29.8%) | 2.02 | 0.17 |
| Employed (full time or part time) | 195 (35.8%) | 211 (29.6%) | 5.41 | 0.02 |
| Educated to higher than high school level | 323 (59.8%) | 347 (49.0%) | 14.38 | <0.001 |
| Family income ≥Can$50,000 per year | 277 (55.1%) | 283 (43.5%) | 15.28 | <0.001 |
| Nonwhite ethnocultural background |
78 (14.2%) | 96 (13.4%) | 0.17 | 0.68 |
| Married | 402 (73.8%) | 508 (71.1%) | 1.05 | 0.31 |
| Living with family | 424 (78.2%) | 543 (76.1%) | 0.83 | 0.38 |
| Clinical | ||||
| Has other medical conditions that prevent exercise | 155 (28.7%) | 246 (37.0%) | 9.35 | 0.003 |
| Mean (SD) BMI (kg/m2) | 27.52 ± 5.21 | 27.54 ± 5.52 | −0.08 | 0.94 |
| Present smoker | 44 (8.0%) | 58 (8.1%) | 0.003 | 0.95 |
| Mean (SD) CCS Class† | 2.38 ± 0.96 | 2.26 ± 1.00 | 1.03 | 0.31 |
| Diabetesa | 129 (23.5%) | 188 (26.3%) | 1.26 | 0.26 |
| Family history of CVDa | 352 (64.1%) | 432 (60.9%) | 1.33 | 0.25 |
| Hypertension a | 333 (60.7%) | 440 (61.5%) | 0.10 | 0.75 |
| Mean (SD) functional status score (DASI) | 39.87 ± 14.46 | 35.21 ± 16.47 | 2.26 | <0.001 |
| Psychosocial and behavioral | ||||
| History of exercise history | 168 (31.2%) | 177 (26.8%) | 2.81 | 0.09 |
| Mean (SD) perceived exercise benefits score (EBBS) | 2.98 ± 0.32 | 2.87 ± 0.38 | 5.57 | <0.001 |
| Mean (SD) perceived exercise barriers score (EBBS) | 2.02 ± 0.38 | 2.13 ± 0.42 | −4.49 | <0.001 |
| Measn (SD) exercise behavior score (PASE) | 132.85 ± 83.18 | 126.55 ± 91.63 | 1.22 | 0.22 |
| Mean (SD) total CR barriers scoreb | 2.07 ± 0.92 | 2.89 ± 0.92 | 0.29 | <0.001 |
| Mean (SD) depressive symptoms score (BDI-II) | 8.90 ± 7.70 | 10.17 ± 8.45 | −2.74 | 0.006 |
| Social support (ESSI), mean ± SD | 28.41 ± 5.92 | 28.28 ± 6.05 | 0.039 | 0.70 |
| Illness perceptions scores (IPQ-R; mean, SD) | ||||
| Personal control | 24.07 ± 3.39 | 22.62 ± 4.01 | 6.81 | <0.001 |
| Illness timeline | 22.35 ± 5.02 | 22.34 ± 4.90 | 0.04 | 0.97 |
| Timeline cyclical | 13.88 ± 3.39 | 13.38 ± 3.45 | 2.55 | 0.01 |
| Consequences | 19.44 ± 4.61 | 20.36 ± 4.89 | −1.76 | 0.08 |
| Curability or controllability of disease | 18.75 ± 2.77 | 17.95 ± 3.06 | 4.78 | <0.001 |
| Health system factors scores (mean, SD) | ||||
| Distance to closest CR site (km) | 17.82 ± 25.26 | 27.98 ± 91.64 | −2.52 | 0.01 |
| Travel time to closest CR (min) | 22.55 ± 24.08 | 31.46 ± 83.06 | −2.44 | 0.02 |
| Number of visits to cardiologist requiredb | 1.63 ± 1.74 | 1.51 ± 1.87 | 1.10 | 0.27 |
| Number of visits to family physician requiredb | 4.77 ± 4.13 | 4.84 ± 4.41 | −2.26 | 0.80 |
Denotes data from physician chart report.
