Abstract
The aged canine (dog) is an excellent model for investigating the neurobiological changes that underlie cognitive impairment and neurodegeneration in humans, as canines and humans undergo similar pathological and behavioural changes with aging. Recent evidence indicates that a combination of environmental enrichment and antioxidant-fortified diet can be used to reduce the rate of age-dependent neuropathology and cognitive decline in aged dogs, although the mechanisms underlying these changes have not been established. We examined the hypothesis that an increase in levels of brain-derived neurotrophic factor (BDNF) is one of the factors underlying improvements in learning and memory. Old, cognitively impaired animals that did not receive any treatment showed a significant decrease in BDNF mRNA in the temporal cortex when compared with the young group. Animals receiving either an antioxidant diet or environmental enrichment displayed intermediate levels of BDNF mRNA. However, dogs receiving both an antioxidant diet and environmental enrichment showed increased levels of BDNF mRNA when compared to untreated aged dogs, approaching levels measured in young animals. BDNF receptor TrkB mRNA levels did not differ between groups. BDNF mRNA levels were positively correlated with improved cognitive performance and inversely correlated with cortical Aβ(1–42) and Aβ(1–40) levels. These findings suggest that environmental enrichment and antioxidant diet interact to maintain brain levels of BDNF, which may lead to improved cognitive performance. This is the first demonstration in a higher animal that non-pharmacological changes in lifestyle in advanced age can up-regulate BDNF to levels approaching those in the young brain.
Keywords: Alzheimer’s disease, neurotrophin, amyloid, diet, environmental enrichment, antioxidant, mRNA
1. Introduction
The preservation of cognitive function throughout life is becoming more critical as our population ages and increasing numbers of individuals are reaching extreme old age. Further, the single biggest risk factor for pathological aging such as Alzheimer disease (AD) and other neurodegenerative diseases is advanced age. Thus, interventions that can promote healthy brain aging may result in reduced risk of developing dementia.
Aged dogs are an ideal model for human aging and are uniquely useful for the development of interventions that improve cognition (Cotman et al., 2002; Cotman and Head, 2008). Aged dogs naturally develop learning and memory impairments and changes in social interaction that can be improved by multiple interventions (Cotman and Head, 2008; Landsberg, et al., 2005). The neuropathology of canine brain aging is similar to human brain aging, as the brains of aged dogs accumulate β-amyloid (Aβ) which follows a distribution similar to that observed in human brain (Cummings et al., 1993; Head et al., 2000; Thal et al., 2002), oxidative damage (Head et al., 2002) and hilar neuron loss in the hippocampus (Siwak-Tapp et al., 2007). Canine Aβ(1–42) has the same amino acid sequence as human Aβ (Selkoe et al., 1987) and accumulates in diffuse plaques (Cummings et al., 1996b); the degree of cognitive impairment in aged dogs correlates with the extent and location of Aβ deposition (Cummings et al., 1996a; Head et al., 1998).
We previously demonstrated that aged, cognitively impaired canines showed reduced age-dependent impairment in discrimination and reversal learning when provided with a socially, physically and cognitively enriched environment or when provided with an antioxidant diet (Milgram et al., 2002a, b; Milgram et al., 2004; Milgram et al., 2005). The combination of the two interventions further improved cognitive test scores when compared with each intervention alone and resulted in reduced oxidative damage in the brain (Opii et al., 2008). Interestingly, preservation of neuron number in the hilus of the hippocampus was specifically linked to the enriched environment treatment group but not to animals receiving the antioxidant diet (Siwak-Tapp et al., 2008). These studies suggest that there may be independent pathways engaged by each treatment that can improve neuronal function.
The beneficial effects of enrichment and diet may be through mechanisms that enhance neuroprotective and neuromodulatory pathways, in particular, through increased brain-derived neurotrophic factor (BDNF). BDNF promotes neuronal survival of AD-affected cell types (Alderson et al., 1990; Ando et al., 2002; Ghosh et al., 1994; Hyman et al., 1991; Knusel et al., 1992; Lindholm et al., 1996), is an activity-dependent modulator of long term potentiation (LTP), and is a key molecule for learning and memory (Bramham and Messaoudi, 2005; Lu, 2003). In people, BDNF mRNA and protein are decreased in the cortex and hippocampus in mild cognitive impairment (MCI) and AD (Ferrer et al., 1999; Garzon et al., 2002; Holsinger et al., 2000; Michalski et al., 2003; Phillips et al., 1991), and decreased BDNF levels correlate with cognitive decline (Peng et al., 2005). In animals, decreased BDNF results in deficits in LTP and memory, and exogenous supplementation of BDNF can rescue memory impairment in transgenic mice with reduced BDNF levels (Nagahara et al., 2009; Blurton-Jones et al., 2009; Korte et al., 1995; Olofsdotter et al., 2000; Patterson et al., 1996), suggesting that increasing BDNF availability in the brain may be a viable strategy to counteract cognitive decline with aging or AD. Importantly, brain BDNF levels can be modulated by non-invasive and non-pharmacological approaches, namely by diet (Duan et al., 2001; Lee et al., 2002; Mattson, 2000), physical activity (Cotman et al., 2007; Neeper et al., 1995), and environmental enrichment (Young et al., 1999; Wolf et al., 2006).
