Abstract
Antibody tests are useful for mapping the distribution of lymphatic filariasis (LF) in countries and regions and for monitoring progress in elimination programs based on mass drug administration (MDA). Prior antibody tests have suffered from poor sensitivity and/or specificity or from a lack of standardization. We conducted a multicenter evaluation of a new commercial ELISA that detects IgG4 antibodies to the recombinant filarial antigen Bm14. Four laboratories tested a shared panel of coded serum or plasma samples that included 55 samples from people with microfilaremic Wuchereria bancrofti or Brugia infections and 26 control samples. Qualitative results were identical in all four test sites. In addition, each laboratory tested samples from their own serum banks. The test detected antibodies in 32 of 36 samples (91%) from people with Brugian filariasis and in 96 of 98 samples (98%) from people with Bancroftian filariasis. Specificity testing showed that many serum or plasma samples from patients with other filarial infections such as onchocerciasis had positive antibody tests. Specificity was otherwise excellent, although 3 of 30 samples from patients with ascariasis and 4 of 51 with strongyloidiasis had positive antibody tests; it is likely that some or all of these people had previously lived in filariasis-endemic areas. Antibody test results obtained with eluates from blood dried on filter paper were similar to those obtained with plasma tested at the same dilution. This test may be helpful for diagnosing LF in patients with clinical signs of filariasis. It may also be a useful tool for use in LF endemic countries to monitor the progress of filariasis elimination programs and for post-MDA surveillance.
Keywords: Filariasis, Brugia, Wuchereria, antibody, serology
1. Introduction
Lymphatic filariasis (also known as elephantiasis; LF) is a disabling and deforming disease that affects some 120 million people in 83 countries; approximately 1.3 billion people are at risk of becoming infected with the nematode parasites (Wuchereria bancrofti and Brugia species) that cause this disease (Ottesen, 2006). The Global Programme to Eliminate Lymphatic Filariasis (GPELF) is using mass drug administration (MDA) to reduce filarial infection rates below those required for sustained transmission with the goal of permanently eliminating LF in all endemic countries by the year 2020 (Ottesen et al.,2008; World Health Organization, 2008). GPELF relies on diagnostic tests to identify and map LF-endemic areas and to monitor the impact of interventions such as MDA. A recent review discussed the value of different diagnostic tests (serum antigen and antibody assays and detection of parasite DNA in vector mosquitoes) for different phases of LF elimination programs (Weil and Ramzy, 2007).
In addition to their value as tools for diagnosing individual patients, filarial antibody tests are useful for identifying endemic areas and for following antibody rates in young children over time as a means of assessing changes in transmission rates following MDA (Ramzy et al.,2006; Weil et al.,2008). Several studies have shown that an ELISA that detects IgG4 antibodies to the recombinant filarial antigen Bm14 (GenBank accession number M95546.1, also known as BmM14, similar to BmSXP-1, GenBank M98813) is a sensitive marker for infections with B. malayi or W. bancrofti (or for heavy exposure to these parasites) (Chandrashekar et al.,1994; Ramzy et al.,1995; Lammie et al.,2004). Since antibodies to Bm14 clear very slowly after infected humans are treated (Helmy et al.,2006), tests like the Bm14 ELISA are especially useful as tools for use in serial surveys of young children to assess changes in LF transmission following MDA (Ramzy et al.,2006; Weil and Ramzy 2007). Known limitations of the test are its cross-reactivity with serum or plasma samples from patients infected with other filarial parasites (eg., Onchocerca volvulus and Loa Loa) and the fact that it was a noncommercial, research grade test (Lammie et al.,2004). A company has recently marketed an ELISA test kit based on Bm14 that employs standardized reagents and an optimized protocol. The purpose of this study was to conduct an independent, multicenter trial to evaluate the performance of the commercial Bm14 antibody test kit.
