Abstract
Stable E-cadherin-based adherens junctions are pivotal in maintaining epithelial tissue integrity and are the major barrier for epithelial cancer metastasis. Proteins of the p120ctn subfamily have emerged recently as critical players for supporting this stability. The identification of the unique juxtamembrane domain (JMD) in E-cadherin that binds directly to δ-catenin/NPRAP/Neurojungin (CTNND2) and p120ctn (CTNND1) provides a common motif for their interactions. Recently, crystallographic resolution of the JMD of p120ctn further highlighted possibilities of intervening between interactions of p120ctn subfamily proteins and E-cadherin for designing anti-cancer therapeutics. For most epithelial cancers, studies have demonstrated a reduction of p120ctn expression or alteration of its subcellular distribution. On the other hand, δ-catenin, a primarily neural-enriched protein in the brain of healthy individuals is upregulated in all cancer types that have been studied to date. Two research articles in this issue of Journal of Pathology increase our understanding of the involvement of these proteins in lung cancer. One reports the identification of rare p120ctn (CTNND1) gene amplification in lung cancer. One mechanism by which δ-catenin and p120ctn may play a role in carcinogenesis is their competitive binding to E-cadherin through the JMD. The other presents the first vigorous characterization of δ-catenin overexpression in lung cancer. Unexpectedly, the authors observed that δ-catenin promotes malignant phenotypes of non-small cell lung cancer by non-competitive binding to E-cadherin with p120ctn in the cytoplasm. Looking towards the future, the understanding of δ-catenin and p120ctn beyond their localization at the cell-cell junction should provide further insight into their roles in cancer pathogenesis.
Keywords: E-cadherin, δ-catenin (CTNND2), p120ctn (CTNND1), juxtamembrane domain (JMD), gene amplification, adherens junction in cancer
E-cadherin-based adherens junctions are one of the most recognized structures in maintaining epithelial tissue integrity and play crucial roles in both embryonic development and cancer [1, 2]. The stabilization of E-cadherin is believed to be controlled by interactions with its associated cytoplasmic proteins called catenins (e.g. α-, β-, γ-, and δ-catenin, APC, and p120ctn).
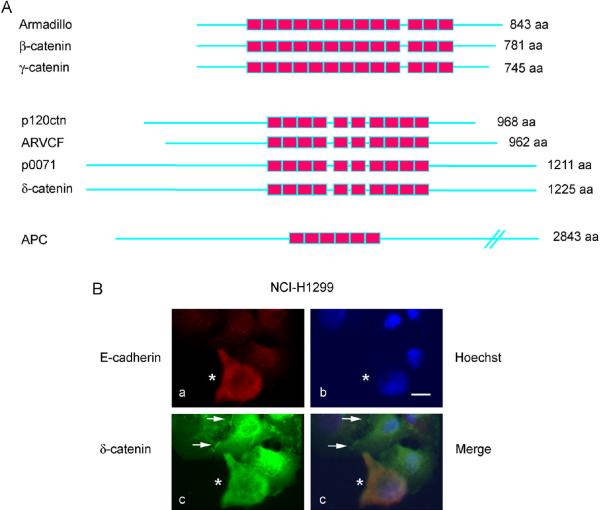
Initially identified as a prominent substrate for non-receptor tyrosine kinase Src, p120ctn (with the gene designation CTNND1) is now widely known as an important E-cadherin-associated protein that is involved in the regulation of E-cadherin stability and cell adhesion [3], gene expression through complex formation with Kaiso [4], and cell motility by modulating Rho family small GTPases [5]. p120ctn is considered as the prototype of subgroup proteins within the β-catenin/armadillo superfamily [6]. While all proteins in this superfamily display distinct 42–43 amino acid repeating sequences called armadillo domains, p120ctn subgroup proteins carry 10 armadillo repeating sequences instead of 13 such repeating units in β-catenin (Fig 1A). Up to now, there are four well-known members in the p120ctn subgroup, i.e. p120ctn, ARVCF (Armadillo Repeat gene deleted in Velo-Cardio-Facial syndrome), δ-catenin (Neuroplakofilin-Related Armadillo Protein [NPRAP]/Neurojungin), and p0071 (plakophilin-4) [7].