The canine model provided us with an opportunity to test the relationship between cognitive impairment in aging, BDNF, and the effects of non-pharmacological interventions in a way that cannot be done in transgenic mice. Genetically unmodified, aged, cognitively impaired canines were subjected to a prolonged (2+ year), multivariable study demonstrating the positive effects of multiple interventions on cognition. In this follow-up study, we investigate whether those interventions that improved cognition also increased BDNF levels. Although previous studies in rodents have unequivocally shown the ability of BDNF to reverse cognitive deficits, there has been no previous demonstration in a higher animal model that long-term environmental enrichment or diet can rescue BDNF levels in aged animals.
2. Methods
2.1. Subjects
Twenty-three beagle dogs ranging in age at the start of the study from 8.05–12.35 yrs (Mean = 10.62 yrs, SD=1.21, 11M/12F) were obtained from the colony at the Lovelace Respiratory Research Institute. Animals were born and maintained in the same environment and all had documented dates of birth and comprehensive medical histories. A second group of 5 young beagle dogs (Mean = 4.1 yrs, SD = 0.43, 3M/2F) from the same colony were also obtained to serve as untreated controls for neurobiological studies; no cognitive data was available for these animals.
2.2. Group Assignments and Study Timeline
All dogs underwent extensive baseline cognitive testing as has been described previously (Milgram et al., 1994). Based on cognitive test scores, animals were ranked in order of cognitive ability and placed into one of four treatment groups such that each group contained animals with equivalent ranges of cognition (e.g. poor to good) and was balanced for sex and age. The treatments investigated were environmental enrichment and anti-oxidant diet, and were tested in the following treatment groups: control environment/control diet (C/C), enriched environment/control diet (E/C), control environment/antioxidant diet (C/A), enriched environment/antioxidant diet (E/A). Twenty-three animals were treated for a period of 1.95–2.83 years (Mean=2.69 years, SD=0.19). At the end of the study, the ages of the dogs in the CC group (Mean=13.95 years SD=0.74), the EC group (Mean=13.3 years SD 0.81), the CA group (13.32 years SD=1.42) and the EA group (12.67 years SD=1.55) were not statistically significantly different (F(3,22)=1.13 p=0.36).
2.3 Environmental Enrichment
The environmental enrichment protocol consisted of housing animals in pairs (social enrichment), providing two 20-minute outdoor walks per week (physical exercise) and continuous cognitive testing (cognitive enrichment). The cognitive enrichment consisted of a landmark discrimination task (Milgram et al., 2002), an oddity discrimination task (Cotman et al., 2002) and a size concept learning task (Tapp et al., 2004).
2.4. Diet
The two foods were formulated to meet the nutrient profile for the American Association of Feed Control Officials recommendations for adult dogs (AAFCO 1999). Control and test diets were identical in composition, other than inclusion of a broad-based antioxidant and mitochondrial cofactor supplementation to the test diet. The control and enriched foods had the following differences in formulation on an asfed basis respectively: dl-alpha-tocopherol acetate (120 ppm vs 1050 ppm), l-carnitine (<20 ppm vs 260 ppm), dl-alpha-lipoic acid (<20 ppm vs 128 ppm), and ascorbic acid as Stay-C (<30 ppm vs 80 ppm). The enriched food additionally contained 1% inclusions of each of the following (1 to 1 exchange for corn): spinach flakes, tomato pomace, grape pomace, carrot granules and citrus pulp.
2.5. Cognitive testing
Learning and memory tasks were used to assess cognition throughout the study as described previously (Milgram et al., 2005). Overall, all treated animals showed significant improvement in spatial attention, complex learning ability and simple discrimination tasks. Further, reversal learning, a measure of prefrontal function, was maintained in treated animals, with the largest effect occurring in the animals receiving the combination treatment. In the last year of the study, dogs were given a test of spatial memory measured using a 3-choice delayed non-match to position task (Nippak et al., 2007). Briefly, each trial began with a sample phase in which a small red block covering a food well was presented at one of three spatial locations, left, center or right. After the dog displaced the block and obtained the reward, the tray was withdrawn. Following a delay, the test phase started with the presentation of both the sample (a block presented in the sample location) and the non-match (an identical red block in one of the two other locations) stimuli. The dog now had to displace the block in the new location in order to obtain reward. If the dog responded to the sample location (incorrect response) the tray was immediately withdrawn and an error was recorded. Animals were allowed to correct their first error for each session. To prevent the animals from using olfactory cues to solve the task, a quantity of food approximately equal to that associated with the non-match was stuck to the bottom of the incorrect stimulus. After a 60 s inter-trial interval, the sample phase of the next trial was initiated. Data were manually collected using a customized computer program that controlled timing, randomization procedures, location of sample and non-match position, and recorded choice-reaction times. Each animal was tested for twelve trials in one daily session. The dogs were initially trained at a 10s delay until they either completed 600 trials (50 sessions) or passed a two-stage criterion. The first stage involved correctly responding on 11/12 trials or better on one day, on 10/12 trials or better over two consecutive days, or on 10/12, 9/12, and 10/12 trials over three successive sessions. To successfully complete the 2nd stage of criterion, they had to respond correctly on at least 70% of the next 36 completed trials (over 3 consecutive sessions). Thus, a minimum of 4 test days were required to achieve the two-stage criterion. Most of the aged animals were unable to achieve the criterion level of performance. Animals that failed to learn were tested at a 5 s delay and were given a maximum of 50 training sessions to pass after which testing was terminated. Animals that passed, however, then graduated to a 10 s delay and were tested until they met the criterion for baseline testing. The total number of errors made to criterion or across the first fifty training sessions was used to measure memory retention (Nippak et al., 2007).