2. Materials and methods
2.1. Tests for antifilarial antibodies
The Filariasis CELISA Test (Cellabs, Brookvale, NSW, Australia) is an indirect ELISA that detects IgG4 antibodies to the recombinant B. malayi antigen Bm14. This test was performed according to the protocol provided by the company with minor modifications. Briefly, serum or plasma and test control samples were tested in duplicate at a dilution of 1:100. The positive kit control sample was also tested at 1:1600 as a weak positive control. Incubation times for the serum or plasma and secondary antibody steps were 2 hr and 45 minutes, respectively. Reactions were stopped 15 min after addition of substrate, and ELISA readers measured optical densities in test wells at 450 nm. Optical density (OD) values were blanked by subtracting OD values obtained with 100 μl dH2O. For some analyses, we converted OD values to units to normalize results between laboratories. Units were defined as the [(mean OD value for the test sample minus the mean OD value for the kit negative control) divided by the (mean OD value for the kit positive control minus the mean OD value for the kit negative control] × 100. Thus, units represent a measure of antibody activity in test samples relative to the kit positive control value defined to contain 100 units. This quantitation is only an approximation, because OD values are not linearly related to antibody concentration.
The Washington University laboratory also tested serum samples with a noncommercial ELISA for IgG4 antibodies to Bm14 (the research laboratory Bm14 test), as previously described (Ramzy et al.,1995).
2.2. Definition of a positive test
Preliminary studies were performed at Washington University to determine a cutoff for the ELISA test. The mean (SD) obtained with 20 non-endemic serum samples (St. Louis, USA) was 0.042 (0.024). Thus, the mean OD plus 3 SD was 0.114. However, we decided to use a conservative cutoff for this study. Therefore, samples that produced mean OD values of > 0.250 were considered to be positive for antibody reactivity.
2.3. Elution of antibodies from dried blood samples
Venous blood was collected in EDTA from donors with no history of exposure to LF. Thirty μl aliquots of blood were centrifuged, and 15 μl of plasma was removed. We then added 15 μl of test samples (serum or plasma) and resuspended the blood. Ten μl aliquots of antibody-spiked blood samples were pipetted onto round filter paper circles (TropBio, Townsville, Australia), and these were dried and stored at 5°C until they were tested. Elution was performed by placing one blood spot in 500 μl of the kit elution buffer in a 1.5 ml tube overnight at 5°C. Tubes were centrifuged the next morning, and eluates (containing an approximate 1:100 dilution of the test serum or plasma present in the blood) were tested by ELISA in parallel with the same serum or plasma samples used to spike the blood diluted 1:100 in sample diluent.
2.4. Serum sets and laboratories
The Washington University laboratory assembled a shared panel of 81 de-identified and coded serum or plasma samples that was tested blindly by the following four laboratories: Washington University (WU), Bernhard Nocht Institute for Tropical Medicine, Hamburg (BNI), Centers for Disease Control and Prevention (CDC), and James Cook University (JCU). The shared panel included serum samples from persons with Wuchereria bancrofti (n=29), B. malayi (n=11), or B. timori (n=15) microfilaremia from different countries (India, Indonesia, Sri Lanka, and Egypt), non-endemic samples from residents of St. Louis, USA (n=20), and samples from patients with strongyloidiasis with no history of exposure to filariasis (n = 6). These samples had been previously tested with the research version of the Bm14 antibody test. Positive samples were selected to represent a range of antibody reactivity. Laboratories were also encouraged to test their own panels of samples from persons with filariasis (with microfilaremia or Mf) and various types of control sera. Dr. Thomas Nutman kindly provided samples obtained from patients with ascariasis collected in an area of Ecuador that is non-endemic for filariasis or onchocerciasis.
3. Results
3.1. Sensitivity of the Bm14 antibody test
Figure 1 compares OD values obtained for 21 serum or plasma samples from patients with LF with the Filariasis CELISA test and the research laboratory Bm14 test. These results show that the CELISA test kit produced higher OD values than the research laboratory test.