Denotes patient self report data measured at follow-up assessment.
Abbreviation: BDI, Beck Depression Inventory; CCS, Canadian Cardiovascular Society; CVD, Cardiovascular Disease; DASI, Duke Activity Status Index; EBBS, Exercise Benefits and Barriers Survey; ESSI, Enriched Social Support Inventory; FT, Full-time; GIS, Geographic Information Systems; PASE, Physical Activity Scale for the Elderly; PT, Part-time.
The mixed logistic regression analysis revealed that positive physician perceptions of CR,, shorter distance to CR and fewer perceived patient CR barriers and greater perceived illness control being associated with making a verified referral, with a trend for cardiologist intention to make referrals (Table 5).
Table 5.
Mixed Logistic Regression Analysis Predicting CR-Verified Referral
| Predictors | Estimate (SE) | P value |
|---|---|---|
| Physicians | ||
| Department/practice generally refers all eligible patients to CR as a standard of care | −0.09 ± 0.10 | 0.36 |
| Intend to refer patients to CR | −0.22 ± 0.12 | 0.06 |
| No standard referral form for CR, making it more effort to refer to sites closest to patients’ homes | 0.06 ± 0.07 | 0.40 |
| Inconvenient to make a referral to CR | 0.07 ± 0.10 | 0.49 |
| Positive physician perception of CR | 0.36 ± 0.16 | 0.03 |
| Patients | ||
| Age | −0.01 ± 0.01 | 0.32 |
| Education to higher than high school level | −0.15 ± 0.18 | 0.40 |
| Family income ≥Can$50,000 | 0.03 ± 0.19 | 0.85 |
| Employed (full time or part time) | 0.21 ± 0.21 | 0.31 |
| History of exercise | −0.18 ± 0.19 | 0.32 |
| Other medical conditions that prevent exercise | 0.00 ± 0.20 | 0.99 |
| Depressive symptoms on BDI-II | −0.01 ± 0.01 | 0.56 |
| Perceive exercise benefits | 0.00 ± 0.29 | 0.99 |
| Distance to closest CR site | −0.00 ± 0.00 | 0.003 |
| Total CR barriers | −0.91 ± 0.10 | <0.001 |
| Illness perceptions (personal control; IPQ-R) | 0.08 ± 0.02 | 0.001 |
| Illness perceptions (timeline cyclical; IPQ-R) | −0.02 ± 0.03 | 0.53 |
| Activity status (DASI) | 0.00 ± 0.01 | 0.62 |
DISCUSSION
Few multilevel studies have been done to assess variations in medical practice and health service use, and even fewer studies examining rehabilitation and specifically CR. We know of one study that has examined CR referral based on patient-level variables and one physician-level variable (sex).29 That study was, however, severely limited because of the small sample located in one site and nonhierarchical analyses. Our study concurrently examines a comprehensive list of multilevel factors affecting CR referral in a broad sample of CAD outpatients and their cardiologists. Although the overall results confirm those in the literature,14,15,30,31 our findings are specific to referral and demonstrate interaction of health system, physician and patient issues in CR referral discussions.
In our model, health system factors at the physician level, and psychosocial and behavioral factors at the patient level were central to achieving verified CR referral. With regard to the former, physician perceptions of CR based on quality, perceived benefit and past experience played key roles. This suggests that means to improve physician perceptions of CR are imperative. Previous data indicated that educating health-care providers regarding the nature and benefits of CR increases referral rates.32 Therefore, increasing awareness among physicians regarding the benefits of CR needs to be pursued.
Important patient-level psychosocial factors were shown to be related to CR referral, specifically the perception of personal control of illness. Adaptive illness perceptions have been identified as important in patients participating in CR14,30, 33 This sense of personal control is likely conveyed to physicians during discussions of CR recovery, ultimately leading to CR referral. Research has shown that when physicians perceive patients as motivated, they are more likely to refer to CR.12 Patients with greater personal control might also be more aware of CR and initiate CR discussions with providers, resulting in a greater referral rate. Future observational research investigating CR referral discussions between patients and providers could shed light on this issue.
