Abstract
Objective
To re-examine the relation of blood glucose monitoring to glycemic control among adolescents with type 1 diabetes and to evaluate the relation of demographic, behavioral, and psychosocial characteristics of adolescents who monitor more and less frequently.
Research Design and Methods
Participants were 132 adolescents with type 1 diabetes (average age = 12 years) and their parents, recruited from Children's Hospital of Pittsburgh. Adolescents were interviewed annually for five consecutive years after routine clinic appointments. At each assessment, data from blood glucose meters were downloaded and glycosylated hemoglobin (HbA1c) was recorded from medical records.
Results
More frequent blood glucose monitoring was related to better glycemic control. Adolescents who monitored more frequently were younger, from higher social status families, on insulin pumps, and had higher self-efficacy. Age-related declines in blood glucose monitoring occurred among adolescents with low self-esteem, high stressful life events, and lower parental support.
Conclusions
Given the importance of blood glucose monitoring for good glycemic control, future research should enhance adolescents' self-efficacy for monitoring and intervene with those who are at risk for age-related declines in blood glucose monitoring.
Keywords: type 1 diabetes, blood glucose monitoring, adolescents
Introduction
Of all the self-care behaviors involved in managing type 1 diabetes, testing blood glucose is clearly one of the most important. In fact, some early intervention studies focused on increasing blood glucose monitoring among adolescents and adults with type 1 diabetes as a way to improve glycemic control—however, results were mixed (1, 2). In the area of adolescents with type 1 diabetes, some, but not all, studies have established a link between blood glucose monitoring and glycemic control. In a study of 300 adolescents (ages 7-16), more frequent blood glucose monitoring as determined by clinician notes in patients' charts was associated with lower HbA1c (3). Another study of adolescents confirmed these results (4) but with a combination of data from meters and logbooks (5). A study of children and adolescents attending a diabetes camp found that frequency of monitoring (determined by parent records based on children's meters) for the two weeks prior to camp was related to lower HbA1c (6).
However, two studies of children with type 1 diabetes did not find a link between glucose monitoring and HbA1c. In one of those studies (children and adolescents ages 2-18), it can be inferred, but was not specifically stated, that monitoring was based on self-report (7). In the other study, it is clear that monitoring was determined by patient diaries (8). The failure to find a relation when data are determined exclusively by self-report is not surprising as patients could easily manufacture or distort the number of times that they monitor blood glucose. This is especially a concern when the patient is a child or adolescent. In the latter study (8), the investigators examined fingertip punctures in a randomly selected group of 86 patients and found that those who had more fingertip punctures had lower HbA1cs, validating their concern that patient diaries were sometimes completed at the clinic appointment.
There are several reasons that frequency of testing is thought to affect glycemic control. First, one assumes that if the results of blood glucose tests are too low or too high, the individual will adjust his or her behavior (i.e., insulin administration, diet) accordingly. To the extent these adjustments are successful, blood glucose values are likely to be in the normal range more often. However, it also is possible that more frequent blood glucose testing is a general sign of good self-care behavior and is correlated with a host of other good health behaviors, such as exercising and eating healthfully, which then improve glycemic control (7). For example, lower rates of blood glucose monitoring have been associated with smoking (9).
There are several reasons why frequency of blood glucose monitoring per se may not be related to glycemic control. First, studies that rely on self-report may not find relations because self-report data are subject to demand characteristics. Patients are likely to overreport the number of times that they test (10). Second, checking blood glucose does not necessarily imply that the data will be used to make insulin or eating adjustments (7), in part because patients are not always certain as to what they should do in response to results of blood glucose tests.
To the extent that there is a link between blood glucose monitoring and glycemic control, there is little data on the characteristics of those who test more rather than less frequently. Among children and adolescents with type 1 diabetes, it appears that older age is associated with less frequent testing (6, 8, 11). Having diabetes for a longer period of time also has been associated with less frequent testing (4), although this relation was not examined independent of age. Other demographic variables that have been linked to more frequent blood glucose testing include being female in a study of adults (9), having married biological parents compared to single, separated or divorced parents in a study of children and adolescents (7), and being white, having higher education and higher income in a study of adults (9).
