Abstract
To have sex, or not to have sex, is a question posed by many microorganisms. In favor of a sexual lifestyle is the associated rearrangement of genetic material that confers potential fitness advantages, including resistance to antimicrobial agents. The asexual lifestyle also has benefits, as it preserves complex combinations of genes that may be optimal for pathogenesis. For this reason, it was thought that several pathogenic fungi favored strictly asexual modes of reproduction. Recent approaches using genome sequencing, population analysis, and experimental techniques have now revised this simplistic picture. It is now apparent that many pathogenic fungi have retained the ability to undergo sexual reproduction, although reproduction is primarily clonal in origin. In this review, we highlight the current understanding of sexual programs in the Candida clade of species. We also examine evidence that sexual-related processes can be used for functions in addition to mating and recombination in these organisms.
Keywords: Recombination, Biofilm, Pheromone, Adhesion, Mating, Fitness
Introduction
The most commonly isolated human fungal pathogen is Candida albicans, an opportunistic fungus that causes both mucosal and systemic infections. C. albicans is a hemiascomycete that diverged from the model organism, Saccharomyces cerevisiae, between 100 and 900 million years ago [1, 2]. Originally believed to be asexual, it was assigned to the genus Candida; a catch-all grouping for yeasts that lacked sexual cycles or spore formation, and yet formed pseudohyphae or true hyphae [3]. It is now recognized that the majority of clinically important Candida species are related and can be grouped into a single clade [4, 5] (Fig. 1). All members of the clade share an altered genetic code, in which the CUG codon is translated as leucine as opposed to serine [6]. This clade still represents a diverse collection of organisms, however, with many different characteristics and lifestyles. While the ability of several of these fungi to cause disease is well documented [7], other aspects of their biology have not been examined in detail. This is particularly true of the sexual programs of these organisms. Long thought to be asexual, it is now becoming evident that several Candida species exhibit unique, or at least highly modified, sexual cycles. Given their importance as human pathogens, it is now imperative to elucidate these sexual processes and determine their potential roles in colonization and infection of the mammalian host.
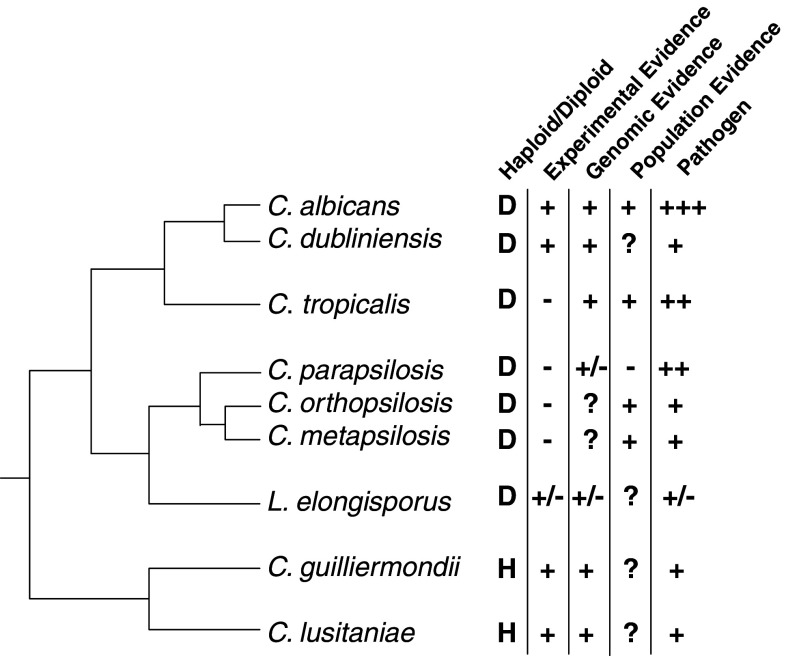
Fig. 1.
Mating and pathogenesis amongst members of the Candida clade. Experimental evidence refers to laboratory evidence of mating. Genomic evidence refers to the presence of genes predicted to be involved in mating from sequencing data. Population evidence refers to evidence of mating based on population structure analysis. Plus/minus indicates that the data is contradictory or inconclusive. Question marks indicate where data is lacking. The phylogenetic tree is for comparison purposes only and is not drawn to scale
Sexual reproduction in Candida albicans
Candida species are the fourth leading cause of nosocomial bloodstream infections in the US, where they are responsible for 8–10% of all such infections [8]. This translates to 10,500–42,000 infections in US hospitals every year due to candidemia [7]. The most common cause of invasive candidiasis is Candida albicans, a commensal of the gastrointestinal tract in 70% of the healthy population [9]. Up until a decade ago, C. albicans was thought to be an asexual yeast, with no potential for undergoing mating or sexual reproduction. However, this paradigm was challenged when Hull and Johnson identified a genetic locus that resembled the classical mating type (MAT) locus in Saccharomyces cerevisiae [10]. The C. albicans mating type-like (MTL) locus contains transcriptional regulators of cell identity similar to those in S. cerevisiae; a1, α1, and α2, as well as an additional regulator, a2. The MTL locus of C. albicans is also much larger than that of S. cerevisiae due to the presence of additional genes that encode phosphatidylinositol kinases (PIK), oxysterol binding proteins (OBP) and poly A polymerases (PAP) [10]. The contribution of these additional genes to mating in C. albicans is not known. The standard laboratory strain of C. albicans, SC5314, was shown to be a diploid a/α cell, and so Hull et al. subsequently used genetic deletions to construct a- and α-type strains to test for potential mating [11]. At the same time, the Magee group utilized sorbose medium to induce homozygosis of chromosome 5 (containing the MTL locus) to generate a/a and α/α derivatives of SC5314 [12]. Both groups were successful in showing mating of C. albicansa and α cells using either an in vitro or in vivo approach, although mating occurred at very low frequencies [11, 12].
Miller and Johnson subsequently discovered an unsuspected link between mating and another phenomenon in C. albicans, that of white-opaque phenotypic switching. White-opaque switching was first described by Slutsky et al. more than 20 years ago, in which a subset of C. albicans strains was shown to switch between two alternative states. In one state, cells were round and gave rise to white, dome-shaped colonies (‘white’ phase), while in the other state cells were elongated and gave rise to flatter, translucent colonies (‘opaque’ phase) [13]. The white and opaque forms differ in many characteristics other than morphology, as white cells are more virulent during systemic infection [14] and secrete a neutrophil chemoattractant [15], while opaque cells are more efficient at colonizing the skin [16] and are less readily phagocytized than white cells [17]. Miller and Johnson further showed that overall regulation of the white-opaque switch involved a1 and α2 regulators encoded at the MTL locus [18]. Cells expressing these factors were blocked from undergoing switching to opaque due to the function of the a1/α2 repressor. As a direct result of this regulation, only strains homozygous for MTL a or MTLα, approximately 3–9% of clinical isolates, are naturally competent for switching [18–20].