The relation between patients’ perception of CR barriers and referral was a novel finding. This finding suggests that patients will identify barriers to CR participation during CR referral discussion with providers, and this information might affect physician referral practices. The most important barriers in our study were transportation issues, time constraints due to family or work responsibilities, available exercise equipment in one’s home, comorbidities and perceiving exercise as tiring or painful. Health-care providers should work with patients to identify and address barriers and facilitators before CR referral. For instance, arranging satisfactory means of transportation, identifying CR programs with classes that fit in with the patients’ daily routine, and discussion of the individualized nature of exercise prescriptions based on a patient’s abilities, comorbidities and preferences might increase CR use.
Few studies have assessed CR utilization based on driving time and distance to the CR center by objective means, such as GIS.10,34 Our results show that the distance the patient has to travel to CR affects physician’s referral practice. Unfortunately, decisions about CR center locations have generally not been made on the basis of regional need, but have emerged through local CR champions, such as physicians. A maldistribution in CR services has, therefore arisen.10 Moreover, patients who reside in rural areas invariably face geographic barriers to health-care facilities, such as CR.35 Even in our sample, where all physicians’ practices were located in a major city region with an extensive CR program, physicians seem to take geography into consideration when making referral decisions. Referral to home-based CR for patients living farther than 30 km or with a greater than 30 min travel time to a CR center10 should be more widely advocated to ensure access to CR services. This mode of CR service delivery has been shown to be efficacious, safe and cost-effective.36,37 Whether cardiologists are aware that home-based CR services exist warrants future study.
Patient sociodemographic and clinical factors were not a major part of CR referral. The fact that characteristics such as sex and ethnocultural background were unrelated to CR referral patterns suggests that inequalities are being overcome. On the contrary, while all eligible patients should be referred for CR, the fact that clinical factors, such as disease severity and risk factor status, were also unrelated to CR utilization is somewhat disheartening. Use of risk factor burden and disease severity information could ensure CR access to cardiac patients who need it most, although all patients in our sample had verified indications for CR, and should have been referred.
Comprehensive reviews of patient-level factors affecting CR utilization have been published,14,15,30,31 however, this study shows that broader physician and health system issues affecting CR must be taken into account. Moreover, while there have been calls to develop means to overcome underutilization of CR services, few interventions have been developed, tested or implemented, particularly at the physician level.38,39 Our group has demonstrated the potential of automatic referral to overcome the physician referral gap by doubling rates of CR utilization.16,17 Automatic referral generally results in approximately 50% patient enrolment, but further means to optimize CR utilization must be explored.
Caution is warranted when interpreting these results, most notably due to the physician response rate. The literature regarding physician response does quell concern over threats to generalizability. In a review of 24 studies, it was demonstrated that nonresponse bias might be less of a concern in physician samples than in other survey samples.40 Other limitations include retention bias in the patient sample. For instance, retained participants were more likely to be older, married, white, have higher income and higher activity status than nonparticipants. Furthermore, although the study was described to cardiologists as broadly examining secondary prevention, and although cardiologists had already seen patients at the time of baseline assessments to minimize threats to study validity, the survey items might have influenced cardiologists’ CR referral practices by the 9 month follow-up. The survey was also lengthy, requiring approximately 45 min for completion, and, therefore, respondent burden could have influenced results. Finally, results may not be generalizable to other health-care systems, particularly those with different referral methods or where CR services are not covered through health insurance.
In conclusion, this study has concurrently examined physician and patient factors affecting CR referral. Attitudes of both patients and physicians towards CR, particularly in relation to patient perceived barriers and control over illness, are important. A combination of health system, patient and physician factors probably affect CR referral decisions. Efforts to improve physician perceptions of CR, referral to home-based CR where geographic barriers are evident and finding solutions to overcome CR barriers with patients could optimize CR referral practice.
KEY POINTS.