Figure 1. δ-Catenin and its potential interactions with E-cadherin in cancer.
A. Schematic illustration of δ-catenin in comparison to other armadillo-domain containing proteins. While β-catenin and γ-catenin display 13 armadillo repeating units and Adenomatous Polyposis Coli (APC) shows 6 such repeating units, δ-catenin, p120ctn, ARVCF, and p0071 carry 10 repeating units. B. Immunofluorescent light microscopy showing partial co-distribution of δ-catenin with E-cadherin in lung cancer cell line NCI-H1299. a. Anti-E-cadherin. b. Hoechst staining to show nuclear morphology. c. EGFP-δ-catenin. d. Merged image of a, b, and c. Stable NCI-H1299 cells expressing EGFP-δ-catenin were transiently transfected with human E-cadherin plasmid and immunostained using mouse anti-E-cadherin. Asterisks indicate the cell transfected with E-cadherin. Arrows point to the cell-cell junctions that can be observed in some cells. Note: ectopic expressed E-cadherin fails to localize to plasma membrane and remains in the cytoplasm. Bar: 10 μm.
While ARVCF and p0071 are clearly distinguished from p120ctn, the designation of δ-catenin has been somewhat confusing. When a partial sequence of δ-catenin was first cloned, it was assigned gene symbol as CTNND2 [8]. CTNND, or the delta subfamily of the catenins, now contains two genes, p120ctn (CTNND1) located on chromosome 11 (q12.1) and δ-catenin (CTNND2) located on chromosome 5 (p15.2). Although they share over 30% amino acid identity in their armadillo domain, the amino- and carboxyl- terminal sequences flanking the armadillo domains are very different from each other. In fact, δ-catenin shares much closer amino acid similarity to p0071 than to p120ctn in mammalian cells.
In epithelial cancers, E-cadherin is often down-regulated with few exceptions (e.g. ovarian cancer) [9]. While many studies attributed the loss of E-cadherin to genetic inactivation or epigenetic silencing, evidence that p120ctn plays important roles in stabilizing E-cadherin at the cell-cell junction is mounting [3]. Therefore, it is not surprising that for most epithelial cancers, studies have demonstrated a concomitant reduction of p120ctn expression or alteration of its subcellular distribution with E-cadherin [10]. Studies of cell cultures as well as animal tissues also support that p120ctn plays tumour suppressive roles. However, in this issue of The Journal of Pathology, Castillo et al [11] investigated gene amplification of two chromosomal regions in lung tumours and identified TFDP1 and CTNND1 as potential oncogenes. The TFDP1 gene, located in 13q34, showed at least 100 times higher overexpression in 3% of the tumours tested. Depletion of TFDP1 expression, by small interfering RNA in lung cancer cell lines carrying TFDP1 amplification, reduced cell viability supporting the E2F-associated transcription factor DP1 [12] as the candidate oncogene at 13q34.
Identification of CTNND1 amplification has important implications for its functions in cancer progression as well. Although the gene copy numbers are not as high as TFDP1, immunohistochemistry to detect p120ctn expression correlated very well with its gene amplification in the 3% tumours that showed the amplification. No evidence was presented that supported the idea that different p120ctn isoforms are the underlying cause for protein overexpression, nor that the overexpressed p120ctn showed predominantly non-membranous, cytoplasmic subcellular distribution in the lung tumours with gene amplification. These observations implied that overexpressed p120ctn might still be able to interact with E-cadherin at the cell-cell junction.
Accumulating evidence indicates that p120ctn could play the role of a tumour suppressor or metastasis promoter, depending on whether it is down-regulated before or after E-cadherin is down-regulated and lost from the cell-cell junction [10]. It was postulated that if E-cadherin loss precedes p120ctn loss, p120ctn could remain stranded in the cytoplasm and promote cell invasion and metastasis through regulation of Rho family small GTPases. Thus, p120ctn may function as a metastasis promoter under these conditions. If p120ctn loss precedes E-cadherin loss, then it may become the initial event leading ultimately to the inactivation of cadherin complex. This is strongly suggested by the observations that the loss of p120ctn destabilizes E-cadherin, which in turn is predicted to reduce levels of α- and β-catenin at the cell-cell junction. Hence, p120ctn acts as a tumour suppressor under this condition.