More recent studies have further elucidated the mechanism underlying the bistable white-opaque switch. In particular, it is now apparent that the a1/α2 complex prevents switching to opaque by blocking expression of white-opaque regulator 1 (Wor1) protein, the master regulator of the switch [21–23]. Other transcriptional regulators involved in the white-opaque switch include Czf1 and Wor2, which favor the opaque state, and Efg1, whose expression favors the white state [24–27]. Zordan et al. suggest a working model in which interlocking transcriptional feedback loops regulate white-opaque bistability [27]. Wor1 and Czf1 act to repress Efg1, while conversely Efg1 acts to repress Wor2 which, in conjunction with Wor1, is critical for stabilization of the opaque state [27]. Understanding the precise interplay between all of these factors will lead to a more detailed picture of this novel regulatory circuit and the regulation of white-opaque switching.
In addition to the complex transcriptional regulation of white-opaque switching in C. albicans, a number of external factors can also affect rates of switching. Temperature [13], oxygen [28, 29] and carbon dioxide [30], as well as genotoxic and oxidative stress [31], have all been shown to influence this transition. Even varying the rate of growth of some strains can affect white-opaque switching [31]. Although the precise mechanism(s) by which these diverse factors influence switching has yet to be established, all appear to impinge on Wor1 at some level as switching to opaque has not been observed in the absence of this protein [29–31]. Recent work in the Soll group has further shown that N-acetylglucosamine can induce switching from white to opaque [32]. Switching is augmented by carbon dioxide and occurs readily even at 37°C, a temperature at which opaque cells are normally unstable, at least in vitro [32]. These conditions may mimic those found in the gastrointestinal (GI) tract where the endogenous microbiota serve as a potential source of N-acetylglucosamine. These results also support earlier reports of stable opaque cells during GI colonization, which can promote C. albicans mating in this niche [28]. Further studies are necessary to see if mating occurs preferentially during GI colonization, or whether other host niches also support switching to opaque and subsequent mating.
Once a and α cells have switched to the opaque state, the conjugation process in C. albicans closely mirrors that in S. cerevisiae. Cells produce sex-specific pheromones, MFa and MFalpha, that are sensed via the cell surface receptors, Ste3 and Ste2, respectively [33–35]. The pheromone secreted by a cells, MFa, is a prenylated 14 amino acid peptide, and is likely to have a limited diffusion range [33]. In contrast, α-pheromone (MFα) is an unmodified 13 or 14 amino acid peptide potentially capable of diffusing longer distances. The combination of one prenylated and one unmodified pheromone for regulating mating appears to be conserved amongst the ascomycetes [33].
Pheromone signaling results in arrest in G1 of the cell cycle and leads to the formation of polarized mating projections [36]. Subsequent cell–cell fusion and nuclear karyogamy produces mononuclear tetraploid cells [37]. Many putative mediators of cell fusion in C. albicans are untested, although it is probable that factors involved in mating and cell fusion in S. cerevisiae have a conserved function in C. albicans, particularly given their increased expression in pheromone-treated cells [34]. C. albicans mating products are typically stable tetraploid cells that can undergo multiple rounds of mitosis, but genome stability can be compromised under certain environmental conditions (discussed below).
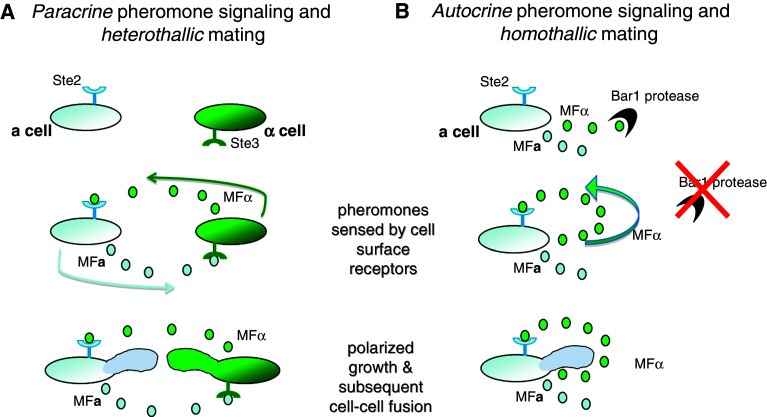
In addition to traditional heterothallic mating between an a cell and an α cell, C. albicans was recently shown to be capable of homothallic mating between cells of the same sex (i.e., a–a or α–α mating). In particular, C. albicans a cells were found to secrete not only the canonical a-pheromone but also functional α-pheromone [38]. Normally, the aspartyl protease Bar1 acts to degrade this α-pheromone and thereby prevents auto-activation of the mating pathway. In the absence of this protease, however, C. albicans a cells initiate mating even though α partner cells are not present. This mechanism involves autocrine pheromone signaling in which α-pheromone secreted by a cells binds to the Ste2 receptor on the same (or neighboring) a cells. Upregulation of the mating program occurs and further augments α-pheromone production (Fig. 2). The induction of the mating response results in a cells being able to fuse with other a cells, providing C. albicans with a mechanism for mating even within unisexual populations. It is postulated that inactivation of Bar1 protease also occurs in certain host niches, thereby activating autocrine signaling and same-sex mating of a cells in vivo [38].
Fig. 2.