There are several limitations of the previous research. First, the majority of studies have focused on adults rather than adolescents. Second, most studies examine the association of blood glucose monitoring to glycemic control at one point in time or over a relatively short timeframe rather than track the association over a longer period of time. Third, studies have not compared the predictive value of self-report measures compared to more objective measures of monitoring or determined whether monitoring is superior to more global measures of self-care in terms of predicting glycemic control. Finally, few studies have examined the characteristics of those who monitor blood glucose more or less frequently.
The first goal of the study was to determine whether blood glucose monitoring, as determined by data downloaded from blood glucose meters, was related to glycemic control. We hypothesized that more frequent monitoring would be related to better glycemic control over time. We examined this relation in a 5-year observational longitudinal study of adolescents with type 1 diabetes, who were interviewed on an annual basis. Thus, we had the opportunity to examine this relation across five occasions and to determine whether the relation changed with age. We used longitudinal growth curve modeling to examine this relation, a significant improvement over ordinary least squares regression (12). This procedure allows one to examine individual variability in rates of change. The rate of change is calculated for each individual and then aggregated across individuals. Because we administered an overall measure of self-care behavior, we were able to compare whether blood glucose monitoring as indicated by data from blood glucose meters was a more important predictor of glycemic control compared to a global index of self-care behavior. The second goal of the study was to examine the demographic and psychosocial correlates of blood glucose testing.
Research Design and Methods
Subjects
Letters of invitation were sent to all adolescents with diabetes who were approximately 11-13 years of age and attending the Children's Hospital of Pittsburgh Diabetes Center (n = 307). Families could return a postcard indicating that they did not want to be contacted by phone about the study. Twenty families returned these postcards, refusing contact about the study without us being able to determine eligibility. We were able to reach 261 of the remaining 287 families by phone and determined that 90 were not eligible. Adolescents were eligible to participate in the study if they were in the 5th, 6th, or 7th grade; had been diagnosed with insulin-treated diabetes for more than one year; and did not have another major chronic illness (e.g., cancer, rheumatoid arthritis). Of the 171 eligible families, 39 refused and 132 agreed. Thus, our effective response rate was 77%.
Protocol
The study was approved by the appropriate Institutional Review Boards. We interviewed adolescents with diabetes immediately before or after their clinic visit in the General Clinical Research Center, which is separate from and not associated with the diabetes clinic. Parent consent and child assent were obtained at the time of the initial interview.
One year later (Time 2 [T2]), we interviewed 127 of the 132 (96%) children. Two years later (Time 3 [T3]), we interviewed 126 (95%) of the children; three years later (Time 4 [T4]), we interviewed 127 (96%) of the children; and four years later (Time 5 [T5]), we interviewed 126 (95%) of the children with diabetes. The majority of parents completed questionnaires at T2 (94%), T3 (90%), T4 (89%), and T5 (92%). The parent was the mother in 92% of cases.
Measures
Self-care behavior
We administered a modification of the widely used 14-item Self-Care Inventory (13) as described in Helgeson et al. (14) to both adolescents and parents. This instrument asks respondents to indicate how well they followed their physician's recommendations for glucose testing, insulin administration, diet, exercise, and other diabetes-related behaviors. Further evidence for the reliability and validity of this instrument has been recently reported, including a high internal consistency, high test-retest reliability, relations to interview-based measures of adherence, and relations to hemoglobin A1c (15). One item on this inventory is especially relevant to blood glucose testing. It asks how often adolescents test their blood glucose. In this study, we examine the total scale as well as this single item.
Monitoring
We downloaded data from adolescents' blood glucose meters which they brought to the clinic. In 16% of the cases, we relied on patient logbooks to document frequency of monitoring either because we had difficulty with the software for downloading the data (78%) or because adolescents forgot to bring their meters to the clinic (22%). We note that the findings reported below were identical when we excluded monitoring based on logbooks. On average, meters (and logbooks) contained about 2 months of data (mean number of days ranged from 59 to 67 across the five waves; SD's ranged from 23 to 50). We calculated the average number of meter readings taken per day. The average number per day over the course of the study was about 4 (means ranged from 3.71 to 3.88; SD's ranged from 1.38 to 1.57).