To account for the unexpected CTNND1 gene amplification reported by Castillo et al [11], one possibility would be that in some cancers where p120ctn is amplified, E-cadherin might already be inactivated or switched for other cadherins that favour metastasis; in that case, amplified p120ctn may actually promote metastasis. It would be interesting to determine the status of E-cadherin and other cadherins and catenins in the lung tumours that showed p120ctn gene amplification. An alternative possibility could be that, in these cancer cells, some mechanisms have developed where cells bypass p120ctn's tumour suppressive functions. Additional questions remain as to whether p120ctn gene amplification occurs in other cancer types as well. Clearly, further studies would be needed to reveal what the molecular changes are in non-small cell lung cancers with amplified p120ctn gene.
Previous studies have characterized β-catenin in great detail in the context of Wnt signalling, cell adhesion, and cancer [13, 14], although the delta subfamily of the catenin proteins such as δ-catenin and p120ctn has received increasing attention only in recent years. As exemplified by several studies that identified δ-catenin gene mutations [15], overexpression in prostate cancer [16, 17], and the effects of its overexpression on the increase in cell survival gene profiles [18]. Additional mechanistic studies highlighted p120ctn functions in mediating inflammatory response in the skin and skin cancer [19, 20] as well as in coordinating antagonism between Rac and Rho with p190RhoGAP interactions in cell-cell adhesion [21]. The progress in this field reached an exciting step when Ishiyama et al [22] reported the determination of the crystal structure of p120ctn in complex with the cadherin juxtamembrane domain (JMD) core peptide at 2.4 Å resolution. The authors proposed that p120ctn regulates the stability of cadherin-mediated cell-cell adhesion by associating with the majority of the E-cadherin JMD, including the endocytic LL motif and tyrosine-phosphorylation sites, through discrete “dynamic” and “static” binding sites. With the identification of the amino acid involvement in the JMD-120ctn complexes, strategies can be applied to design molecular therapeutics that strengthen or interfere with cadherin-p120ctn interactions.
Through the armadillo repeat sequences, p120ctn binds to this unique JMD on E-cadherin [23–25]. This binding site is in contrast to the distal cytoplasmic tail domain of E-cadherin that β-catenin interacts with. The JMD has been implicated in a variety of cellular processes, including cadherin clustering and adhesive strengthening [23], promotion of axon outgrowth [26], and suppression of cell motility [27]. The significance of the p120ctn-JMD interaction is underscored by the fact that the JMD is highly conserved among classical (type I), type II, and certain invertebrate cadherins [28] as well as the binding of other p120ctn subgroup proteins, such as δ-catenin, to E-cadherin through the same JMD domain [29].
So far, at least one study has reported that δ-catenin affects the localization and stability of p120ctn by competitively interacting with E-cadherin [30]. In xenografted prostate cancer CWR22Rv-1 cells, it was found that the cells overexpressing δ-catenin contain less p120ctn at the cell-cell junction than do control cells, and that this causes the relocalization of p120ctn from the plasma membrane to the cytosol. Thus, for δ-catenin and p120ctn, successful binding by one to E-cadherin adversely affects the stability of the other. However, in this issue of The Journal of Pathology, Zhang et al [31] showed that δ-catenin promotes a malignant phenotype in non-small cell lung cancer by non-competitive binding to E-cadherin with p120ctn in the cytoplasm.
While the article of Zhang et al [31] was under review, a study by DeBusk et al indicated that the level of δ-catenin (albeit with a spurious molecular weight designation) was elevated in lung cancer [32]. In the current issue of The Journal of Pathology, Zhang et al [31] examined the expression of δ-catenin by immunohistochemistry in 115 cases of non-small cell lung cancer (NSCLC) specimens (including 65 cases with follow-up records and 50 cases with paired lymph node metastases lesions). These more vigorous studies allowed the authors to reach several important conclusions: a. δ-catenin is overexpressed in lung cancer, and the positive expression rate of δ-catenin was significantly increased in adenocarcinoma, stage III–IV, paired lymph node metastasis lesions, and primary tumours with lymph node metastasis, and b. postoperative survival period of patients with δ-catenin positive expression was shorter than the negative ones.