2.6 Aβ(1–40) and Aβ(1–42) quantification
Aβ(1–40) and Aβ(1–42) were extracted and quantified by enzyme linked immunosorbant assay using previously published methods (Nistor et al., 2007). Briefly, Aβ was extracted from approximately 200 mg of frozen temporal cortex in 0.1M Tris pH 6.8 with 1% SDS and a Protease Inhibitor Cocktail Kit (MP Biochemicals Inc., Westburry, NJ) containing 0.4 mg/ml AEBSF 1mg/ml EDTA, 1µg/ml each leupeptin and pepstatin A in 1ml buffer/150 mg wet weight tissue using a Potter Elevehjem 10 ml Wheaton glass tube. The homogenate was centrifuged at 4°C and 100,000×g for 1h. The pellet was resuspended in 70% formic acid and sonicated on ice. After centrifugation as above, the supernatant was assayed in triplicate on ELISA plates coated with a monoclonal anti-Aβ1–16 antibody (kindly provided by Dr. William Van Nostrand, Stony Brook University, Stony Brook, NY). Detection was by monoclonal HRP-conjugated antibodies anti-Aβ(1–40) (MM32-13.1.1) and anti-Aβ(1–42) (MM40-21.3.1) (kindly provided by Dr. Christopher Eckman, Mayo Clinic Jacksonville, Jacksonville, FL). Values were normalized to the wet weight of the tissue for individual animals.
2.7 RNA isolation, DNAse treatment, reverse transcription, and real-time quantitative PCR (qRT-PCR)
20–40 mg frozen shavings of temporal cortices from the twenty-eight dogs were sonicated on ice 3 × 5 sec, without thawing, in 1ml/100 mg wet weight tissue cold Trizol™ (Life Technologies, Inc., Gaithersburg, MD, U.S.A.). RNA was isolated from the Trizol homogenate by centrifugation at 12,000×g for 15 min. 70% ethanol was added to the aqueous phase containing RNA, followed by passage over RNeasy™ spin columns (Qiagen, Mississauga, Ontario, Canada), DNAse treatment on the column and elution, according to the manufacturer’s instructions. RNA concentration and integrity were determined by spectrophotometry and agarose gel electrophoresis. A260/A280 ratios were > 1.7. There were no intra- or inter-group differences in RNA yields.
1µg RNA was reverse transcribed at 25°C for 10 min, 42°C for 50 min and 70°C for 15 min, following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, U.S.A.). Briefly, 20 µl of reaction mix contained 250 ng of random primers, 0.5 mM dNTPs (0.5 mM each of dATP, dTTP, dCTP, and dGTP), 1X first-strand buffer, 0.05 mM DTT, 2 U of RNaseOUT, and 200 U of Superscript II (Invitrogen). As a negative control, water was added instead of the enzyme ("no RT" control).
Real-time PCR was performed in the Stratagene MX3000P (La Jolla, CA, U.S.A.) using the DNA binding dye SYBR green™ (Platinum SYBR Green qPCR SuperMix UDG; Invitrogen). The 20 µl PCR mix contained 1X qPCR SuperMix (Invitrogen), forward and reverse primers, 30 nM ROX reference dye (Invitrogen), and cDNA from 50 ng of RNA or reference standard for absolute quantification. A "no template" control lacking cDNA was included.
Primers for canine BDNF, full-length TrkB and β-actin were designed using Primer3 software and NCBI accession numbers AB105074, AY514746.1 and AF021873 & NM_001003349, respectively. Full-length TrkB primers were chosen within exons 18 and 19. Primers were not homologous to any other sequence in NCBI. For canine BDNF and TrkB detection, 300 nM forward and reverse primers were used (BDNF forward primer 5’ CTG CAA ACA TGT CGA TGA GG 3’, BDNF reverse primer 5' ATG GGA TTG CAC TTG GTC TC 3’, product 211 bp; TrkB forward primer 5’ ATG TCT GGA GCC TGG GAG T 3’, TrkB reverse primer 5’ CCC TGC GTG ATG CAT TCT AT 3’, product 103 bp), and 150 nM forward and reverse primers were used for β-actin (forward primer, 5' GGC ATC CTG ACC CTC AAG TA 3’; reverse primer, 5' ACA TAC ATG GCT GGG GTG TT 3’; product, 215 bp). The thermal profile was: 2 min at 50°C, 2 min at 95°C followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s (BDNF and TrkB) or 95°C for 15 s, 58°C for 30 s, and 72°C for 30 s (β-actin).
cDNA standards were obtained by PCR using the above primers and spectrophotometric quantification of gel-purified product. Standard curves were run in triplicate with six 10-fold serial dilutions starting at 4.3 × 106 copies of BDNF, 8.86 × 106 copies of TrkB or 4.2 × 106 copies of β-actin cDNA. Each sample, no-RT and no-template control was also run in triplicate. Following qRT-PCR, a dissociation curve was added to verify that no secondary products had formed. Only experiments with R2 > 0.990 and PCR efficiency between 90 and 100% were used for analysis. Levels of BDNF, TrkB and β-actin (a housekeeping gene used to check integrity of RNA) were determined by absolute quantification using MXPro Software v. 3.00 (Stratagene).
