Abstract
Compared with apoE3, apoE4 is associated with increased risk to develop age-related cognitive decline, particularly in women. In this study, young, middle-aged, and old female mice expressing human apoE under control of the mouse apoE promoter were behaviorally analyzed. Cognitive performance in the water maze decreased with age in all mice. Compared with apoE2 and apoE3 mice, apoE4 mice showed better cognitive performance and higher measures of anxiety than apoE2 and apoE3 mice. Measures of anxiety correlated with cognitive performance in the water maze and passive avoidance tests and might have contributed to the enhanced cognitive performance of the apoE4 mice. ApoE4 mice showed better water maze learning and higher cortical apoE levels than mice expressing apoE4 in astrocytes under control of the GFAP promoter. This was not seen in apoE3 mice. There were no line differences in either genotype in spatial memory retention in the probe trial following the last day of hidden platform training. Thus, the promoter used to express apoE4 critically modulates its effects on brain function.
Keywords: Aging, Anxiety, Apolipoprotein E, Cognition
In the brain, apolipoprotein E (apoE) plays an important role in lipid transport and metabolism and repair following brain injury (Arendt et al., 1997; Mahley, 1988). The three major human apoE isoforms are encoded by distinct alleles (ε2, ε3, and ε4; Mahley, 1988). Compared with ε2 and ε3, ε4 increases the risk of developing Alzheimer's disease (AD), particularly in women (Farrer et al., 1997; Roses, 1994; Roses, 1995; Saunders et al., 1993). Compared with ε3, ε4 also increases the risk of developing cognitive impairments following various environmental challenges, including neurotrauma (Brichtova and Kozak, 2008; Crawford et al., 2002; Nathoo et al., 2003) and ischemia (Guangda et al., 1999; Raber, 2004).
The apoE4 protein also modulates cognitive function in nondemented elderly individuals. ApoE4 is associated with impaired attention, working memory, and spatial memory in elderly people (Berteau-Pavy et al., 2007; Greenwood et al., 2005a; Greenwood et al., 2005b). In contrast to these findings, however, Alexander et al. showed that apoE4 is associated with enhanced verbal fluency in elderly people (Alexander et al., 2007). The discrepancy in these results might relate to the cognitive test used.
The effects of apoE4 on cognitive function at younger ages are less clear. ApoE4 was reported to protect against lead toxicity and to improve mental development in infants (Wright et al., 2003). Other studies found no association between general cognitive ability and apoE4 in children over the age of 6 (Plomin et al., 1995; Shaw et al., 2007; Turic et al., 2001; Yu et al., 2000). Similarly, there was no association between apoE4 and verbal and non-verbal reasoning as assessed in the Moray House test in 11 yr old children (Deary et al., 2002). However, when these study participants were tested again at age 80, apoE4 was associated with cognitive decline (Deary et al., 2002). Furthermore, 19–21 yr old apoE4 women showed similar WAIS IQ scores to non-apoE4 women (Yu et al., 2000). In contrast to these findings, 7–10 yr old apoE4 children did not show spatial memory retention in the Memory Island spatial navigation test (Acevedo et al., 2010) and 16–30 yr old apoE4 carriers performed less well on a maze test (Alexander et al., 2007). These data suggest that in contrast to general cognitive ability and verbal and non-verbal reasoning, spatial learning and memory might be particularly sensitive to detect negative effects of apoE4 at younger ages.