Heterothallic and homothallic mating in Candida albicans. A Paracrine pheromone signaling drives heterothallic mating in C. albicans. Opaque MTLα cells secrete α pheromone (MFα), which is sensed by the cell surface receptor, Ste2, on MTL a cells. Conversely, opaque MTL a cells secrete a pheromone (MFa), which is sensed by the receptor, Ste3, on MTLα cells. This inter-cellular pheromone signaling results in upregulation of mating genes, induction of polarized growth, and a-α cell fusion. B Autocrine pheromone signaling drives homothallic mating in C. albicans. It is now recognized that opaque MTL a cells secrete not only the canonical MFa pheromone but also MFα pheromone. The Bar1 aspartyl protease degrades MFα, but in the absence of this protease MFα accumulates and binds to the Ste2 receptor, resulting in auto-activation of the mating response. Subsequent same-sex conjugation of C. albicans MTL a cells occurs resulting in formation of tetraploid a cells
Homothallic mating is not limited to a-type cells of C. albicans, as both a–a and α–α mating products were obtained upon co-incubation of wild-type a and α cells, in addition to a–α products [38]. Same-sex mating is presumably due to the high concentrations of both a- and α-pheromones produced in these mixed populations of cells. Furthermore, α cells were able to induce mating in a cells even when acting at a distance, or when present only in limiting amounts [38]. Taken together, these studies indicate that pheromone induction of the mating response, whether involving a or α cells, is necessary and sufficient for same-sex fusion of C. albicans cells.
The parasexual cycle of C. albicans
Most sexual organisms complete a mating cycle and decrease their ploidy via meiosis; DNA replication is followed by two successive rounds of DNA division, effectively halving the number of chromosomes in the cell. In higher eukaryotes, this process results in the formation of gametes, which then fuse to form the diploid embryo. In fungi, meiosis is also used to reduce the ploidy of the cell and is again accompanied by high rates of homologous recombination. Surprisingly, however, a traditional meiosis has not been identified in C. albicans, and instead ploidy is reduced through a mechanism of concerted chromosome loss [39]. Although tetraploid C. albicans cells are generally stable on most media, if grown under select conditions (e.g., S. cerevisiae pre-sporulation medium at 37°C) genomic instability is induced and cells with lower ploidy are generated. Interestingly, most of the progeny formed by this parasexual cycle are not true diploids, with many aneuploid products recovered from the process [39, 40]. Instability of tetraploid cells was similarly observed during infection of a mammalian host using a mouse model of systemic candidiasis. These results suggest that the parasexual program of concerted chromosome loss may also occur in vivo [41].
The parasexual cycle can generate recombinant products similar to a traditional meiosis, as a subset of parasexual progeny showed multiple recombination events [40]. In addition, this process appears to utilize at least one ‘meiosis-specific gene’, that of SPO11. In many eukaryotes, Spo11 serves to introduce DNA double-strand breaks that initiate meiotic recombination (for review see [42]). In C. albicans, Spo11 is similarly required for recombination during the parasexual process of concerted chromosome loss. It remains to be seen if additional genes that function exclusively in meiotic recombination in other species are also required for parasexual recombination in C. albicans. We note that it is also possible that a traditional meiosis has yet to be discovered in C. albicans, or that the parasexual cycle involves a subset of cells undergoing meiosis and recombination while the remainder undergo a form of mitotic nondisjunction. Clearly, much remains to be uncovered about this critical step in the parasexual cycle of C. albicans.
Regardless of the mechanism, the parasexual program produces recombinant C. albicans strains with altered genotypes to the parental types [40]. Progeny strains exhibit varied properties in filamentation, growth rate, and white-opaque phenotypic switching [31, 40]. Further characterization of these progeny will provide new insights into how changes at the genomic level can lead to changes in C. albicans virulence. One particular area of interest is that of resistance to antifungal drugs, as it has already been established that aneuploid chromosomes (e.g., trisomies of chromosome 5) can increase resistance to the antifungal drug, fluconazole [43]. It therefore seems likely that a subset of parasexual aneuploid strains will also exhibit increased resistance to the azole class of drugs. Whether the parasexual mating cycle generates drug-resistant isolates of C. albicans in the clinic is a question for future studies.
Sex in C. albicans: unanswered questions
Despite the progress made in understanding the mating cycle of C. albicans since its discovery 10 years ago, many questions remain. Where are the niches where C. albicans undergoes white-opaque switching, mating, and parasexual chromosome loss in vivo? And are these processes facilitated by interactions with the host microbiota? Population analysis of natural isolates of C. albicans show that these strains exhibit mostly clonal modes of reproduction, although levels of recombination suggest either occasional mating or mating events have occurred in the recent past [20]. Whether same-sex mating is occurring in natural populations and with what frequency has yet to be investigated.
Another important question relates to the evolution of the Bar1 protease and how it emerged to regulate the balance between homothallic (same-sex) mating and heterothallic (opposite-sex mating) in C. albicans. In S. cerevisiae, Bar1 also acts to degrade α-pheromone [44], but there is no evidence that it inhibits same-sex mating within populations of MAT a cells. One difference between C. albicans and S. cerevisiae that is relevant here is that many S. cerevisiae cells undergo mating-type switching but C. albicans strains do not [45]. Thus, S. cerevisiae strains expressing HO endonuclease are competent for mating-type switching and thus exhibit pseudo-homothallism and inbreeding within the population. Given that mating-type switching does not occur in C. albicans, potential autocrine signaling and same-sex mating offer an alternative mechanism by which these strains can undergo sexual reproduction even in unisexual populations. Clearly, it will be revealing to see if Bar1 also regulates homothallic mating in other species from the Candida clade, and if this protease evolved to limit such mating events in yeast.
How does mating take place if the majority of natural isolates are a/α and hence unable to switch to opaque? One interesting possibility is that even a/α cells may be able to form opaques under certain conditions in vivo. Decreased expressed of HBR1 (hemoglobin response gene 1) has been shown to alter expression of the MTL transcription factors a1, α1, and α2. The result of altered MTL expression is that a/α cells behave phenotypically as a cells, and are therefore able to switch to opaque and undergo mating [46]. It is therefore possible that limiting Hbr1 expression in vivo, perhaps in response to a decrease in hemoglobin levels, could permit mating by a/α cells. This hypothesis is intriguing given the recent discovery of a mechanism for same-sex mating in MTL a cells, as it suggests that a/α cells may be able to undergo homothallic mating in the mammalian host.
What advantages could a parasexual cycle provide for C. albicans in contrast to a traditional meiotic program? One hypothesis is that a conventional fungal meiosis results in the formation of spores, and creating these highly antigenic structures may be undesirable for a organism with a commensal lifestyle [47]. A second possibility is that the aneuploid products formed by the parasexual process provide an advantage for C. albicans, which has shown a remarkable ability to utilize aneuploid chromosomes. In fact, aneuploidy is a common method by which C. albicans can adapt to a variety of environmental stresses. By proceeding through a parasexual cycle rather than a traditional meiosis C. albicans may allow multiple aneuploid combinations to be generated, and those with increased fitness could be selected for after completion of the mating process.