Glycemic control
Glycemic control was measured with hemoglobin A1C (HbA1c) obtained at the clinic appointment measured by HPLC (Tosoh Instruments) with normal range of 4.6-6.1%.
Psychosocial variables
We used well-established measures of psychosocial variables, all of which have well-documented reliability and validity as provided in the references below. We measured self-efficacy in children with two items from the Multidimensional Diabetes Questionnaire (16)—one that reflected blood glucose testing self-efficacy and one that reflected ability to control blood glucose. Children also completed measures of depressive symptoms (17); self-esteem (18), stressful life events (19); parent relationship quality (20); and diabetes-specific support (21, 22).
Data Analysis
First, we used multi-level modeling or longitudinal growth curve modeling (SPSS) to examine the relation of blood glucose monitoring to glycemic control (12). This procedure allows us to examine the concurrent association between the two variables at all 5 waves of assessment by taking advantage of all available data, including data from participants who did not complete all assessments. Then, we examined whether self-reports of self-care predicted glycemic control and whether monitoring and self-reports of self-care independently predicted glycemic control. Second, we used multi-level modeling to examine individual predictors of monitoring. We examined demographic variables, self-reports of self-care, and psychosocial variables in separate analyses. To ensure that psychosocial predictors of monitoring were independent of demographic correlates, we statistically controlled for age, body mass index, social status (23), and insulin delivery method in these analyses. We also examined interactions of psychosocial variables with age to determine if they were more potent predictors of monitoring for younger or older adolescents.
Results
Patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics at Baseline (n = 132)
| Sex | 53% female | ||
| Age (years) | M = 12.10 | SD = .77 | range = 10.73 – 14.21 |
| Diabetes duration (years) | M = 4.91 | SD = 2.09 | range = 1 – 13 |
| Insulin delivery method | 26% pump | ||
| 72% multiple daily injection | |||
| 2% two injections per day | |||
| Race/ethnicity | 93% Caucasian | ||
| 2% African American | |||
| 1% Asian | |||
| 1% American Indian | |||
| 3% mixed race | |||
| Hollingshead social status | M = 41.97* | SD = 11.05 | range = 17 – 66 |
| HbA1c% | M = 8.04 | SD = 1.31 | range = 6 – 13 |
This average reflects lower end of technical workers, medium business, minor professionals.
Monitoring and Glycemic Control
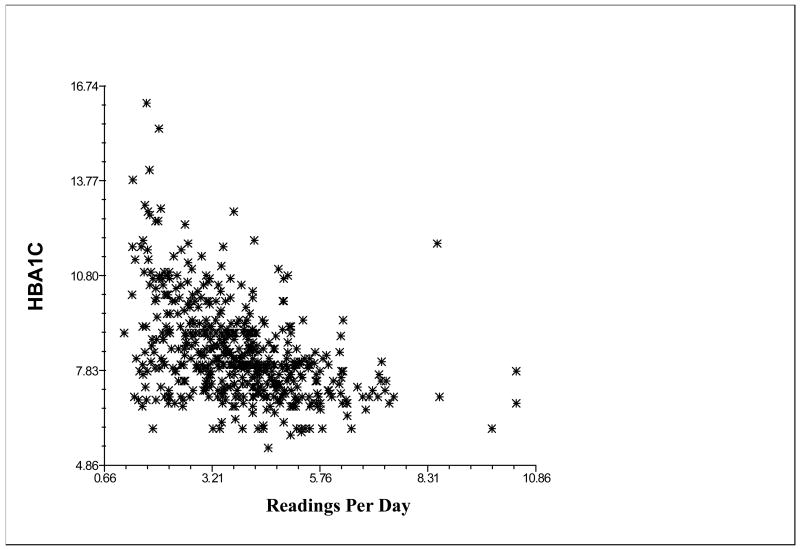
Confirming our primary hypothesis, more frequent monitoring was related to better glycemic control (B = -.32, p < .001), even when age was statistically controlled (see Figure 1). Monitoring did not interact with age to predict glycemic control. The average HbA1c for an adolescent who monitored an average of 2 times per day was 9.07%, whereas the average HbA1c for an adolescent who monitored 5 times per day was 8.12%.