2.8 Statistical Analyses
BDNF and TrkB mRNA copy numbers were compared across the 5 groups using a one-way analysis of variance (ANOVA); for BDNF analysis, post hoc comparisons were with a one-sided Dunnett’s test with the prediction that untreated aged dogs would have the lowest copy number overall. Additional cross group comparisons were conducted using the least significant difference (LSD) post hoc procedure. Pearson or Spearman rank correlation coefficients were calculated for BDNF mRNA copy number compared with either cognitive test error scores or Aβ measures. Data analysis was performed with SPSS v.15 (SPSS, Chicago, IL, U.S.A.) for Windows and an alpha level of 0.05. For Aβ analyses, raw values were log transformed to test for linearity.
3. Results
3.1. Cortical BDNF and TrkB mRNA levels
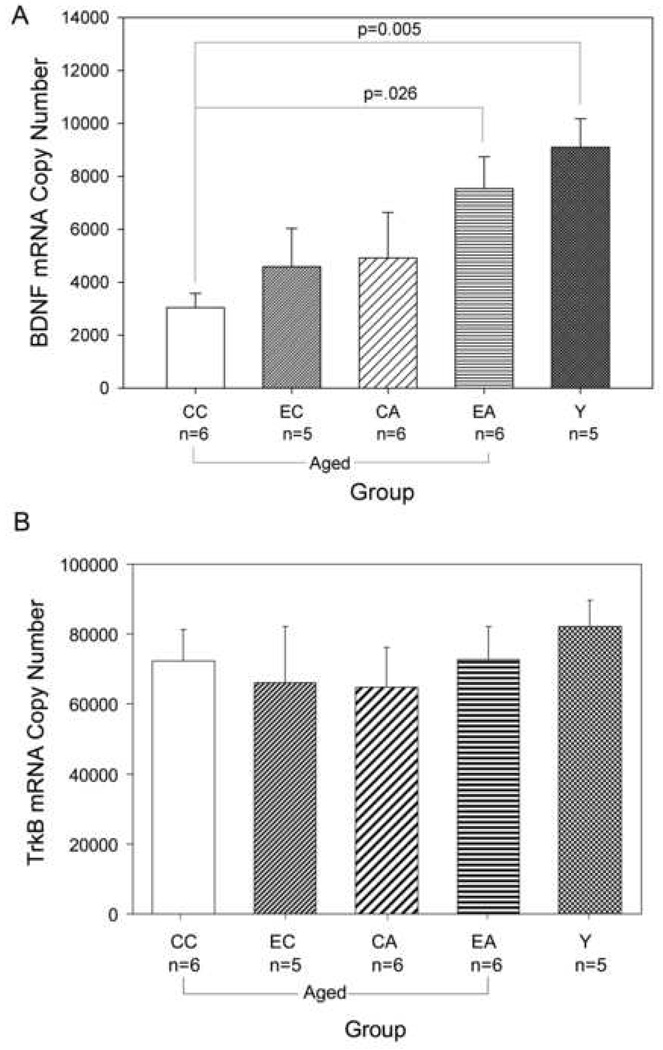
To test whether aged, cognitively impaired dogs exhibited lower BDNF levels than young dogs, we measured BDNF mRNA levels in post mortem cortical tissue by qRT-PCR. BDNF mRNA levels exhibited a significant main effect of group (F(4,27)=3.66 p=0.019). Aged dogs housed in control environmental conditions and receiving standard senior dog food (CC group) exhibited statistically significantly lower BDNF mRNA copy numbers when compared to young dogs (Dunnett’s test p=0.005, Fig.1A).
Figure 1. A. BDNF mRNA levels in the five canine groups.
BDNF mRNA copy numbers per 50 ng total RNA. CC: control environment/ control diet; EC: behavioral enrichment/control diet; CA: control environment/ antioxidant diet; EA: behavioral enrichment/antioxidant diet; Y: young canines; * = p<0.05 (one-way ANOVA and post hoc one sided Dunnett’s test). n=5–6 per group. B. Full-length TrkB mRNA levels do not differ between the five canine groups. TrkB mRNA copy numbers per 50 ng total RNA. CC: control environment/ control diet; EC: behavioral enrichment/control diet; CA: control environment/antioxidant diet; EA: behavioral enrichment/ antioxidant diet; Y: young canines; p=0.83 (one-way ANOVA). n=5–6 per group.
We next tested whether interventions that improved cognitive function in aged canines would also rescue BDNF levels. Canines exposed to an enriched environment alone (CC compared to EC – Dunnett’s test p=0.97) or receiving an antioxidant-enriched diet alone (CC compared to CA – Dunnett’s test p=0.98) displayed intermediate but not significantly different BDNF mRNA copy numbers relative to untreated aged controls (Fig. 1A). However, animals treated with the combination of an enriched environment and receiving an antioxidant-enriched diet (EA) showed significantly higher BDNF mRNA copy numbers than the untreated aged controls (CC compared to EA, Dunnett’s test p=0.026). Importantly, BDNF mRNA levels in the EA group were not significantly different from BDNF levels in the young dogs (Dunnett’s test p=0.429), whereas the CC (Dunnett’s test p=0.005), the CA (Dunnett’s test p=0.046), and the EC (Dunnett’s test p=0.039) groups all had significantly lower BDNF mRNA copy numbers compared to young animals. The EA group was not significantly different from either the EC group (LSD p=0.131) or the CA group (LSD p=0.158).
We then investigated whether changes in BDNF expression were associated with comparable changes in expression of its receptor, TrkB. Cortical levels of full-length TrkB (assayed using primers within the kinase domain) did not differ between groups (F(4,27)=0.37 p=0.83 Fig. 1B) indicating that there was no change with age or with dietary or environmental intervention.