Increased measures of anxiety are frequently associated with AD (de Toledo et al., 2004; Gustavson and Cummings, 2004) and altered anxiety levels might contribute to performance on cognitive tests. Anxiety symptoms, which are more common among AD patients with a younger age at onset (under age 65; (Porter et al., 2003)), are inversely related to Mini-Mental State Examination (MMSE) scores and correlated with disability in daily activities (Gustavson and Cummings, 2004; Porter et al., 2003). As apoE4 is a risk factor for developing AD at an earlier age (Farrer et al., 1997), it might contribute to this effect (Raber, 2007). In probable AD (PRAD) patients, we found that female and male ε4/ε4 individuals have higher anxiety scores than sex-matched ε3/ε3 individuals (Robertson et al., 2005, Pritchard et al., 2007). Non-cognitive behavioral changes, which include increased anxiety levels, are the major cause of institutionalization of AD patients and a major concern for their caregivers (Gilley et al., 1991; Morris et al., 1996; Rabins et al., 1982) and although non-cognitive behavioral changes are a negative predictor of survival and quality of life for AD patients (Barclay et al., 1985; Martin et al., 1994), they have received much less attention than the cognitive impairments in AD. Most pharmacological strategies for controlling behavioral changes, including treatment with benzodiazepines, cause deterioration in mental performance and motor function (Burvill et al., 1991). However, caregivers often demand therapeutic intervention regardless of the effects on cognitive and motor function, underlining the need to increase our understanding of the potential differential effects of apoE isoforms on anxiety. Furthermore, the differential effects of apoE isoforms on measures of anxiety might not be limited to AD. For example, in patients with chronic hepatitis C undergoing treatment with interferon, individuals with at least one ε4 allele show more symptoms of anxiety than those without an ε4 allele (Gochee et al., 2004).
The effects of apoE on cognitive function have been modeled in mice lacking murine apoE (Apoe−/−) and expressing human apoE in neurons under control of the neuron-specific enolase (NSE) promoter or in astrocytes under control of the glial fibrillary acidic protein (GFAP) promoter. In these mouse models, the effects of apoE4 expression on cognition and behavior are modulated by sex. For example, 6 mo old NSE-apoE4 female, but not male, mice show impaired object recognition and spatial learning and memory in the water maze compared with age-matched NSE-apoE3, Apoe−/−, and wild type C57BL/6J female mice (Raber et al., 1998; Raber et al., 2000b; Raber et al., 2002). Similarly, 6 mo old GFAP-apoE4 female mice show impaired spatial memory retention in the water maze compared with GFAP-apoE3 female mice (van Meer et al., 2007) and impaired object recognition that was not observed in 6 mo old Apoe−/− mice (Raber et al., 2002). The detrimental effects of apoE4 on water maze learning and memory in female mice was more pronounced at 18 mo of age (Raber et al., 2000). At this older age, however, male apoE4 mice still did not show cognitive impairments in the water maze. This gender-dependent effect on age-related cognitive decline (ACD) might not be limited to apoE4. In C57BL/6J wild type mice, the age-related decline in water maze performance and passive avoidance memory retention was also more pronounced in female than male mice (Benice et al., 2006).
Previously, we reported higher measures of anxiety in 6 mo old Apoe−/− male mice than in sex-matched wild type controls (Raber et al., 2000a). Consistent with the PRAD data, there were isoform-dependent effects of apoE on measures of anxiety in 6–8-mo old male Apoe−/−, NSE- and GFAP-apoE3 and apoE4 mice (Raber et al., 2000b; Robertson et al., 2005). Apoe−/− and apoE4 male mice showed increased measures of anxiety, whereas apoE3 mice behaved like wild type controls. These differential effects were age-dependent, as they were not seen in 2–4-mo old male mice.
The effects of apoE on measures of anxiety and cognitive function can also be studied in mice expressing human apoE isoforms under control of the mouse apoE promoter (targeted replacement (TR) mice; Knouff et al., 1999; Sullivan et al., 1997; Sullivan et al., 2004). In contrast to the NSE-apoE and GFAP-apoE models, in the TR mice apoE is expressed under control of the mouse apoE promoter and therefore expressed in both the brain and the periphery. In the brain, the use of different promoters to express apoE and important physiological feedback loops might modulate the anatomical specificity and levels of apoE expression and subsequently the effects of apoE on brain function. In addition, the presence or absence of apoE in the periphery might also modulate the effects of apoE on brain function. For example, in the adrenal gland, apoE mRNA is inversely related to steroidogenesis (Nicosia et al., 1992; Prack et al., 1991) and Apoe−/− mice show increased adrenal glucocorticoid content and release following anxiety testing or restraint stress (Raber et al., 2000). Enhanced glucocorticoid release following anxiety testing and restraint stress were also seen in mice expressing apoE3 or apoE4 only in the brain (Robertson et al., 2005). Thus, the behavioral phenotype of mice expressing apoE under control of the NSE or GFAP promoter might be different from that of the TR mice.