Figure 1.
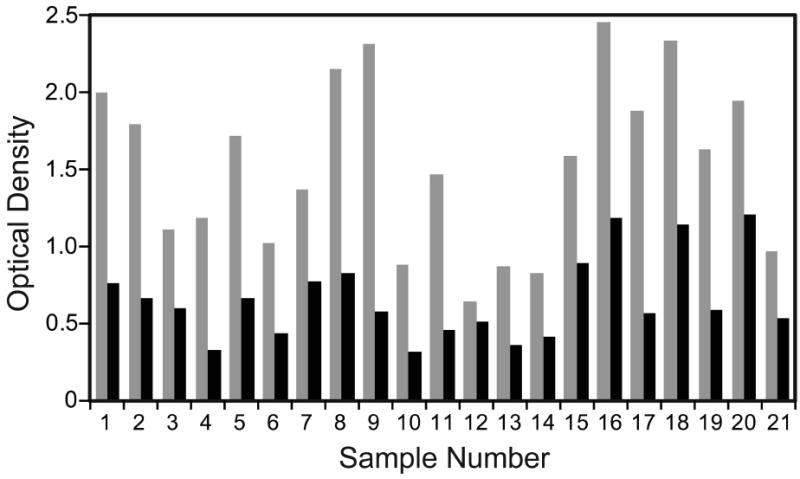
Relative sensitivity of the commercial and research laboratory filariasis antibody tests. The figure compares OD values obtained for 21 serum or plasma samples from microfilaremic subjects with lymphatic filariasis by the Filariasis CELISA test (gray bars) and the research laboratory Bm14 ELISA (black bars).
Antibody results obtained with the Filariasis CELISA test for different patient groups are shown in Figure 2. Sensitivity was evaluated with Mf-positive filariasis serum or plasma samples from two study laboratories (WU and CDC). These included samples in the shared panel and other samples from serum banks at these laboratories. Thirty-two of 35 sera (91%) from people with B. malayi or B. timori infections had positive antibody tests. These sera were from India and Indonesia. Ninety-six of 98 serum or plasma samples (98%) from people with W. bancrofti microfilaremia had positive tests. These samples were from India, Egypt, Papua New Guinea, Sri Lanka, Haiti, and Kenya. The difference in % positivity with Brugia and Wuchereria samples was not statistically significant (P = 0.22).
Figure 2.
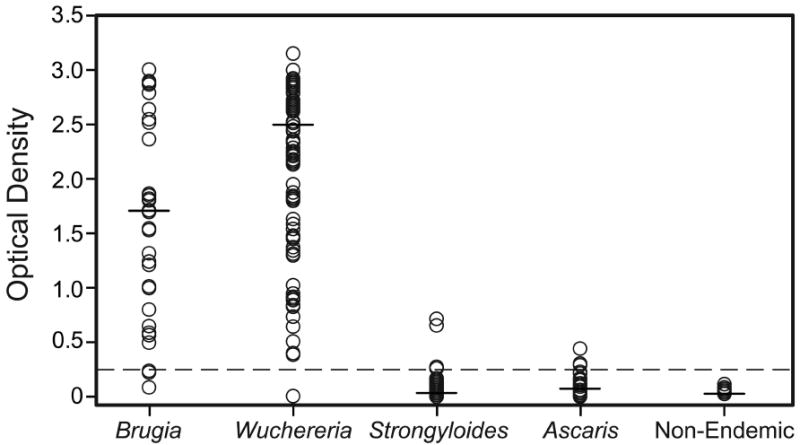
Filariasis CELISA results (optical density) by serum or plasma category. Horizontal lines show median values. The cutoff OD value of 0.250 is shown with a dashed horizontal line.