A one-way ANOVA revealed that mRNA levels of β-actin, a housekeeping gene, did not differ significantly across the five groups (F(4,27)=0.47 p=0.76).
3.2 BDNF mRNA copy number is correlated with Aβ(1–40) and Aβ(1–42) levels and cognitive test scores
Like human subjects, the aged canine naturally accumulates Aβ in the absence of any transgene. In man, increased amyloid accumulation correlates with reduced BDNF levels and decreased cognitive function (Peng et al., 2005). We therefore tested, in the aged canine, whether amyloid accumulation in temporal cortex correlated with BDNF levels in the same region of the brain and with cognitive status.
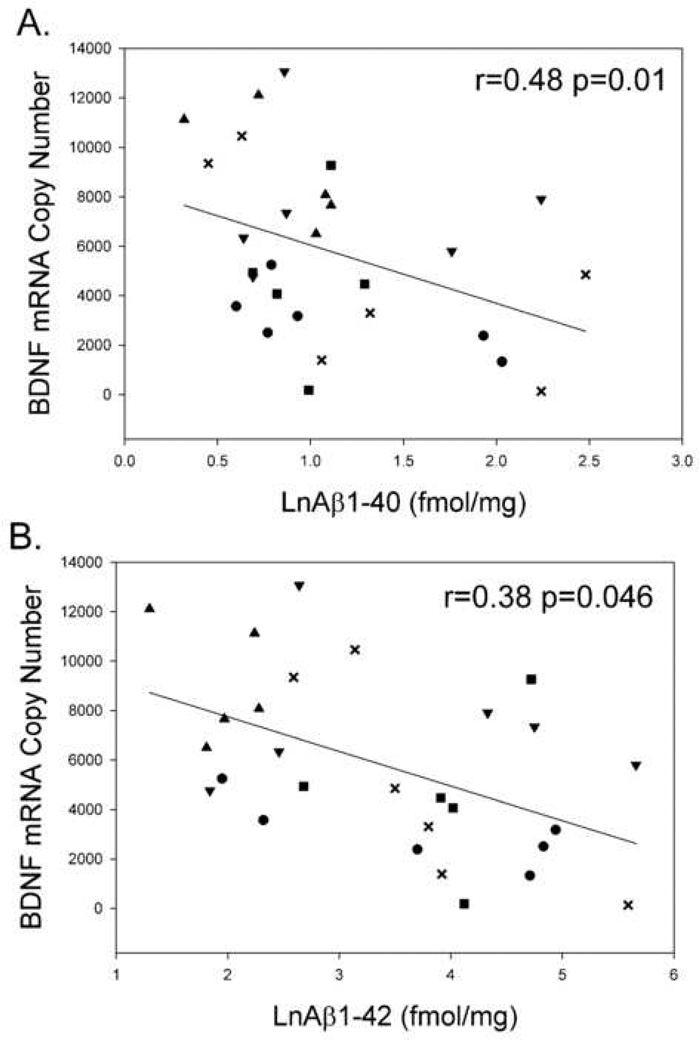
Aβ(1–42) but not Aβ(1–40) levels, measured by ELISA in temporal cortex, were significantly lower in young dogs compared to untreated aged dogs (t(9)=2.39 p=0.04). Although there was a trend towards lower Aβ in aged animals provided with an enriched environment, these differences did not reach statistical significance due to large individual variability (Pop et al., 2003). Nevertheless, across all animals, BDNF mRNA copy number in the temporal cortex was inversely correlated with both Aβ(1–42) (r=−0.38 p=.046 n=28) and Aβ(1–40) (r = −0.48 p=0.01 n=28) (Fig. 2), revealing that higher brain Aβ was associated with lower BDNF mRNA copy number.
Figure 2. BDNF mRNA levels are inversely correlated with Aβ.
A. BDNF mRNA copy number is lower in animals with higher levels of Aβ1–40. B. BDNF mRNA copy number is lowest in animals with higher Aβ1–42. Note that the x axes use log transformed raw Aβ values. Correlations are Pearson correlations. Symbols represent (▲)-Y, (●)-CC, (■)-EC, (X)-CA, (▼)-EA.