Figure 1.
Relation of number of meter readings taken per day to hemoglobin A1c.
Next, we examined the relations of self-care behavior variables to HbA1c and determined whether blood glucose monitoring predicted HbA1c when self-care behavior was statistically controlled. The global self-care index predicted HbA1c (B = -.86, p < .001), as did the specific item on blood glucose testing (B = -.29, p < .001). When blood glucose monitoring and the self-care index were entered into the same analysis, both emerged as independent predictors of HbA1c (self-report: B = -.19, p = .001; monitoring: B = -.29, p < .001).
Predictors of Monitoring
Demographics
Blood glucose monitoring frequency did not significantly change over the course of the five years. Adolescents tested between 3 and 4 times a day (T1 3.88; T2 3.89; T3 3.81; T4 3.81; T5 3.71). Age revealed a weak relation to monitoring, showing a marginal decrease with age (B = -.06, p = .08). Monitoring frequency was not related to sex or diabetes duration, but more frequent monitoring was related to higher social status (B = .02, p < .05), using an insulin pump (B = .49, p = .001), and lower absolute body mass index (or percentiles; -.09, p < .001).
Self-report of self-care behavior
Child report of better global self-care behavior was related to more frequent monitoring (B = .60, p < .001). Interestingly, parent report of better global self-care behavior was even more strongly related to more frequent monitoring (B = .74, p < .001). Child report of the one item on frequency of blood glucose testing was also related to monitoring frequency (B = .33, p < .001), as was parent report of frequency of testing (B = .39, p < .001), but these relations were not as strong as the relations reported above to the global self-care index.
Psychosocial predictors
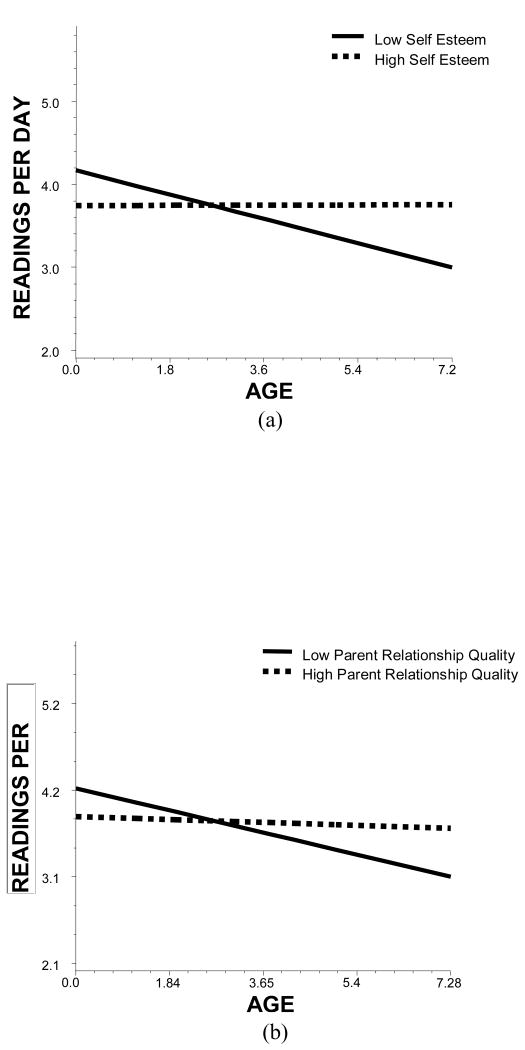
Self-efficacy about testing and self-efficacy to control blood glucose were both related to more frequent monitoring (B = .01, p < .001; B = .01, p < .05; respectively), and did not interact with age. Depressive symptoms were not related to monitoring, but self-esteem interacted with age to predict monitoring (B = .20, p < .01), such that monitoring declined with age among those with low self-esteem but not high self-esteem (see Figure 2a). Stressful life events also interacted with age, such that monitoring declined with age for those with more but not fewer stressful life events, similar to the pattern shown in Figure 2a (B = -.03, p < .05). Parent relationship quality and parent support also each interacted with age (B = .14, p < .05; B = .11, p < .05, respectively). Monitoring declined with age among those who had a poor relationship with their parents but not those who had a good relationship (see Figure 2b). Monitoring also declined with age among those who received less diabetes support from parents but not those who received more support, similar to the interaction shown in Figure 2b.