Effects of apoE on cognitive function in the TR mice have been reported but the results are inconsistent. While Mathis and co-workers reported impaired spatial memory retention in the water maze of apoE4 female mice at 4–5 mo and 15–18 mo of age (Bour et al., 2008; Grootendorst et al., 2005), we did not see impairments in spatial memory retention in the water maze of apoE4 female or male mice at 5 mo of age following sham-irradiation (one time anesthesia) at 2 mo of age (Villasana et al., 2006). Similarly, while Mathis and co-workers reported no difference in passive avoidance memory retention of apoE3 and apoE4 mice at 4–5 mo of age but impaired passive avoidance retention of both male and female apoE4 mice at older ages (Bour et al., 2008; Grootendorst et al., 2005), we found enhanced passive avoidance memory retention of apoE3 and apoE4 male mice at 5 mo of age compared with sex-matched apoE2 mice following sham-irradiation at 2 mo of age (Villasana et al., 2008). Although the results were divergent, these data support that female mice are more susceptible to the effects of apoE4, consistent with the increased susceptibility of apoE4 female than male mice to develop cognitive impairment following 137Cesium irradiation (Villasana et al., 2006). In the current study, we examined whether apoE has isoform-dependent effects on measures of anxiety in female TR mice and whether these effects are age-dependent and associated with alterations in cognitive performance and levels of apoE expression in brain areas involved in the regulation of anxiety and cognitive performance, the amygdala, hippocampus, and cortex. In addition, we compared the water maze performance and apoE expression levels of young TR female mice with that of age-, sex-, and genotype-matched GFAP-apoE mice.
1. Methods
1.1. Animals
Human APOE TR mice expressing human apoE2, apoE3, or apoE4, under control of the mouse apoE promoter and on the C57BL/6J background, were provided by Dr Patrick Sullivan for breeding of the mice for this study (Knouff et al., 1999; Sullivan et al., 1997). The colony was maintained by homozygous breeding. Young (6–8 mo), middle-aged (10–13 mo) and old (14–22 mo) female apoE2 (n = 31–43), apoE3 (n = 34–40), and apoE4 (n = 25–39) mice were used for behavioral testing. In addition, young (6–8 mo) GFAP-apoE3 (n = 9) and GFAP-apoE4 (n = 8) female mice were used for water maze testing. The mice were maintained on a 12 h light/dark schedule (lights on at 6:00). Behavioral testing took place during the light cycle. Laboratory chow (PicoLab Rodent diet 20, # 5,053; PMI Nutrition International, St. Louis MO, USA) and water were given ad libitum. All procedures conformed to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University.
1.2. Behavioral testing
The sequence of behavioral testing was open field activity, elevated zero maze, elevated plus maze, Morris water maze, and passive avoidance. This order of testing was used to begin with the least stressful tests and end with those thought to be most stressful. Deaths or illnesses in the mice resulted in some mice only being included in some of the tests. Table 1 shows the number of mice for each genotype and age group included in each behavioral test.
Table 1.
Number of apoE targeted replacement mice within each genotype and age group included in the behavioral tests
| Behavioral test | Age | ApoE2a | ApoE3a | ApoE4a |
|---|---|---|---|---|
| Open field | Youngb | 9 | 10 | 9 |
| Middle-agedc | 7 | 8 | 10 | |
| Oldd | 27 | 20 | 17 | |
| Elevated zero maze | Youngb | 9 | 10 | 9 |
| Middle-agedc | 6 | 8 | 11 | |
| Oldd | 25 | 17 | 17 | |
| Elevated plus maze | Youngb | 8 | 10 | 9 |
| Middle-agedc | 7 | 8 | 12 | |
| Oldd | 26 | 22 | 18 | |
| Morris water maze | Youngb | 8 | 10 | 9 |
| Middle-agedc | 6 | 8 | 9 | |
| Oldd | 19 | 17 | 8 | |
| Passive avoidance | Youngb | 8 | 10 | 9 |
| Middle-agedc | 7 | 7 | 10 | |
| Oldd | 25 | 21 | 16 |
ApoE = apolipoprotein E.