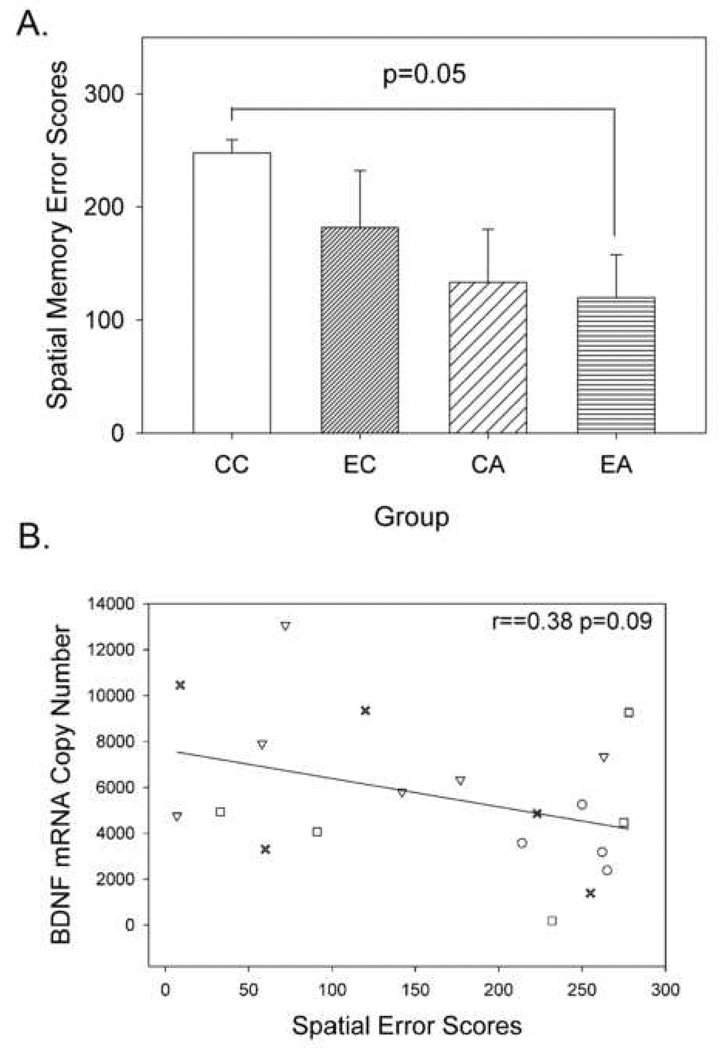
Because BDNF is a central player in plasticity and learning mechanisms, we hypothesized that BDNF mRNA copy number would also be inversely correlated with performance during cognitive testing. As previously reported (Nippak et al., 2007), aged animals receiving the combined intervention (antioxidant diet and environmental enrichment) made fewer errors than aged untreated animals in a 3-choice delayed non-match to position spatial memory task. (Dunnett’s post hoc test, p=0.050, Fig. 3A). As predicted, there was a trend towards higher BDNF mRNA levels in dogs exhibiting fewer errors (r = −0.38, p = 0.09, n = 23, Fig. 3B). Overall, these results reveal that poorer cognitive performance is associated with lower BDNF and higher Aβ, suggesting a link between BDNF, Aβ levels and cognitive function in the aged canine model.
Figure 3. A. Spatial memory scores using a 3-choice delayed non-match to sample task.
Dogs provided with the combination treatment showed a significant reduction in spatial memory error scores (Dunnett’s test comparison of EA to CC group). CC: control environment/ control diet; EC: behavioral enrichment/control diet; CA: control environment/antioxidant diet; EA: behavioral enrichment/ antioxidant diet. n=5–6 per group. B. Trend towards an association between BDNF mRNA levels and spatial error scores. Animals that performed more poorly and learned the task with more errors generally showed lower BDNF mRNA copy numbers. Symbols represent (▲)-Y, (●)-CC, (■)-EC, (X)-CA, (▼)-EA (Pearson correlation, r = −0.38, p = 0.09).
4. Discussion
In this study, we show that BDNF mRNA levels in the canine temporal cortex are significantly decreased in aged, cognitively impaired animals compared with young dogs, and that a combination of environmental enrichment and antioxidant dietary intervention can increase BDNF mRNA in aged animals to levels approaching those seen in young dogs. Further, higher BDNF mRNA levels are correlated with lower brain Aβ and improved spatial memory performance.
The mechanism underlying improved cognitive function with environmental enrichment and dietary intervention in the canine brain is likely to be multifaceted. Many studies in rodents have demonstrated that physical activity, one of the components of the environmental enrichment treatment, promotes neurogenesis and synaptic plasticity (Farmer et al., 2004; van Praag et al., 1999), modulates BDNF mRNA and protein levels (Adlard et al., 2005; Cotman & Berchtold, 2002; Berchtold et al., 2005; Vaynmann et al., 2003), decreases Aβ in transgenic AD mice (Um et al., 2008) and improves cognitive function (Cotman et al., 2007), suggesting that exercise may be a key component of enrichment. Rodents housed in enriched environments display increased hippocampal BDNF mRNA (Falkenberg et al., 1992) and CREB immunoreactivity (Williams et al., 2001) and perform better on tests of spatial learning and memory than rats housed in standard or isolated conditions (Zhu et al., 2006). Similarly, a flavonoid-rich diet improves spatial working memory in aged rats by activating CREB and BDNF in the hippocampus (Williams et al., 2008). In this study, we reveal that the combined treatment of diet and enrichment, but not either intervention alone, significantly increases BDNF mRNA in the aged canine brain. Interestingly, dogs receiving environmental enrichment or antioxidant dietary intervention alone exhibited BDNF levels intermediate between aged untreated and young dogs. These data suggest that diet and environmental enrichment can interact to increase BDNF and reduce cognitive impairment. Previous studies have shown that diet improves mitochondrial function and reduces mitochondrial reactive oxygen species (Head, 2009; Head et al., 2009). We propose that improved mitochondrial function as a result of the antioxidant diet allows the aged brain to better respond to environmental enrichment. In other words, improved mitochondrial function may be permissive for BDNF expression, to our knowledge, a new concept in the field of intervention research for brain aging.