Surprisingly, these authors found no competition between δ-catenin and p120ctn for binding to E-cadherin in two lung cancer cell lines. These observations raised some interesting questions. Do δ-catenin and p120ctn show competitive binding to E-cadherin only in certain cancer cell types? The authors argued that the reason δ-catenin and p120ctn compete for binding to E-cadherin in CWR22Rv-1 prostate cancer cells reported by Yang et al [30] may be due to the membrane expression of E-cadherin, p120ctn, and δ-catenin in these cell lines. This interpretation suggests that this competition may become lost because these proteins are primarily in the cytoplasm of the lung cancer cell lines they studied. Although this scenario is possible, δ-catenin overexpressed in CWR22Rv-1 cells as well as in some lung cancer cell lines, such as NCI-H1299, also showed both membrane and cytoplasmic distributions ([17]. See also Fig 1B). In addition, although E-cadherin distribution in NCI-H1299 is not junctional, it is not entirely cytosolic either, because the strong immunoreactivity is observed at the perinuclear region, indicating a Golgi and endoplasmic reticulum (ER) distribution. As E-cadherin is a transmembrane protein, this localization is not unreasonable unless E-cadherin is processed in such a way that the JMD is cleaved and only the carboxyl terminal sequences are retained inside the cell. However, the data from Zhang et al [31] is not consistent with this notion because the Western blots showed a full-length E-cadherin expressed in these cell lines. Another possibility is that there are perhaps different pools of E-cadherin, and that this protein is localized to different compartments of the cells due to differential distribution of p120ctn and δ-catenin. This hypothesis implies that p120ctn and δ-catenin play non-overlapping roles in cancer. Indeed, p120ctn does not contain the PDZ binding domain at its carboxyl terminus like that of δ-catenin, and the majority of amino- and carboxyl sequences flanking the armadillo domains are very different between the two proteins [7].
There are indications that p120ctn and δ-catenin are involved in similar as well as different cellular functions and some of their modulatory roles are not directly related to the cell-cell junction. Regarding JMD, p120ctn and δ-catenin utilize part of the armadillo domains to achieve the binding to cadherins. It is worthy of note that these domains are also important for their interactions with the presenilin protein, which is part of the γ-secretase complex involved in Alzheimer's disease [8, 33–35]. Presenilin appears to compete with p120ctn for E-cadherin binding through JMD [36]. Clearly, further investigation into the JMD interactions with p120ctn, δ-catenin, and perhaps presenilin will facilitate research progresses in both Alzheimer's disease and cancer mechanisms.
Finally, studies have suggested non-overlapping functions between p120ctn and δ-catenin. For example, mutant δ-catenin mice show cognitive and synaptic dysfunction [37]. δ-Catenin interacts with sphingosine-kinase 1 [38] and Wnt-glycogen synthase kinase (GSK) axis [39, 40], modulates angiogenesis under pathological condition [32], and influences cell survival through modulating Pax6-caspase-PARP axis, Bcl-XL, and survivin expression [18, 41]. δ-Catenin is enriched in the postsynaptic dendrites of neurons [42–44], excreted into extracellular milieu [45], and predicted to localize in both cytoplasmic and nuclear compartments [29]. Given its potentially significant involvements in several neurologic disorders and cancers [16, 17, 31, 46, 47], exploitation into δ-catenin as well as its comparison to p120ctn promises to provide important insights into their regulation and functions in cell signalling and cancer.
Acknowledgement
The author's research was supported in part by the United States National Institutes of Health/National Cancer Institute (CA111891) and Department of Defense (PC040569).