Young = 6–8 mo of age.
Middle-aged = 10–13 mo of age.
Old = 14–22 mo of age.
1.2.1. Open field activity
Exploratory activity was assessed in the open field. Mice (N = 117) were placed in a lit open arena with clear Plexiglas walls (40.6 × 40.6 cm) equipped with infrared photocells interfaced with a computer (Hamilton-Kinder, Poway, CA). Distance moved was recorded for a single 10-min session. The open field arena was cleaned with 5% acetic acid between mice.
1.2.2. Elevated zero maze
Anxiety-like behavior was assessed in the elevated zero maze and elevated plus maze. The elevated zero maze consisted of two open and two closed areas (35.5 cm in length, each; Hamilton-Kinder, Poway, CA). The closed areas were surrounded by opaque walls (15 cm tall), with no walls surrounding the open areas. Mice (N = 112) were placed into the maze facing one of the closed areas and were allowed to explore for a single 10-minute trial. More anxious mice will spend more time in the closed areas of the maze (Shepherd et al., 1994). Distance moved and the percentage of time in the open and closed areas were measured with a video tracking system (Noldus, Leesburg, VA). The zero maze was cleaned with 5% acetic acid between mice.
1.2.3. Elevated plus maze
Anxiety-like behavior was also assessed in the elevated plus maze. The maze consisted of two open and two closed arms equipped with infrared photocells interfaced with a computer (34 cm in length, each; Hamilton-Kinder, Poway, CA). The closed arms were surrounded by black walls (15 cm tall), with no walls surrounding the open arms. Mice (N = 120) were placed into the intersection of the maze facing one of the closed arms and were allowed to explore for a single 10-min trial. More anxious mice will spend more time in the closed arms of the maze (Pellow et al., 1985). Distance moved and the percentage of time in the open and closed arms were measured. The maze was cleaned with 5% acetic acid between mice.
1.2.4. Morris water maze
Spatial learning and memory requiring navigation was assessed in the Morris water maze. A circular pool (diameter 140 cm) was filled with water and divided into four imaginary quadrants for the purpose of testing and probe trial data analysis. Mice (N = 94) were assigned to four testing groups using a randomized block design. Each group was trained to find a platform in a different quadrant of the pool to avoid any potential quadrant bias. Mice were first trained to swim to a platform with a visible beacon marking the location of the platform (nonspatial learning, days 1–2) and then trained to swim to a platform hidden beneath opaque water (spatial learning, days 3–5) in two daily sessions, spaced 3.5 h apart and each consisting of 3 sixty-second trials (10 min intertrial intervals). During visible platform training, the platform was moved to a different quadrant of the pool for each session. During hidden platform training, the platform remained in the same quadrant. Mice were placed into the pool facing the wall in nine different locations around the pool. The starting location changed from trial to trial. Mice had to rely on spatial cues in the room to find the hidden platform. Mice that failed to find the platform within the 60-sec trial were led to the platform and allowed to remain on the platform for 3 sec.
Swimming patterns were recorded using a video tracking system (Noldus, Leesburg, VA), set at six samples/seconds. The distance from the mouse to the platform was summed across all samples during the trial (cumulative distance to the platform). Mice with better spatial learning will swim closer to the platform location during the trial and thus will have lower cumulative distance scores (Benice et al., 2006; Gallagher et al., 1993). The time to find the platform (latency) and the average swim speeds were also analyzed. Sixty second probe trials (no platform), designed to examine the extent of spatial discrimination learning, were performed 1 h after the last hidden platform training session on days 3–5. For the probe trials, mice were placed in the quadrant opposite from the target quadrant (where the platform was located during hidden platform sessions).