Comparative analysis of the MTL locus in the Candida clade
Cell-type identity in yeast is controlled by transcription factors expressed at the MAT/MTL locus. In the case of C. albicans, a2 is required for expression of a-specific genes, α1 is required for expression of α-specific genes, and a1/α2 acts to inhibit mating (and white-opaque switching) in a/α cells [48]. This appears to be the ancestral configuration for cell-type control in the hemiascomycete yeast. In contrast, in S. cerevisiae the a2 gene has been lost and a-specific genes are expressed by default. In addition, α cells repress a-specific genes through the action of α2 and Mcm1, although α-specific genes are under the control of the α1 gene, similar to C. albicans [49]. All of the Candida clade species also contain homologs of the PAP, PIK, and OBP genes at the MTL locus, while these genes are absent from the MTL loci of other hemiascomycete yeast, including S. cerevisiae [5].
Examination of the MTL locus amongst the Candida clade has revealed a surprising variation in the genes present at this locus. For example, in the branch of the tree containing Candida guilliermondii and Debaryomyces hansenii the α2 gene has been lost, while C. guilliermondii has also lost the a1 gene [5]. The absence of these factors is not due to loss of mating in these species, as both C. guilliermondii and D. hansenii undergo sexual reproduction. Similarly, C. parapsilosis lacks an intact a1 gene, although mating has yet to been identified in this species (discussed below). The most striking change in MTL configuration, however, has occurred in Lodderomyces elongisporus. This species also belongs to the Candida clade and was recently isolated from bloodstream infections [50], yet is lacking all four MTL transcription factors. Despite this deficiency, L. elongisporus is still thought to undergo a complete sexual cycle. These changes at the MTL loci further underline the plasticity of sexual regulation, and will require further studies to establish how cell type is defined in species with altered complements of transcriptional regulators.
Evidence for mating and sexual reproduction in the Candida clade
Several Candida species have now been shown to undergo mating and meiosis, while for others population studies indicate that sexual reproduction is at least a possibility. The species most closely related to C. albicans, Candida dubliniensis, exhibits strong similarities in its mating program, including the necessity for white-opaque switching [51]. So far, these are the only two species in which white-opaque switching has been observed. Although many fungal species contain homologs of WOR1, it appears that the function of this gene varies significantly from species to species. For example, expression of C. albicans WOR1 in S. cerevisiae resulted in increased adherence to polystyrene [52]. In contrast, in the distantly related basidiomycete Histoplasma capsulatum, the WOR1 homolog RYP1 is involved in regulating the yeast-hyphal transition [53, 54]. Furthermore, in the filamentous ascomycete Fusarium oxysporum, the WOR1 homolog SGE1 does not regulate a morphological switch, but is a putative regulator of genes necessary for infection of the plant host [55]. Clearly then, Wor1 homologs can function in a variety of pathways, but aside from these few examples little is known about this family of transcription factors, and function has not been described in other species from the Candida clade.
In the case of C. dubliniensis, work from the Soll group has demonstrated that MTL a/α, MTL a/a, and MTLα/α strains exist, with a higher proportion of natural a/a or α/α isolates than C. albicans. It seems likely that the mating cycle in C. dubliniensis is completed by a similar parasexual mechanism to that of C. albicans, and in fact opaque cells of C. albicans and C. dubliniensis can readily mate with one another, although it is not clear that such interspecies crosses occur in nature [51]. C. dubliniensis is also a comparatively rare pathogen of humans despite its close relationship to C. albicans, and genomic studies indicate this may be due to expansion of the TLO and IFA gene families only in the C. albicans lineage [56]. These families encode for putative transcription factors and transmembrane proteins respectively, with TLO genes linked to the control of morphogenesis in both C. albicans and C. dubliniensis [56].
The haploid species Candida lusitaniae and Candida guilliermondii are the closest relatives to C. albicans that have defined sexual cycles that cumulate in meiosis and the formation of ascospores [5, 57]. Both Candida species are potential human pathogens, but are only rarely encountered in the clinic [7]. In fact, the haploid species from the Candida clade (Cl, Cg, and Dh) are infrequent pathogens when compared to the diploid Candida species (Ca, Cp, and Ct, in particular) (see Fig. 1). It remains to be seen if ploidy changes enhanced their development as human pathogens, perhaps by promoting the expansion of gene families associated with virulence.
A recent study by Reedy and Heitman examined the mating cycle of C. lusitaniae in detail [57]. Despite this species lacking many of the genes necessary for meiosis in S. cerevisiae (including the master regulator IME1 and multiple components of the synaptonemal complex), C. lusitaniae was able to undergo efficient sporulation and associated recombination. Spo11 was integral to both processes, consistent with C. lusitaniae exhibiting a true meiotic program [57]. Curiously, sporulation in C. lusitaniae resulted in the formation of dyads as opposed to tetrads typically formed during S. cerevisiae sporulation. Furthermore, a large fraction (~30%) of C. lusitaniae spores contained aneuploid or diploid nuclei, rather than true haploid nuclei. It is possible that the absence of many conserved meiotic components has reduced the fidelity of meiosis in C. lusitaniae, thereby leading to increased rates of chromosome nondisjunction. Further understanding of the molecular mechanism of meiosis in C. lusitaniae may therefore shed light on the most fundamental aspects of this process.
The only diploid species from the Candida clade shown to undergo mating (other than C. albicans/C. dubliniensis) is the homothallic organism L. elongisporus. This species is a conundrum; reports suggest it can form ascus structures from a single yeast cell (an indication of homothallic mating) [50, 58], and yet the MTL locus has lost all of the transcription factors that regulate mating in other yeast [5]. If L. elongisporus does mate, it may involve a mechanism of autocrine pheromone signaling analogous to that in C. albicans ([38] and Fig. 2). The L. elongisporus genome contains only one type of pheromone gene (encoding α-pheromone) and one pheromone receptor gene (homolog of STE2, encoding the α-pheromone receptor). Confirmation that autocrine pheromone signaling regulates mating in L. elongisporus would also suggest that this mechanism is likely to be encountered in the mating cycles of other homothallic fungi.
Are there truly asexual species in the Candida clade?