Cardiac rehabilitation (CR) is shown to improve patient outcomes, yet many physicians do not refer their patients when indicated
Through multilevel analysis, this study shows that both physician and patient factors have roles in CR referral
The most relevant perceptions of physicians relate to CR programs’ quality and benefit, and for patients relate to CR barriers
Patients might try to convey concerns during pre-referral CR discussions, and physicians should be trained to identify and address them
Distance from the patient’s home to the CR site was related to physician referral practice, despite the availability of home-based services
Particular attention on improving physician perceptions of CR, referral to home-based CR where geographic barriers are evident and finding solutions to overcome CR barriers with patients could improve CR referral practice
Acknowledgments
This research was funded by the Canadian Institutes of Health Research (CIHR) grant # MOP-74431. SL Grace is supported by CIHR award # MSH-80489, and S Gravely-Witte is supported by the Ontario Women’s Health Council/CIHR Institute of Gender and Health. J Brual is supported by the Heart and Stroke Foundation of Ontario. We thank Ms Sheena Kayaniyil for assistance with data entry, and Dr Georges Monette for assistance with hierarchical modeling.
References
- 1.Jolliffe JA, et al. Exercise-based rehabilitation for coronary heart disease (Cochrane review) Cochrane Database Syst Rev. 2001;1 doi: 10.1002/14651858.CD001800. Art. No: CD001800. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RS, et al. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Brown A, et al. Canadian Coordinating Office for Health Technology Assessment: Report no. 34. 2003. Exercise-based cardiac rehabilitation programs for coronary artery disease: A systematic clinical and economic review. [Google Scholar]
- 4.Canadian Association of Cardiac Rehabilitation. CACRC 2004. Winnipeg; Manitoba: 2004. Canadian guidelines for cardiac rehabilitation and cardiovascular disease prevention: Enhancing the science, refining the art. [Google Scholar]
- 5.Thomas RJ, et al. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services endorsed by the American College of Chest Physicians, American College of Sports Medicine, American Physical Therapy Association, Canadian Association of Cardiac Rehabilitation, European Association for Cardiovascular Prevention and Rehabilitation, Inter-American Heart Foundation, National Association of Clinical Nurse Specialists, Preventive Cardiovascular Nurses Association, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2007;14:1400–1433. doi: 10.1016/j.jacc.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Bittner V, et al. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol. 1999;83:252–255. doi: 10.1016/s0002-9149(98)00830-3. [DOI] [PubMed] [Google Scholar]
- 7.Wyer S, et al. Predicting attendance at cardiac rehabilitation: A review and recommendations. Coronary Health Care. 2001;5:171–177. [Google Scholar]
- 8.Grace SL, et al. Cardiac rehabilitation II: Referral and participation. Gen Hosp Psychiatry. 2002;24:127–134. doi: 10.1016/s0163-8343(02)00179-2. [DOI] [PubMed] [Google Scholar]
- 9.Bunker SJ, Goble AJ. Cardiac rehabilitation: Under-referral and underutilisation. Med J Aust. 2002;179:332–333. doi: 10.5694/j.1326-5377.2003.tb05583.x. [DOI] [PubMed] [Google Scholar]
- 10.Cardiac Care Network. Cardiac Care Network. Toronto, Ontario: 2002. The ontario cardiac rehabilitation pilot project: Report and recommendations. [Google Scholar]
- 11.Suter P, et al. Views of arkansas physicians on cardiac rehabilitation. J Cardiopulm Rehabil. 1992;12:32–35. [Google Scholar]
- 12.Grace SL, et al. Physician management preferences for cardiac patients: Factors affecting referral to cardiac rehabilitation. Can J Cardiol. 2004;20:1101–1107. [PubMed] [Google Scholar]
- 13.Scott LB, Allen JK. Providers’ perceptions of factors affecting women’s referral to outpatient cardiac rehabilitation programs: an exploratory study. J Cardiopulm Rehabil. 2004;24:387–91. doi: 10.1097/00008483-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Jackson L, et al. Getting the most out of cardiac rehabilitation: A review of referral and adherence predictors. Heart. 2005;91:10–14. doi: 10.1136/hrt.2004.045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott LA, et al. Why are women missing from outpatient cardiac rehabilitation programs? A review of multilevel factors affecting referral, enrollment, and completion. J Womens Health. 2002;11:773–791. doi: 10.1089/15409990260430927. [DOI] [PubMed] [Google Scholar]