3.2. Specificity of the Bm14 antibody test
Specificity results are also shown in Figure 2. All 20 nonendemic normal serum or plasma samples in the shared panel were negative at all study sites; the range of OD values obtained in different laboratories for individual control samples in the shared panel was -0.014 to 0.135. The mean OD plus 3 SD obtained with the panel of negative samples in the 4 test laboratories ranged from 0.082 to 0.164.
All six samples in the shared panel from patients with strongyloidiasis were negative at all study sites. Two of 19 samples tested at CDC from Brazilian patients with strongyloidiasis were weakly positive in the Bm14 test with OD values of 0.271 and 0.654. One of 19 samples from patients with strongyloidiasis tested at the JCU lab was positive with an OD value of 0.711. One of 7 samples from expatriate patients with strongyloidiasis tested at BNI was positive with an OD of 0.262. Thus, 4 of 51 samples from patients with strongyloidiasis had positive Bm14 antibody tests (specificity 92.2%). It is possible that some or all of the patients with positive tests had been exposed to filarial infections in the past. One of 25 samples submitted to CDC for Strongyloides serology (data not shown in Figure 2) was positive by the Bm14 test with an OD value of 1.939. This person had previously lived in Liberia and Ghana and may have been exposed to filarial infections.
Five of 5 samples from patients with ascariasis tested at CDC were negative with OD values < 0.1. Three of 15 samples from Turkish patients with ascariasis tested at BNI were positive with OD values of 0.289-0.440. Travel histories were not available for these patients. None of 10 Ecuadoran samples from patients with ascariasis tested at WU were positive. None of 6 nonendemic (USA) samples with rheumatoid factor titers ≥ 1:64 were positive.
The BNI laboratory reported that 19 of 25 samples (76%) from onchocerciasis patients living in an area of Ghana without bancroftian filariasis had positive antibody tests. Twenty-two of 24 samples (92%) from an area in Uganda that is highly endemic for Mansonella perstans and M. streptocerca had positive antibody tests. There is some onchocerciasis in this area but no W. bancrofti. In contrast, only 3 of 12 M. perstans samples from a different area in Uganda with low-level onchocerciasis endemicity had positive antibody tests.
3.3. Interlaboratory reproducibility
Qualitative results for the 81 samples in the shared serum panel were identical in all four laboratories. OD values for filariasis samples varied between laboratories with a mean coefficient of variation (CV) of 0.16 (SD 0.15); CV values for antibody units were similar (mean 0.15, SD 0.14).
3.4. Antibody tests of blood dried on filter paper
OD values obtained with eluates from blood dried on filter paper were similar to those obtained with serum (Figure 3).
Figure 3.
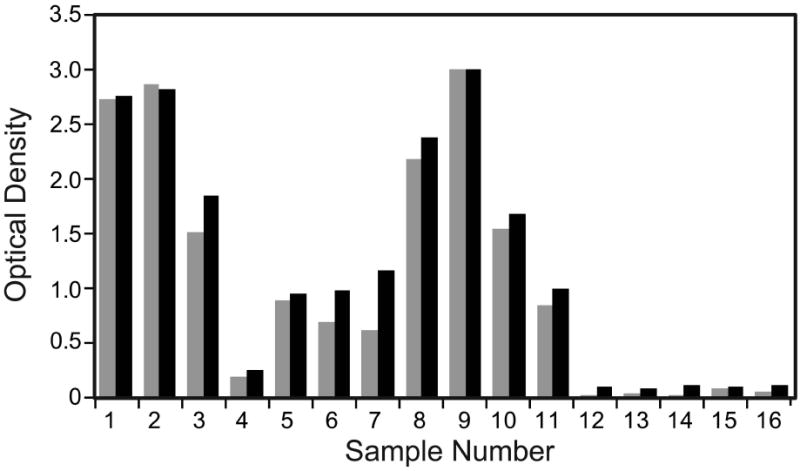
Antibody results obtained with serum or plasma samples and eluates from dried blood samples. The figure compares optical densities (OD values) obtained with the Filariasis CELISA with serum or plasma samples (gray bars) and filter paper blood eluates (black) for filariasis serum or plasma samples (sample numbers 1-11) and control samples (12-16).