Sexual cycles have yet to be identified for several Candida clade species, including the important human pathogens C. parapsilosis and C. tropicalis. Recent population studies have indicated that some ‘asexual’ Candida species may be recombining, or have at least undergone sexual recombination in the recent past. In the case of C. tropicalis, this species contains a similar repertoire of mating and meiosis genes to C. albicans with two notable exceptions. One, C. tropicalis lacks the GTT1 gene involved in spore assembly, and two, this species contains ten genes all predicted to encode a-pheromones [5]. It remains to be seen if the genes implicated in mating and meiosis are retained in C. tropicalis because of a yet undiscovered sexual cycle, or because they have been reprogrammed for a different function. The presence of large stretches of homozygosity within the genome, however, suggests that a sexual cycle could exist [5]. In addition, multi-locus sequence typing (MLST) studies of C. tropicalis isolates resemble those of C. albicans; isolates exhibit a largely clonal mode of reproduction but with sufficient recombination to implicate sexual reproduction [59].
For the closely related species C. parapsilosis, the picture is even less clear. C. parapsilosis fails to encode a functional a1 gene [60], although absence of a1 does not preclude sexual reproduction as seen in C. guilliermondii. However, the low numbers of SNPs (single nucleotide polymorphisms) and the lack of long stretches of homozygosity indicate the absence of heterothallic reproduction, although a modified homothallic cycle cannot be ruled out [5]. In contrast, two closely related species to C. parapsilosis, C. metapsilosis and C. orthopsilosis, have population structures that are highly recombinant consistent with a sexual cycle [61, 62]. For example, amplified fragment length polymorphism analysis (AFLP) of C. metapsilosis isolates showed a high percentage (80%) of polymorphic bands and pairwise genetic distances were consistent with recombination and possible sexual reproduction. In contrast, C. parapsilosis showed a predominance (>80%) of monomorphic bands, supporting a strictly clonal population structure [61]. The third family member, C. orthopsilosis, shows an intermediate level of polymorphic AFLP fragments (75%) and analysis of pairwise genetic distances provides evidence for both clonality and recombination [62]. Clearly, further analysis of these species, including direct experimental approaches, will be needed to confirm if sexual reproduction is occurring in these species.
Alternative uses for sexual machinery in Candida species
The benefits for organisms undergoing sexual reproduction are thought to include increased recombination and hence the ability to adapt to changing conditions [63]. It is now evident, however, that the complex molecular processes involved in sexual reproduction can also be beneficial for other functions in the cell. In extreme examples, it is possible that sexual machinery has been retained for uses other than mating and recombination. This discussion is particularly relevant to species from the Candida clade, where mating may have been lost in members of the clade but mating-related functions retained for processes relevant for pathogenesis.
One example of this functional overlap is the white-opaque phenotypic switch in C. albicans; switching to opaque is necessary for mating but opaque cells are also better suited for evasion of the host immune system. As mentioned earlier, white cells, but not opaque cells, secrete a chemoattractant for polymorphonuclear lymphocytes (PMNs), and white cells are also phagocytosed more efficiently than opaque cells by macrophages [15, 17]. Thus, independent of its role in regulating mating, switching to the opaque form may provide a direct benefit for C. albicans cells by enabling them to avoid clearance by host defenses [64]. One caveat to this argument, however, is that opaque cells appear to be more sensitive to oxidative stress, and so under some conditions may actually be more susceptible to killing by phagocytic cells than white cells [65].
A second example of the use of the mating apparatus for a non-reproductive function involves the pheromone-signaling pathway in yeast. In both S. cerevisiae and C. albicans, components of the pheromone MAPK (mitogen-activated protein kinase) cascade are also required for filamentation in response to environmental cues [66, 67]. The outputs of these two pathways appear very different at first sight; one results in activation of the mating program while the other leads to hyphal (or pseudohyphal) growth. However, this signaling configuration may be beneficial in some instances, as low concentrations of pheromone induce S. cerevisiae to undergo a form of filamentous growth that can also enhance their ability to find a mating partner [68].
In C. albicans, downstream targets of the pheromone-signaling cascade have also been shown to play an important function in non-mating cells. In the opaque state, cells can both secrete pheromones and respond morphologically to pheromones by undergoing polarized growth. In contrast, white cells do not obviously secrete pheromones but can still mount their own unique response to pheromones produced by opaque cells. White cells exhibit increased adhesion and biofilm formation on synthetic surfaces due to the upregulation of multiple factors that are not induced in opaque cells [69–72]. It is speculated that the differential response of white and opaque cells may have evolved to promote mating in the mammalian host. Biofilm formation by responding white cells could stabilize pheromone gradients produced by opaque cells and enable potential opaque partners to come together across greater distances [69]. Recent studies have suggested that this mechanism is not limited to mixed populations of mating cells, but that pure populations of MTL a white cells may also be able to auto-activate the pheromone response and thereby enhance their ability to form biofilms [71, 72]. The mechanism by which white a cells undergo auto-activation of pheromone signaling has yet to be demonstrated, but could involve autocrine signaling similar to that uncovered in opaque cells (see Fig. 2; [38]).
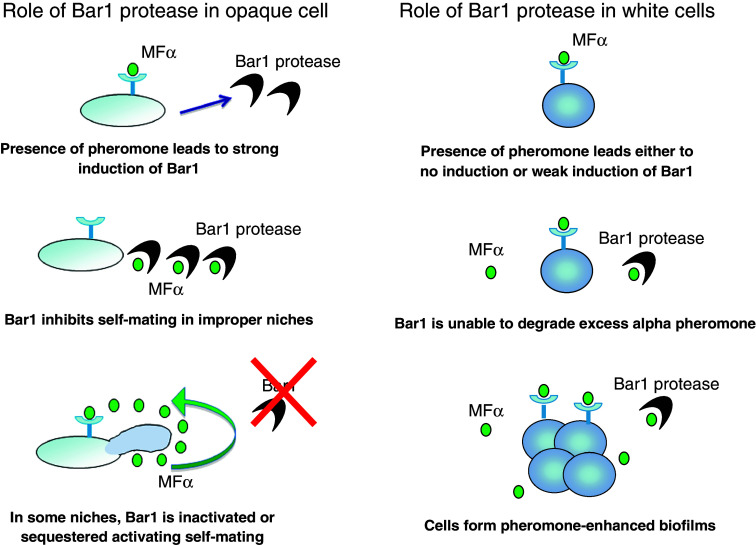
A closer inspection of white and opaque cells suggests that the Bar1 protease may play an important but differential role in regulating the response to pheromone in these two cell types. The BAR1 gene is highly induced in opaque a cells treated with α-pheromone, while it is either not induced or is induced only weakly in white cells [73, 74]. As a result, high levels of Bar1 act to inhibit same-sex mating between opaque a cells, while inhibition or sequestration of Bar1 is predicted to promote self-mating in certain host niches (Fig. 3). In contrast, the limited expression of Bar1 in white cells may be easily overcome by α-pheromone, leading to activation of the signaling cascade and more efficient biofilm formation. White a cells may therefore be generally sensitive to α-pheromone due to the lower levels of Bar1 secreted, a model that is now testable. Additional studies will be necessary to determine whether white cells are able to respond to pheromone produced endogenously (i.e., via autocrine signaling), or whether white cells require the presence of pheromone-secreting opaque cells in order to undergo enhanced biofilm formation.