- 16.Grace SL, et al. Automatic referral to cardiac rehabilitation. Med Care. 2004;42:661–669. doi: 10.1097/01.mlr.0000129901.05299.aa. [DOI] [PubMed] [Google Scholar]
- 17.Grace SL, et al. A prospective comparison of cardiac rehabilitation enrolment following automatic versus usual referral. JRM. 2007;39:239–245. doi: 10.2340/16501977-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hlatky MA, et al. A brief self-administered questionnaire to determine functional capacity (the duke activity status index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 19.Washburn RA, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 20.Sechrist KR, et al. Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health. 1987;10:357–365. doi: 10.1002/nur.4770100603. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, et al. Beck Depression Inventory-II Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 23.Frasure-Smith N, et al. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 24.Mitchell PH, et al. A short social support measure for patients recovering from myocardial infarction: The ENRICHD social support inventory. J Cardiopulm Rehabil. 2003;23:398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Moss-Morris R, et al. The revised illness perception questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. [Google Scholar]
- 26.SPSS for windows 13.0. SPSS Inc; Chicago, Il, USA: [Google Scholar]
- 27.R: A language and environment for statistical computing. R Development Core Team; 2006. http://www.R-project.org. [Google Scholar]
- 28.Pinheiro J, et al. NLME software 3.3.1. 2006. Nlme: Linear and nonlinear mixed effects models. [Google Scholar]
- 29.Stiller JJ, Holt MM. Factors influencing referral of cardiac patients for cardiac rehabilitation. Rehabil Nurs. 2004;29:18–23. doi: 10.1002/j.2048-7940.2004.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 30.Cooper AF, et al. Factors associated with cardiac rehabilitation attendance: A systematic review of the literature. Clin Rehabil. 2002;16:541–552. doi: 10.1191/0269215502cr524oa. [DOI] [PubMed] [Google Scholar]
- 31.Daly J, et al. Barriers to participation in and adherence to cardiac rehabilitation programs: A critical literature review. Prog Cardiovasc Nurs. 2002;17:8–17. doi: 10.1111/j.0889-7204.2002.00614.x. [DOI] [PubMed] [Google Scholar]
- 32.Caulin-Glaser T, Schmeizl R. Impact of educational initiatives on gender referrals to cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation. 2000;20:302. doi: 10.1097/00008483-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Petrie KJ, et al. Role of patients’ view of their illness in predicting return to work and functioning after myocardial infarction: Longitudinal study. BMJ. 1996;312:1191–1194. doi: 10.1136/bmj.312.7040.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melville MR, et al. Cardiac rehabilitation: Socially deprived patients are less likely to attend but patients ineligible for thrombolysis are less likely to be invited. Heart. 1999;82:373–377. doi: 10.1136/hrt.82.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson JE, et al. Rural residents’ use of cardiac rehabilitation programs. Public Health Nurs. 1998;15:288–296. doi: 10.1111/j.1525-1446.1998.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 36.Taylor RS, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: A cost effectiveness analysis. Int J Cardiol. 2007;119:196–201. doi: 10.1016/j.ijcard.2006.07.218. [DOI] [PubMed] [Google Scholar]
- 37.Jolly K, et al. Home-based cardiac rehabilitation compared with centre-based rehabilitation and usual care: A systematic review and meta-analysis. Int J Cardiol. 2006;111:343–351. doi: 10.1016/j.ijcard.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Beswick AD, et al. Improving uptake and adherence in cardiac rehabilitation: Literature review. J Adv Nurs. 2005;49:538–555. doi: 10.1111/j.1365-2648.2004.03327.x. [DOI] [PubMed] [Google Scholar]
- 39.LaBresh KA, et al. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164:203–209. doi: 10.1001/archinte.164.2.203. [DOI] [PubMed] [Google Scholar]
- 40.Kellerman SE, Herold J. Physician response to surveys. A review of the literature. Am J Prev Med. 2001;20:61–67. doi: 10.1016/s0749-3797(00)00258-0. [DOI] [PubMed] [Google Scholar]