Additionally, our results in the aged canine model are consistent with the relationship between BDNF, Aβ levels, and cognitive function that has been established in rodent models. BDNF is well-established to be a key molecule in synaptic plasticity underlying learning and memory. It has become clear that a decrease in BDNF availability can compromise neuronal plasticity and function, while restoring BDNF levels can reverse these deficits. BDNF availability and signalling are decreased in the presence of Aβ, which down-regulates BDNF and impairs BDNF signal transduction in cell culture models (Tong et al., 2001, 2004; Garzon & Fahnestock, 2007). In addition, Aβ impairs retrograde axonal transport of BDNF (Poon et al., 2009). In parallel, in transgenic mouse models of AD as well as in MCI and AD subjects, high Aβ levels are correlated with decreased BDNF levels and with cognitive deficits (Peng et al., 2005, 2009). Consistent with this literature, our data demonstrate that increases in Aβ(1–40) and particularly Aβ(1–42) are correlated with decreased BDNF mRNA in the aged canine brain. In addition, higher BDNF mRNA levels correlate with improved cognitive performance in these animals. Thus, it is likely that in the aged canine, increased Aβ contributes to poor cognitive performance in part via decreased BDNF, while environmental enrichment and an antioxidant diet increase BDNF, thus contributing to improved neuronal function and cognition.
Previously we have shown that synaptic dysfunction in AD may result in part from BDNF signalling deficits (Tong et al., 2001, 2004; Poon et al., 2009). Either decreased BDNF availability or reduced levels of TrkB, the BDNF receptor, could reduce BDNF signalling. In this study, we evaluated the effects of age and diet/environmental enrichment on gene expression of both BDNF and TrkB in the canine temporal cortex. While BDNF mRNA levels were decreased, there was no effect of age or of either intervention on TrkB gene expression. Protein levels of BDNF and TrkB could not be evaluated in parallel because currently available antibodies do not recognize canine BDNF or TrkB. However, previous studies in the AD brain have demonstrated that declines in BDNF mRNA are comparable to declines in BDNF protein (Holsinger et al., 2000; Peng et al., 2005), and the amount of BDNF mRNA decrease seen in this study in the aged, cognitively impaired canine is similar to the amount of BDNF loss seen in MCI and AD. In addition, previous studies have demonstrated that TrkB protein levels are not altered in aging in the rat hippocampus (Silhol et al., 2005) or in the human parietal cortex in AD (Savaskan et al., 2000), consistent with our data on TrkB levels in temporal cortex of the aged cognitively impaired canine. It will be important to evaluate protein levels of BDNF and TrkB in the canine model when the tools become available. Overall, the presence of normal TrkB levels in the aged, cognitively impaired canine suggests that increasing BDNF may be a useful therapeutic option for improving cognitive status, as it may increase signalling via existing TrkB receptors.
Our results are significant in view of a growing awareness that the aged canine represents a higher animal model for studying human aging with neurodegeneration and cognitive impairment. The canine, by virtue of its natural accumulation of amyloid and cognitive impairment with aging, may offer unique insights into the development of therapeutic strategies to slow or halt cognitive decline and allow studies not possible in rodents. Although previous studies in rodents have unequivocally shown the ability of BDNF to reverse cognitive deficits, there has been no previous demonstration that long-term environmental enrichment or diet in a higher animal model can rescue age-related declines in BDNF. This study thus is the first demonstration in a higher animal that non-pharmacological changes in lifestyle can interact to up-regulate BDNF to levels approaching those in the young brain. Improved cognition in the canine model concurrent with increased BDNF levels suggests that these dietary and behavioural interventions may be effective in human MCI and AD subjects.
Acknowledgements
Supported by grant #IIRG-07-59038 from the Alzheimer’s Association to MF and grant #AG12694 from NIH/NIA to CWC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Ando S, Kobayashi S, Waki H, Kon K, Fukui F, Tadenuma T, Iwamoto M, Takeda Y, Izumiyama N, Watanabe K, Nakamura H. Animal model of dementia induced by entorhinal synaptic damage and partial restoration of cognitive deficits by BDNF and carnitine. J Neurosci Res. 2002;70:519–527. doi: 10.1002/jnr.10443. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23(5):809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15(4):685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Su JH, Cotman CW, White R, Russell MJ. Beta-amyloid accumulation in aged canine brain: a model of plaque formation in Alzheimer's disease. Neurobiol Aging. 1993;14:547–560. doi: 10.1016/0197-4580(93)90038-d. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. 1996a;66(1):11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Satou T, Head E, Milgram NW, Cole GM, Savage MJ, Podlisny MB, Selkoe DJ, Siman R, Greenberg BD, Cotman CW. Diffuse plaques contain C-terminal A beta 42 and not A beta 40: evidence from cats and dogs. Neurobiol Aging. 1996b;17(4):653–659. doi: 10.1016/0197-4580(96)00062-0. [DOI] [PubMed] [Google Scholar]
- Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci. 2001;16(1):1–12. doi: 10.1385/JMN:16:1:1. [DOI] [PubMed] [Google Scholar]
- Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci Lett. 1992;138(1):153–156. doi: 10.1016/0304-3940(92)90494-r. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fernandez CI, Collazo J, Bauza Y, Castellanos MR, Lopez O. Environmental enrichment-behavior-oxidative stress interactions in the aged rat: issues for a therapeutic approach in human aging. Ann N Y Acad Sci. 2004;1019:53–57. doi: 10.1196/annals.1297.012. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Marin C, Rey MJ, Ribalta T, Goutan E, Blanco R, Tolosa E, Martí E. BDNF and full-length and truncated TrkB expression in Alzheimer disease, Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58(7):729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Garzon D, Yu G, Fahnestock M. A new brain-derived neurotrophic factor transcript and decrease in brain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer's disease parietal cortex. J Neurochem. 2002;82(5):1058–1064. doi: 10.1046/j.1471-4159.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- Garzon DJ, Fahnestock M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J Neurosci. 