Fig. 3.
Model for the role of Bar1 protease in white and opaque MTL a cells of C. albicans. Opaque cells exposed to pheromone highly upregulate the expression of the BAR1 gene. This upregulation results in sufficient secretion of Bar1 protease to prevent auto-activation of the mating program. However, it is predicted that in certain niches Bar1 activity is sequestered/degraded allowing for opaque cells to initiate autocrine pheromone signaling and undergo efficient same-sex mating. In contrast, when white cells are exposed to pheromone, they do not respond by upregulating expression of BAR1. It is therefore possible that the level of secreted Bar1 is insufficient to degrade exogenous α-pheromone, making these cells highly sensitive to the effects of pheromone and allowing for pheromone-enhanced biofilm formation to occur
Overall, these experiments indicate an unexpectedly close link between the mechanisms involved in mating, phenotypic switching, and biofilm formation in C. albicans. Given that biofilm formation is both a major virulence determinant for C. albicans and also mediates drug resistance [75–77], these studies will have important ramifications for understanding the wider role of pheromone signaling in pathogenesis.
Conclusions
The Candida clade of species provides a challenging backdrop for understanding sexual reproduction. While originally designated as obligate asexual organisms, this clade is now recognized as containing fully sexual species, parasexual species, and those with population structures that are consistent with yet undiscovered sexual cycles. It therefore seems likely that strictly asexual species are the exception, rather than the rule, in this collection of medically important fungi.
Furthermore, even within the Candida clade there is now evidence for a wide variety of mechanisms regulating sexual biology. The most dramatic example of this is seen in C. albicans and C. dubliniensis where mating is uniquely regulated by white-opaque switching. Additional differences are seen at the MTL loci, where transcriptional regulators of cell identity have been lost in some species, or else reprogrammed to work via alternative transcriptional circuits. The molecular machinery involved in directing meiosis in species such as C. lusitaniae is also quite distinct from that in model yeast. Taken together, these findings emphasize the plasticity inherent in sexual programming, and that studies of the Candida clade of species are likely to unveil further revelations about mechanisms regulating mating and meiosis.
Finally, we emphasize that accumulating evidence shows that sexual biology is not limited to the generation of recombinant progeny. Candida species have not only evolved distinct ways of undergoing sexual reproduction but often utilize sexual processes for other functions associated with their diverse lifestyles. In several cases, there are now direct observations linking mating-related mechanisms with pathogenesis, particularly with respect to their roles in filamentation, adhesion, and biofilm formation. Given the importance of these mechanisms for infection, we propose that the pleiotropic use of sexual processes is also likely to be uncovered when analyzing the function of ‘mating-specific’ genes in other microbial organisms.
Acknowledgments
Work in the author’s laboratory was supported by a PATH award from the Burroughs Wellcome Fund (RJB) as well as the NIH (R21AI081560 to RJB and F31DE019752 to KA). We thank Racquel Sherwood for comments on the manuscript.
References
- 1.Gargas A, Taylor JW. Phylogeny of Discomycetes and early radiations of the apothecial Ascomycotina inferred from SSU rDNA sequence data. Exp Mycol. 1995;19:7–15. doi: 10.1006/emyc.1995.1002. [DOI] [PubMed] [Google Scholar]
- 2.Hedges SB. The origin and evolution of model organisms. Nat Rev Genet. 2002;3:838–849. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- 3.Heitman J, Kronstad JW, Taylor JW, Casselton LA (2007) Sex in fungi: molecular determination and evolutionary implications. ASM Press
- 4.Butler G. Fungal sex and pathogenesis. Clin Microbiol Rev. 2010;23:140–159. doi: 10.1128/CMR.00053-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, Agrafioti I, Arnaud MB, Bates S, Brown AJ, Brunke S, Costanzo MC, Fitzpatrick DA, de Groot PW, Harris D, Hoyer LL, Hube B, Klis FM, Kodira C, Lennard N, Logue ME, Martin R, Neiman AM, Nikolaou E, Quail MA, Quinn J, Santos MC, Schmitzberger FF, Sherlock G, Shah P, Silverstein KA, Skrzypek MS, Soll D, Staggs R, Stansfield I, Stumpf MP, Sudbery PE, Srikantha T, Zeng Q, Berman J, Berriman M, Heitman J, Gow NA, Lorenz MC, Birren BW, Kellis M, Cuomo CA. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos MA, Tuite MF. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans . Nucleic Acids Res. 1995;23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 9.Ruhnke M, Maschmeyer G. Management of mycoses in patients with hematologic disease and cancer—review of the literature. Eur J Med Res. 2002;7:227–235. [PubMed] [Google Scholar]
- 10.Hull CM, Johnson AD. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans . Science. 1999;285:1271–1275. doi: 10.1126/science.285.5431.1271. [DOI] [PubMed] [Google Scholar]
- 11.Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- 12.Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- 13.Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. “White-opaque transition”: a second high-frequency switching system in Candida albicans . J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kvaal CA, Srikantha T, Soll DR. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun. 1997;65:4468–4475. doi: 10.1128/iai.65.11.4468-4475.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger J, Wessels D, Lockhart SR, Soll DR. Release of a potent polymorphonuclear leukocyte chemoattractant is regulated by white-opaque switching in Candida albicans . Infect Immun. 2004;72:667–677. doi: 10.1128/IAI.72.2.667-677.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun. 1999;67:6652–6662. doi: 10.1128/iai.67.12.6652-6662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohse MB, Johnson AD. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS One. 2008;3:e1473. doi: 10.1371/journal.pone.0001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/S0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- 19.Lockhart SR, Pujol C, Daniels KJ, Miller MG, Johnson AD, Pfaller MA, Soll DR. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics. 2002;162:737–745. doi: 10.1093/genetics/162.