Figure 2.
(a) Relation of age to number of blood glucose tests performed for patients who are low (25th percentile; solid line) and high (75th percentile; dashed line) in self-esteem. (b) Relation of age to number of blood glucose tests performed for patients who are low (25th percentile; solid line) and high (75th percentile; dashed line) in parent relationship quality.
Conclusions
Frequency of blood glucose monitoring as determined by data downloaded from blood glucose meters is related to better glycemic control among adolescents with type 1 diabetes over time. However, self-reports of self-care more generally also predicted better glycemic control, independently of monitoring frequency. Thus, there appear to be multiple aspects of self-care that have implications for glycemic control.
Given the importance of blood glucose monitoring for good glycemic control, we explored the demographic, behavioral, and psychosocial correlates of monitoring. Unlike previous research (6, 8, 11), we found only a weak trend for monitoring to decline with age. Consistent with prior research (9), monitoring was less frequent among adolescents from lower social status families. Monitoring also was related to global self-care behavior, signifying that those who monitor blood glucose more frequently are likely to engage in good self-care more generally. The age-related declines in monitoring were moderated by several psychosocial variables. Monitoring was more likely to decline with age among adolescents with low self-esteem, adolescents who had experienced more stressful life events in the past year, and adolescents who had a poorer quality relationship with parents or received less parent support for their diabetes. The challenge to make frequent glucose monitoring effective is to ensure that the families use the information obtained to make appropriate treatment decisions. This aspect was not measured directly in this study, but it is assumed that this is the mechanism by which parental support is helpful in maintaining better glycemic control. Future research should examine whether adolescents and parents have a plan in place to respond to low and high blood glucose readings.
Acknowledgments
The authors acknowledge the support of grant R01 DK60586 from the National Institutes of Health to conduct this work and the support of the Pediatric Clinical and Translational Research Center at Children's Hospital of Pittsburgh (GCRC grant, 5MO1RR00084). Portions of these data were presented at the 69th Annual Meeting of the American Diabetes Association (New Orleans, June, 2009). We appreciate the assistance of Pamela Snyder with the data analyses and of all the Carnegie Mellon research assistants who conducted these interviews. We also are grateful to the children and their parents for their cooperation.
Contributor Information
Vicki S. Helgeson, Carnegie Mellon University, Pittsburgh, PA 15213
Erin Honcharuk, Carnegie Mellon University, Pittsburgh, PA 15213.
Dorothy Becker, Children's Hospital of Pittsburgh, Pittsburgh, PA 15213
Oscar Escobar, Children's Hospital of Pittsburgh, Pittsburgh, PA 15213
Linda Siminerio, University of Pittsburgh Medical Center, Pittsburgh, PA 15213
References
- 1.Mann NP, Noronha JL, Johnston DI. A prospective study to evaluate the benefits of long-term self-monitoring of blood glucose in diabetic children. Diabetes Care. 1984;7:322–326. doi: 10.2337/diacare.7.4.322. [DOI] [PubMed] [Google Scholar]
- 2.Schiffrin A, Belmonte M. Multiple daily self-glucose monitoring: Its essential role in long-term glucose control in insulin-dependent diabetic patients treated with pump and multiple subcutaneous injections. Diabetes Care. 1982;5:479–484. doi: 10.2337/diacare.5.5.479. [DOI] [PubMed] [Google Scholar]
- 3.Levine BS, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LMB. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139:197–203. doi: 10.1067/mpd.2001.116283. [DOI] [PubMed] [Google Scholar]