4. Discussion
The purpose of this study was to evaluate the performance of the Filariasis CELISA antibody test kit. This was an independent evaluation that was not sponsored by the manufacturer, although Cellabs did provide kits at no cost for the study. The exercise was strengthened by participation of 4 different laboratories in 3 continents. The laboratories tested a shared panel of coded sera, and each laboratory performed supplemental testing with samples in their own collections.
The CELISA test has several advantages over the research laboratory Bm14 ELISA. First, the kit is manufactured under GMP conditions with standardized reagents. This should improve reproducibility of the test. Secondly, the package insert from the manufacturer states that the kit has a shelf life of 12 months if it is stored at 5° C. The research laboratory test is not a kit, and its components do not have a shelf life rating. Finally, the Filariasis CELISA kit is more sensitive than the research laboratory Bm14 test. This may be due to optimized reagent concentrations or to the fact that the kit uses a more reactive horseradish peroxidase substrate than the research test (3,3’,5,5’-tetramethylbenzidine vs o-phenylenediamine).
The Bm14 CELISA had high sensitivity with samples from people infected with W. bancrofti or Brugia species. This is consistent with results previously reported for the research laboratory version of the test (Chandrashekar et al.,1994; Ramzy et al.,1995; Lammie et al.,2004). Specificity testing confirmed that the test is often positive with samples from people with other filarial infections such as onchocerciasis. Results for samples from people in two areas of Uganda who had M. perstans infections were inconsistent and inconclusive. It is possible that some of the positive tests in these people were due to coinfection or exposure to other filarial parasites. Most samples from patients with strongyloidiasis or ascariasis were antibody negative, and we cannot be sure that persons with positive tests had not been exposed to filarial infections. For example, sporadic cases of LF are still seen in southern Turkey (Cengiz et al.,2006). Therefore, we doubt that intestinal nematodes infections are associated with positive Bm14 antibody tests.
The present study used a cutoff value of 0.25 for a positive test. The kit protocol recommends using a cutoff of 0.4 for definite positives and retesting samples with OD values in an indeterminant range of 0.200-0.400. The higher cutoff would not have made much difference in this study; only one positive filariasis serum had an OD value between 0.250 and 0.400. Two stronglyoidiasis samples and two ascariasis samples had OD values in this indeterminant zone. We also analyzed the data based on antibody units to normalize results based on the net OD values obtained with the positive control on each plate. This did not affect our results. We found that the OD cutoff of 0.25 produced the same results as a cutoff of 10 units; only one positive filariasis serum would have been reclassified as antibody negative with the higher cutoff values of OD 0.4 or 15 units. This sensitivity analysis is encouraging, because it shows that the test is robust and that the results were not affected much by different cutoff values in this range.
Similar antibody test results were obtained with serum and with dried filter paper blood samples. The ability to test filter paper blood samples will improve the field utility of the test. However, this study tested blood samples that were spiked with positive sera. Additional studies are needed to directly compare blood and serum samples from the same subjects and to test the stability of antibodies in dried blood samples stored under different conditions.
In conclusion, the Bm14 CELISA appears to be an excellent test for the specific detection of antibody responses to filarial parasites. Physicians may find this test useful to support a diagnosis of filariasis in patients with a history of exposure to filariasis and clinical signs of the disease. The antibody test was sensitive and specific for filarial infections in this study. However, prior studies have shown that antifilarial antibodies are common in healthy people living LF-endemic areas who have negative tests for microfilaremia and filarial antigenemia and no clinical signs of filariasis (so-called “endemic normals”) (Weil et al.,1996). This high sensitivity makes antibody testing especially useful as an epidemiological tool for assessing levels of infection and/or exposure to filarial parasites in populations. This test has attractive features for use in LF endemic countries, namely robustness, high throughput, and the ability to test filter paper blood samples. These features should help to make antibody monitoring a practical monitoring option for late stages of filariasis elimination programs and for post-MDA surveillance.