2007;27(10):2628–2635. doi: 10.1523/JNEUROSCI.5053-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Head E. Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem Res. 2009;34(4):670–678. doi: 10.1007/s11064-008-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Head E, Liu J, Hagen TM, Muggenburg BA, Milgram NW, Ames BN, Cotman CW. Oxidative damage increases with age in a canine model of human brain aging. J Neurochem. 2002;82(2):375–381. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21(1):89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- Head E, Nukala VN, Fenoglio KA, Muggenburg BA, Cotman CW, Sullivan PG. Effects of age, dietary, and behavioral enrichment on brain mitochondria in a canine model of human aging. Exp Neurol. 2009;220(1):171–176. doi: 10.1016/j.expneurol.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res. 2000;76(2):347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Knusel B, Beck KD, Winslow JW, Rosenthal A, Burton LE, Widmer HR, Nikolics K, Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992;12:4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(19):8856–8860. doi: 10.1073/pnas.92.19.8856. PMCID: PMC41066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg G, Araujo JA. Behavior problems in geriatric pets. Vet Clin North Am Small Anim Pract. 2005;35(3):675–698. doi: 10.1016/j.cvsm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80(3):539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Carroll P, Tzimagiogis G, Thoenen H. Autocrine-paracrine regulation of hippocampal neuron survival by IGF-1 and the neurotrophins BDNF, NT-3 and NT-4. Eur J Neurosci. 1996;8:1452–1460. doi: 10.1111/j.1460-9568.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski B, Fahnestock M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer's disease. Brain Res Mol Brain Res. 2003;111(1–2):148–154. doi: 10.1016/s0169-328x(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Muggenburg B, Holowachuk D, Murphey H, Estrada J, Ikeda-Douglas CJ, Zicker SC, Cotman CW. Landmark discrimination learning in the dog: effects of age, an antioxidant fortified food, and cognitive strategy. Neurosci Biobehav Rev. 2002;26(6):679–695. doi: 10.1016/s0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Weiner E, Thomas E. Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav Neurosci. 1994;108(1):57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas C, Murphey H, Muggenberg BA, Siwak CT, Tapp PD, Lowry SR, Cotman CW. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp Gerontol. 2004;39(5):753–765. doi: 10.1016/j.exger.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26(1):77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002;23(5):737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nippak PM, Mendelson J, Muggenburg B, Milgram NW. Enhanced spatial ability in aged dogs following dietary and behavioural enrichment. Neurobiol Learn Mem. 2007;87(4):610–623. doi: 10.1016/j.nlm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Nistor M, Don M, Parekh M, Sarsoza F, Goodus M, Lopez GE, Kawas C, Leverenz J, Doran E, Lott IT, Hill M, Head E. Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain. Neurobiol Aging. 2007;28(10):1493–1506. doi: 10.1016/j.neurobiolaging.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsdotter K, Lindvall O, Asztely F. Increased synaptic inhibition in dentate gyrus of mice with reduced levels of endogenous brain-derived neurotrophic factor. Neuroscience. 2000;101(3):531–539. doi: 10.1016/s0306-4522(00)00428-0. [DOI] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer's disease. Neurobiol Aging. 2008;29(1):51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. PMCID: PMC2203613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16(6):1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93(6):1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, Francis B, Mount HTJ, Mufson EJ, Salehi A, Fahnestock M. Decreased brain-derived neurotrophic factor depends upon amyloid aggregation state in transgenic mouse models of Alzheimer's disease. J. Neurosci. 2009;29(29):9321–9329. doi: 10.1523/JNEUROSCI.4736-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7(5):695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Poon WW, Blurton-Jones M, Tu CH, Feinberg LM, Chabrier MA, Harris JW, Jeon NL, Cotman CW. beta-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol Aging Jun 18. 2009 doi: 10.1016/j.neurobiolaging.2009.05.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V, Head E, Nistor M, Milgram NW, Muggenburg BA, Cotman CW. Program No. 525.4.2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Reduced Ab deposition with long-term antioxidant diet treatment in aged canines. Online. [Google Scholar]
- Savaskan E, Müller-Spahn F, Olivieri G, Bruttel S, Otten U, Rosenberg C, Hulette C, Hock C. Alterations in trk A, trk B and trk C receptor immunoreactivities in parietal cortex and cerebellum in Alzheimer's disease. Eur Neurol. 2000;44(3):172–180. doi: 10.1159/000008229. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132(3):613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88(2):249–259. doi: 10.1016/j.nlm.2007.05.001. PMCID: PMC2173881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol Aging. 2008;29:39–50. doi: 10.1016/j.neurobiolaging.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Head E, Cotman CW, Murphey H, Muggenburg BA, Ikeda-Douglas C, Milgram NW. Concept abstraction in the aging dog: development of a protocol using successive discrimination and size concept tasks. Behav Brain Res. 2004;153(1):199–210. doi: 10.1016/j.bbr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Tong L, Balazs R, Thornton PL, Cotman CW. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004;24(30):6799–6809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Thornton PL, Balazs R, Cotman CW. Beta -amyloid-(1–42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival is not compromised. J Biol Chem. 2001;276(20):17301–17306. doi: 10.1074/jbc.M010450200. [DOI] [PubMed] [Google Scholar]
- Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, Hwang DY, Cho JY. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22(4):529–539. [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, McCoy JG. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol Behav. 2001;73(4):649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45(3):295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60(12):1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5(4):448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res. 2006;169(1):10–20. doi: 10.1016/j.bbr.2005.11.024. [DOI] [PubMed] [Google Scholar]