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odds FC, Bougnoux ME, Shaw DJ, Bain JM, Davidson AD, Diogo D, Jacobsen MD, Lecomte M, Li SY, Tavanti A, Maiden MC, Gow NA, d’Enfert C. Molecular phylogenetics of Candida albicans . Eukaryot Cell. 2007;6:1041–1052. doi: 10.1128/EC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikantha T, Borneman AR, Daniels KJ, Pujol C, Wu W, Seringhaus MR, Gerstein M, Yi S, Snyder M, Soll DR. TOS9 regulates white-opaque switching in Candida albicans . Eukaryot Cell. 2006;5:1674–1687. doi: 10.1128/EC.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans . Proc Natl Acad Sci USA. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zordan RE, Galgoczy DJ, Johnson AD. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci USA. 2006;103:12807–12812. doi: 10.1073/pnas.0605138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lachke SA, Srikantha T, Soll DR. The regulation of EFG1 in white-opaque switching in Candida albicans involves overlapping promoters. Mol Microbiol. 2003;48:523–536. doi: 10.1046/j.1365-2958.2003.t01-1-03448.x. [DOI] [PubMed] [Google Scholar]
- 25.Srikantha T, Tsai LK, Daniels K, Soll DR. EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J Bacteriol. 2000;182:1580–1591. doi: 10.1128/JB.182.6.1580-1591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinces MD, Haas C, Kumamoto CA. Expression of the Candida albicans morphogenesis regulator gene CZF1 and its regulation by Efg1p and Czf1p. Eukaryot Cell. 2006;5:825–835. doi: 10.1128/EC.5.5.825-835.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zordan RE, Miller MG, Galgoczy DJ, Tuch BB, Johnson AD. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans . PLoS Biol. 2007;5:e256. doi: 10.1371/journal.pbio.0050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumitru R, Navarathna DH, Semighini CP, Elowsky CG, Dumitru RV, Dignard D, Whiteway M, Atkin AL, Nickerson KW. In vivo and in vitro anaerobic mating in Candida albicans . Eukaryot Cell. 2007;6:465–472. doi: 10.1128/EC.00316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez-Zavala B, Reuss O, Park YN, Ohlsen K, Morschhauser J. Environmental induction of white-opaque switching in Candida albicans . PLoS Pathog. 2008;4:e1000089. doi: 10.1371/journal.ppat.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang G, Srikantha T, Sahni N, Yi S, Soll DR. CO2 regulates white-to-opaque switching in Candida albicans . Curr Biol. 2009;19:330–334. doi: 10.1016/j.cub.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alby K, Bennett RJ. Stress-induced phenotypic switching in Candida albicans . Mol Biol Cell. 2009;20:3178–3191. doi: 10.1091/mbc.E09-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang G, Yi S, Sahni N, Daniels KJ, Srikantha T, Soll DR. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010;6:e1000806. doi: 10.1371/journal.ppat.1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dignard D, El-Naggar AL, Logue ME, Butler G, Whiteway M. Identification and characterization of MFA1, the gene encoding Candida albicans a-factor pheromone. Eukaryot Cell. 2007;6:487–494. doi: 10.1128/EC.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett RJ, Uhl MA, Miller MG, Johnson AD. Identification and characterization of a Candida albicans mating pheromone. Mol Cell Biol. 2003;23:8189–8201. doi: 10.1128/MCB.23.22.8189-8201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panwar SL, Legrand M, Dignard D, Whiteway M, Magee PT. MFalpha1, the gene encoding the alpha mating pheromone of Candida albicans . Eukaryot Cell. 2003;2:1350–1360. doi: 10.1128/EC.2.6.1350-1360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lockhart SR, Daniels KJ, Zhao R, Wessels D, Soll DR. Cell biology of mating in Candida albicans . Eukaryot Cell. 2003;2:49–61. doi: 10.1128/EC.2.1.49-61.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett RJ, Miller MG, Chua PR, Maxon ME, Johnson AD. Nuclear fusion occurs during mating in Candida albicans and is dependent on the KAR3 gene. Mol Microbiol. 2005;55:1046–1059. doi: 10.1111/j.1365-2958.2005.04466.x. [DOI] [PubMed] [Google Scholar]
- 38.Alby K, Schaefer D, Bennett RJ. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans . Nature. 2009;460:890–893. doi: 10.1038/nature08252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett RJ, Johnson AD. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 2003;22:2505–2515. doi: 10.1093/emboj/cdg235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forche A, Alby K, Schaefer D, Johnson AD, Berman J, Bennett RJ. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 2008;6:e110. doi: 10.1371/journal.pbio.0060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim AS, Magee BB, Sheppard DC, Yang M, Kauffman S, Becker J, Edwards JE, Jr, Magee PT. Effects of ploidy and mating type on virulence of Candida albicans . Infect Immun. 2005;73:7366–7374. doi: 10.1128/IAI.73.11.7366-7374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keeney S, Neale MJ. Initiation of meiotic recombination by formation of DNA double-strand breaks: mechanism and regulation. Biochem Soc Trans. 2006;34:523–525. doi: 10.1042/BST0340523. [DOI] [PubMed] [Google Scholar]
- 43.Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans . Science. 2006;313:367–370. doi: 10.1126/science.1128242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manney TR. Expression of the BAR1 gene in Saccharomyces cerevisiae: induction by the alpha mating pheromone of an activity associated with a secreted protein. J Bacteriol. 1983;155:291–301. doi: 10.1128/jb.155.1.291-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler G, Kenny C, Fagan A, Kurischko C, Gaillardin C, Wolfe KH. Evolution of the MAT locus and its Ho endonuclease in yeast species. Proc Natl Acad Sci USA. 2004;101:1632–1637. doi: 10.1073/pnas.0304170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pendrak ML, Yan SS, Roberts DD. Hemoglobin regulates expression of an activator of mating-type locus alpha genes in Candida albicans . Eukaryot Cell. 2004;3:764–775. doi: 10.1128/EC.3.3.764-775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen K, Heitman J. Sex and virulence of human pathogenic fungi. Adv Genet. 2007;57:143–173. doi: 10.1016/S0065-2660(06)57004-X. [DOI] [PubMed] [Google Scholar]
- 48.Tsong AE, Miller MG, Raisner RM, Johnson AD. Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell. 2003;115:389–399. doi: 10.1016/S0092-8674(03)00885-7. [DOI] [PubMed] [Google Scholar]