References
- 1.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 2.Makrilia N, Kollias A, Manolopoulos L, et al. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 2009;27:1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Roy FM, McCrea PD. A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer. 2005;5:956–964. doi: 10.1038/nrc1752. [DOI] [PubMed] [Google Scholar]
- 5.Anastasiadis PZ. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 7.Hatzfeld M. The p120 family of cell adhesion molecules. Eur J Cell Biol. 2005;84:205–214. doi: 10.1016/j.ejcb.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Liyanage U, Medina M, et al. Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 1997;8:2085–2090. doi: 10.1097/00001756-199705260-00054. [DOI] [PubMed] [Google Scholar]
- 9.Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1:a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 11.Castillo SD, Angulo B, Suarez-Gauthier A, et al. Gene amplification of the transcription factor DP1 and CTNND1 in human lung cancer. J Pathology. 2010 doi: 10.1002/path.2732. DOI: 10.1002/path.2732. [DOI] [PubMed] [Google Scholar]
- 12.Bandara LR, Lam EW, Sørensen TS, et al. DP-1: a cell cycle-regulated and phosphorylated component of transcription factor DRTF1/E2F which is functionally important for recognition by pRb and the adenovirus E4 orf 6/7 protein. EMBO J. 1994;13:3104–3114. doi: 10.1002/j.1460-2075.1994.tb06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris TJ, Peifer M. Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234–237. doi: 10.1016/j.tcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Chen YH, Hong H, et al. Increased nucleotide polymorphic changes in the 5'-untranslated region of delta-catenin (CTNND2) gene in prostate cancer. Oncogene. 2009;28:555–564. doi: 10.1038/onc.2008.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger MJ, Tebay MA, Keith PA, et al. Expression analysis of delta-catenin and prostate-specific membrane antigen: their potential as diagnostic markers for prostate cancer. Int J Cancer. 2002;100:228–237. doi: 10.1002/ijc.10468. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q, Dobbs LJ, Gregory CW, et al. Increased expression of delta-catenin/neural plakophilin-related armadillo protein is associated with the down-regulation and redistribution of E-cadherin and p120ctn in human prostate cancer. Hum Pathol. 2005;36:1037–1048. doi: 10.1016/j.humpath.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y, Abdallah A, Lu JP, et al. delta-Catenin promotes prostate cancer cell growth and progression by altering cell cycle and survival gene profiles. Mol Cancer. 2009;8:19. doi: 10.1186/1476-4598-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Moreno M, Davis MA, Wong E, et al. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Moreno M, Song W, Pasolli HA, et al. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci USA. 2008;105:15399–15404. doi: 10.1073/pnas.0807301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wildenberg GA, Dohn MR, Carnahan RH, et al. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 22.Ishiyama N, Lee SH, Liu S, et al. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozawa M, Kemler R. The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J Cell Biol. 1998;142:1605–1613. doi: 10.1083/jcb.142.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkubo T, Ozawa M. p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J Biol Chem. 1999;274:21409–21415. doi: 10.1074/jbc.274.30.21409. [DOI] [PubMed] [Google Scholar]
- 26.Riehl R, Johnson K, Bradley R, et al. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Paradies NE, Fedor-Chaiken M, et al. E-cadherin mediates adhesion and suppresses cell motility via distinct mechanisms. J. Cell Sci. 1997;110:345–356. doi: 10.1242/jcs.110.3.345. [DOI] [PubMed] [Google Scholar]
- 28.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 29.Lu Q, Paredes M, Medina M, et al. delta-catenin, an adhesive junction-associated protein which promotes cell scattering. J Cell Biol. 1999;144:519–532. doi: 10.1083/jcb.144.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang I, Chang O, Lu Q, et al. Delta-catenin affects the localization and stability of p120-catenin by competitively interacting with E-cadherin. Mol Cells. 2010;29:233–237. doi: 10.1007/s10059-010-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Zhang D, Wang Y, et al. δ-Catenin promotes malignant phenotype of non-small cell lung cancer by non-competitive binding to E-cadherin with p120ctn in cytoplasm. J Pathology. 2010 doi: 10.1002/path.2742. DOI: 10.1002/path.2742. [DOI] [PubMed] [Google Scholar]