Acknowledgments
We would like to thank Cellabs Pty Ltd for providing Filariasis CELISA kits for this evaluation. This work was supported in part by NIH grant AI 65715 and by Barnes-Jewish Hospital Foundation Grant No. 01251-0608.
Footnotes
Disclosure The Bm14 antibody test evaluated in this article uses reagents licensed from Barnes-Jewish Hospital, an affiliation of G.J. Weil. All royalties from sales of this test are donated to the Barnes-Jewish Hospital Foundation, a registered not-for-profit organization (http://www.barnesjewish.org/groups/default).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cengiz N, Savas L, Uslu Y, Anarat A. Filariasis in a child from southern Turkey: a case report. Turk J Peds. 2006;48:152–154. [PubMed] [Google Scholar]
- Chandrashekar R, Curtis KC, Ramzy RM, Liftis F, Li B-W, Weil GJ. Molecular cloning of Brugia malayi antigens for diagnosis of lymphatic filariasis. Mol Biochem Parasitol. 1994;64:261–274. doi: 10.1016/0166-6851(94)00035-2. [DOI] [PubMed] [Google Scholar]
- Helmy H, Weil GJ, Ellethy AS, Ahmed ES, El Setouhy M, Ramzy RMR. Bancroftian filariasis: Effect of repeated treatment with diethycarbamazine and albendazole on microfilaremia, antigenemia, and anti-filarial antibodies. Trans R Soc Trop Med Hyg. 2006;100:656–662. doi: 10.1016/j.trstmh.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Lammie P, Weil G, Rahmah N, Kaliraj P, Steel C, Goodman D, Lakshmikanthan V, Ottesen E. Recombinant antigen based assays for the diagnosis and surveillance of lymphatic filariasis - a multicenter trial. Filaria Journal. 2004;3:9. doi: 10.1186/1475-2883-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen EA. Lymphatic filariasis: Treatment, control, and elimination. Adv Parasitol. 2006;61:395–441. doi: 10.1016/S0065-308X(05)61010-X. [DOI] [PubMed] [Google Scholar]
- Ottesen EA, Hooper PJ, Bradley M, Biswas G. The Global Programme to Eliminate Lymphatic Filariasis: Health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzy R, Helmy H, Faris R, Gad A, Chandrashekar R, Weil G. Evaluation of a recombinant antigen-based antibody assay for diagnosis of bancroftian filariasis in Egypt. Ann Trop Med Parasitol. 1995;89:443–446. doi: 10.1080/00034983.1995.11812974. [DOI] [PubMed] [Google Scholar]
- Ramzy RMR, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. Effect of yearly mass drug administration with diethycarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367:992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- Weil GJ, Kastens W, Susapu M, Laney SJ, Williams SA, King CL, Kazura JW, Bockarie MJ. The impact of repeated rounds of mass drug administration with diethylcarbamazine plus albendazole on bancroftian filariasis in Papua New Guinea. PLoS Negl Trop Dis. 2008;3:e344. doi: 10.1371/journal.pntd.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil GJ, Ramzy RM, Chandrashekar R, Gad AM, Lowrie RC, Jr, Faris R. Parasite antigenemia without microfilaremia in bancroftian filariasis. Am J Trop Med Hyg. 1996;55:333–337. doi: 10.4269/ajtmh.1996.55.333. [DOI] [PubMed] [Google Scholar]
- Weil GJ, Ramzy RMR. Diagnostic tools for filariasis elimination programmes. Trends Parasitol. 2007;23:78–82. doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global programme to eliminate lymphatic filariasis: Progress report on mass drug administration in 2007. Wkly Epidemiol Rec. 2008;83:333–341. [Google Scholar]