- 49.Tsong AE, Tuch BB, Li H, Johnson AD. Evolution of alternative transcriptional circuits with identical logic. Nature. 2006;443:415–420. doi: 10.1038/nature05099. [DOI] [PubMed] [Google Scholar]
- 50.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. Lodderomyces elongisporus masquerading as Candida parapsilosis as a cause of bloodstream infections. J Clin Microbiol. 2008;46:374–376. doi: 10.1128/JCM.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pujol C, Daniels KJ, Lockhart SR, Srikantha T, Radke JB, Geiger J, Soll DR. The closely related species Candida albicans and Candida dubliniensis can mate. Eukaryot Cell. 2004;3:1015–1027. doi: 10.1128/EC.3.4.1015-1027.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li F, Palecek SP. Identification of Candida albicans genes that induce Saccharomyces cerevisiae cell adhesion and morphogenesis. Biotechnol Prog. 2005;21:1601–1609. doi: 10.1021/bp050236c. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen VQ, Sil A. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci USA. 2008;105:4880–4885. doi: 10.1073/pnas.0710448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webster RH, Sil A. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum . Proc Natl Acad Sci USA. 2008;105:14573–14578. doi: 10.1073/pnas.0806221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michielse CB, van Wijk R, Reijnen L, Manders EM, Boas S, Olivain C, Alabouvette C, Rep M. The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 2009;5:e1000637. doi: 10.1371/journal.ppat.1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson AP, Gamble JA, Yeomans T, Moran GP, Saunders D, Harris D, Aslett M, Barrell JF, Butler G, Citiulo F, Coleman DC, de Groot PW, Goodwin TJ, Quail MA, McQuillan J, Munro CA, Pain A, Poulter RT, Rajandream MA, Renauld H, Spiering MJ, Tivey A, Gow NA, Barrell B, Sullivan DJ, Berriman M. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans . Genome Res. 2009;19:2231–2244. doi: 10.1101/gr.097501.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reedy JL, Floyd AM, Heitman J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr Biol. 2009;19:891–899. doi: 10.1016/j.cub.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Walt JP. Lodderomyces, a new genus of the Saccharomycetaceae. Antonie Van Leeuwenhoek. 1966;32:1–5. doi: 10.1007/BF02097439. [DOI] [PubMed] [Google Scholar]
- 59.Jacobsen MD, Davidson AD, Li SY, Shaw DJ, Gow NA, Odds FC. Molecular phylogenetic analysis of Candida tropicalis isolates by multi-locus sequence typing. Fungal Genet Biol. 2008;45:1040–1042. doi: 10.1016/j.fgb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Logue ME, Wong S, Wolfe KH, Butler G. A genome sequence survey shows that the pathogenic yeast Candida parapsilosis has a defective MTLa1 allele at its mating type locus. Eukaryot Cell. 2005;4:1009–1017. doi: 10.1128/EC.4.6.1009-1017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hensgens LA, Tavanti A, Mogavero S, Ghelardi E, Senesi S. AFLP genotyping of Candida metapsilosis clinical isolates: evidence for recombination. Fungal Genet Biol. 2009;46:750–758. doi: 10.1016/j.fgb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Tavanti A, Hensgens LA, Ghelardi E, Campa M, Senesi S. Genotyping of Candida orthopsilosis clinical isolates by amplification fragment length polymorphism reveals genetic diversity among independent isolates and strain maintenance within patients. J Clin Microbiol. 2007;45:1455–1462. doi: 10.1128/JCM.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goddard MR, Godfray HC, Burt A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature. 2005;434:636–640. doi: 10.1038/nature03405. [DOI] [PubMed] [Google Scholar]
- 64.Magee PT, Magee BB. Through a glass opaquely: the biological significance of mating in Candida albicans . Curr Opin Microbiol. 2004;7:661–665. doi: 10.1016/j.mib.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Kolotila MP, Diamond RD. Effects of neutrophils and in vitro oxidants on survival and phenotypic switching of Candida albicans WO-1. Infect Immun. 1990;58:1174–1179. doi: 10.1128/iai.58.5.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magee BB, Legrand M, Alarco AM, Raymond M, Magee PT. Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans . Mol Microbiol. 2002;46:1345–1351. doi: 10.1046/j.1365-2958.2002.03263.x. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz MA, Madhani HD. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae . Annu Rev Genet. 2004;38:725–748. doi: 10.1146/annurev.genet.39.073003.112634. [DOI] [PubMed] [Google Scholar]
- 68.Erdman S, Snyder M. A filamentous growth response mediated by the yeast mating pathway. Genetics. 2001;159:919–928. doi: 10.1093/genetics/159.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. Opaque cells signal white cells to form biofilms in Candida albicans . EMBO J. 2006;25:2240–2252. doi: 10.1038/sj.emboj.7601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahni N, Yi S, Pujol C, Soll DR. The white cell response to pheromone is a general characteristic of Candida albicans strains. Eukaryot Cell. 2009;8:251–256. doi: 10.1128/EC.00320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi S, Sahni N, Pujol C, Daniels KJ, Srikantha T, Ma N, Soll DR. A Candida albicans-specific region of the alpha-pheromone receptor plays a selective role in the white cell pheromone response. Mol Microbiol. 2009;71:925–947. doi: 10.1111/j.1365-2958.2008.06575.x. [DOI] [PubMed] [Google Scholar]
- 72.Sahni N, Yi S, Daniels KJ, Srikantha T, Pujol C, Soll DR. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans . PLoS Pathog. 2009;5:e1000601. doi: 10.1371/journal.ppat.1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaefer D, Cote P, Whiteway M, Bennett RJ. Barrier activity in Candida albicans mediates pheromone degradation and promotes mating. Eukaryot Cell. 2007;6:907–918. doi: 10.1128/EC.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennett RJ, Johnson AD. The role of nutrient regulation and the Gpa2 protein in the mating pheromone response of C. albicans . Mol Microbiol. 2006;62:100–119. doi: 10.1111/j.1365-2958.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 75.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramage G, VandeWalle K, Bachmann SP, Wickes BL, Lopez-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46:3634–3636. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramage G, Wickes BL, Lopez-Ribot JL. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am Clin Lab. 2001;20:42–44. [PubMed] [Google Scholar]