- 32.DeBusk LM, Boelte K, Min Y, et al. Heterozygous deficiency of delta-catenin impairs pathological angiogenesis. J Exp Med. 2010;207:77–84. doi: 10.1084/jem.20091097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanahashi H, Tabira T. Isolation of human delta-catenin and its binding specificity with presenilin 1. Neuroreport. 1999;10:563–568. doi: 10.1097/00001756-199902250-00022. [DOI] [PubMed] [Google Scholar]
- 34.Levesque G, Yu G, Nishimura M, et al. Presenilins interact with armadillo proteins including neural-specific plakophilin-related protein and beta-catenin. J Neurochem. 1999;72:999–1008. doi: 10.1046/j.1471-4159.1999.0720999.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Bareiss S, Kim KK, et al. Presenilin-1 inhibits delta-catenin-induced cellular branching and promotes delta-catenin processing and turnover. Biochem Biophys Res Commun. 2006;351:903–908. doi: 10.1016/j.bbrc.2006.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baki L, Marambaud P, Efthimiopoulos S, et al. Presenilin-1 binds cytoplasmic epithelial cadherin, inhibits cadherin/p120 association, and regulates stability and function of the cadherin/catenin adhesion complex. Proc Natl Acad Sci USA. 2001;98:2381–2386. doi: 10.1073/pnas.041603398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Israely I, Costa RM, Xie CW, et al. Deletion of the neuron-specific protein delta-catenin leads to severe cognitive and synaptic dysfunction. Curr Biol. 2004;14:1657–1663. doi: 10.1016/j.cub.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 38.Fujita T, Okada T, Hayashi S, et al. Delta-catenin/NPRAP (neural plakophilin-related armadillo repeat protein) interacts with and activates sphingosine kinase 1. Biochem J. 2004;382:717–723. doi: 10.1042/BJ20040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh M, Kim H, Yang I, et al. GSK-3 phosphorylates delta-catenin and negatively regulates its stability via ubiquitination/proteosome-mediated proteolysis. J Biol Chem. 2009;284:28579–28589. doi: 10.1074/jbc.M109.002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bareiss S, Kim K, Lu Q. δ-Catenin/NPRAP: A new member of the glycogen synthase kinase-3β signaling complex that promotes β-catenin turnover in neurons. J Neurosci Res. 2010 doi: 10.1002/jnr.22414. DOI: 10.1002/jnr.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Lu JP, Suter DM, et al. Isoform- and dose-sensitive feedback interactions between paired box 6 gene and delta-catenin in cell differentiation and death. Exp Cell Res. 2010;316:1070–1081. doi: 10.1016/j.yexcr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones SB, Lanford GW, Chen YH, et al. Glutamate-induced delta-catenin redistribution and dissociation from postsynaptic receptor complexes. Neuroscience. 2002;115:1009–1021. doi: 10.1016/s0306-4522(02)00532-8. [DOI] [PubMed] [Google Scholar]
- 43.Silverman JB, Restituito S, Lu W, et al. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci. 2007;27:8505–8516. doi: 10.1523/JNEUROSCI.1395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu-Elneel K, Ochiishi T, Medina M, et al. A delta-catenin signaling pathway leading to dendritic protrusions. J Biol Chem. 2008;283:32781–32791. doi: 10.1074/jbc.M804688200. [DOI] [PubMed] [Google Scholar]
- 45.Lu Q, Zhang J, Allison R, et al. Identification of extracellular delta-catenin accumulation for prostate cancer detection. Prostate. 2009;69:411–418. doi: 10.1002/pros.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medina M, Marinescu RC, Overhauser J, et al. Hemizygosity of delta-catenin (CTNND2) is associated with severe mental retardation in cri-du-chat syndrome. Genomics. 2000;63:157–164. doi: 10.1006/geno.1999.6090. [DOI] [PubMed] [Google Scholar]
- 47.Vrijenhoek T, Buizer-Voskamp JE, van der Stelt I, et al. Recurrent CNVs disrupt three candidate genes in schizophrenia patients. Am J Hum Genet. 2008;83:504–510. doi: 10.1016/j.ajhg.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]