- 4.Dorchy H, Roggemans MP, Willems D. Glycated hemoglobin and related factors in diabetic children and adolescents under 18 years of age: a Belgian experience. Diabetes Care. 1997;20:3–6. doi: 10.2337/diacare.20.1.2. [DOI] [PubMed] [Google Scholar]
- 5.Dorchy H. Personal communication. 2008 [Google Scholar]
- 6.Haller MJ, Stalvey MS, Silverstein JH. Predictors of control of diabetes: Monitoring may be the key. J Pediatr. 2004;144:660–661. doi: 10.1016/j.jpeds.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Urbach SL, LaFranchi S, Lambert L, Lapidus JA, Daneman D, Becker TM. Predictors of glucose control in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2005;6:69–74. doi: 10.1111/j.1399-543X.2005.00104.x. [DOI] [PubMed] [Google Scholar]
- 8.Belmonte M, Schiffrin A, Dufresne J, Suissa S, Goldman H, Polychronakos C. Impact of SMBG on control of diabetes as measured by HbA1: 3 year survey of a juvenile IDDM clinic. Diabetes Care. 1988;11:484–488. doi: 10.2337/diacare.11.6.484. [DOI] [PubMed] [Google Scholar]
- 9.Karter AJ, Ackerson LM, Darbinian JA, D'Agostino RB, Ferrara A, Liu J, Selby JV. Self-monitoring of blood glucose levels and glycemic control: The northern California kaiser permanent diabetes registry. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler O, Kolopp M, Got I, Genton P, Debry G, Drouin P. Reliability of self-monitoring of blood glucose by CSII-treated patients with type I diabetes. Diabetes Care. 1989;12:184–188. doi: 10.2337/diacare.12.3.184. [DOI] [PubMed] [Google Scholar]
- 11.Evans JMM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: Observational study with diabetes database. BMJ. 1999;319:83–86. doi: 10.1136/bmj.319.7202.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer JD, Willett JB. Applied Longitudinal Data Analysis. New York: Oxford University Press; 2003. [Google Scholar]
- 13.La Greca AM, Swales T, Klemp S, Madigan S. Self-care behaviors among adolescents with diabetes. Paper presented at the Ninth Annual Convention of the Society for Behavioral Medicine. [Google Scholar]
- 14.Helgeson VS, Snyder PR, Escobar O, Siminerio L, Becker D. Comparison of adolescents with and without diabetes on indices of psychosocial functioning for three years. J Pediatr Psychol. 2007;32:794–806. doi: 10.1093/jpepsy/jsm020. [DOI] [PubMed] [Google Scholar]
- 15.Lewin AB, LaGreca AM, Geffken GR, Williams LB, Duke DC, Storch EA, Silverstein JH. Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: The Self-Care Inventory (SCI) J Pediatr Psychol. 2009;34:999–1007. doi: 10.1093/jpepsy/jsp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbot F, Nouwen A, Gingras J, Gosselin M, Audet J. The assessment of diabetes-related cognitive and social factors: The Multidimensional Diabetes Questionnaire. J Behav Med. 1997;20:291–312. doi: 10.1023/a:1025508928696. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs M. Children's Depression Inventory: Technical manual. North Tonawanda, NY: Multi-Health Systems, Inc; 2001. [Google Scholar]
- 18.Harter S. Manual for the self-perception profile for children. Denver: University of Denver; 1985. [Google Scholar]
- 19.Yeaworth RC, York J, Hussey MA, Ingle ME, Goodwin T. The development of an adolescent life change event scale. The development of an adolescent life change event scale. 1980;15:91–97. [PubMed] [Google Scholar]
- 20.Kerr M, Stattin H. What parents know, how they know it, and several forms of adolescent adjustment: Further support for a reinterpretation of monitoring. Dev Psychol. 2000;36:366–380. [PubMed] [Google Scholar]
- 21.Schafer LC, McCaul KD, Glasgow RE. Supportive and nonsupportive family behaviors: Relationships to adherence and metabolic control in persons with Type I Diabetes. Diabetes Care. 1986;9:179–185. doi: 10.2337/diacare.9.2.179. [DOI] [PubMed] [Google Scholar]
- 22.McKelvey J, Waller DA, North AJ, Marks JF, Schreiner B, Travis LB, Murphy JN. Reliability and validity of the Diabetes Family Behavior Scale (DFBS) Diabetes Educ. 1993;19:125–132. doi: 10.1177/014572179301900206. [DOI] [PubMed] [Google Scholar]
- 23.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]