1.2.5. Passive avoidance
Emotional learning and memory were assessed in a passive avoidance test. Mice (N = 113) were individually placed into one compartment of a chamber containing two equally sized sound attenuating compartments (24.5 × 19 × 23 cm), separated by a gate (Hamilton-Kinder, Poway, CA). After a 5-sec acclimation period, a house light turned on in the chamber containing the mouse and the connecting gate between the chambers opened. In general, mice will step quickly through the gate and enter the dark compartment because mice prefer to be in the dark. Upon entering the dark compartment, the mice received a brief foot shock (0.3 mA for 3 sec), and were immediately removed from the chamber. If the mouse remained in the light compartment for the duration of the trial (120 sec), the gate closed and the mouse was removed from the light compartment. The next trial began after an intertrial interval of 180 sec. Mice were trained until they met a learning criterion of three consecutive trials without entering the dark compartment, or up to 10 trials, whichever came first. The chambers were cleaned with 5% acetic acid between trials for each mouse and between testing of individual mice. After a 24 h retention period, the mice were placed back into the light compartment, and the time to re-enter the dark compartment (latency) was measured up to 300 sec. No shock was administered during the testing phase if the mouse entered the dark compartment before 300 sec had elapsed. The number of trials to reach criterion during training and the latency to re-enter the dark compartment 24 h later were measured.
1.3. ApoE Western blot analyses
Young TR mice expressing human apoE2, apoE3, or apoE4 and GFAP-apoE3 and GFAP-apoE4 female mice (n = 14) and old TR female mice expressing human apoE2, apoE3, or apoE4 (n = 9) were killed by cervical dislocation and their brains quickly removed. An Apoe−/− mouse was killed similarly to serve as a negative control for apoE expression on all western blots. Following a cut along the midline, one hemibrain was used to dissect the amygdala as described (Raber et al., 1997). The other hemibrain was used to dissect the hippocampus and cortex, as described (Bongers et al., 2004). The tissues were homogenized using 1 mL (cortex samples) or 0.5 mL (hippocampus or amygdala samples) of RIPA buffer (Pierce Pharmaceuticals, Rockford, IL) containing a cocktail of protease inhibitors (Sigma, Saint Louis, MO). Homogenized tissues were spun at 12,000g for 15 min, and the supernatants were transferred to new tubes and kept for analysis. Protein concentrations were determined using a Nanodrop Spectrometer (Nanodrop ND-1000).
For each sample and lane, 40 μg of protein was used for western blot analysis. Proteins were first denatured for 15 min by boiling them in a solution of Laemmli's buffer containing 2-mercaptoethanol (Sigma-Aldrich). Pre-prepared gels (Criterion Bio-Rad Ready Gels, 4–15% Tris×HCl, 18 well) were placed in the electrophoresis apparatus. In addition to the tissue samples, one lane was used for the Precision plus Protein Western Standard (Bio-Rad). Gels were run using a Bio-Rad Power Pac for 60 min at 175 V. Subsequently, proteins were transferred to PVDF membranes for 90 min at 30 V.
Once proteins were transferred to the membranes, the membranes were placed in blocking buffer (5% dry-milk in PBS containing 0.5% Tween 20 (TBS-Tween)) for 1 h. Membranes were washed with the TBS-Tween buffer (4 × 5 min) and incubated with the dry-milk buffer containing β-actin antibody (Santa Cruz Biotechnology, raised in mouse, 0.5 μg/mL) for 12 h at 4 °C. Membranes were washed with TBS-Tween buffer (4 × 5 min) and incubated in the dry-milk buffer containing the secondary antibody for detecting the primary antibody against β-actin, donkey anti-mouse-HRP (Santa Cruz, 1 μg/mL) for 1 h and the secondary antibody for detecting the protein ladder, Precision protein StrepTactin-HRP (Bio-Rad; 5 μL). Subsequently, the membranes were incubated in Immun-Star HRP (Bio-Rad) for 5 min and imaged (FluorChem Q, Alpha Innotech, San Leandro, CA). After imaging, the antibodies were stripped from the membranes using stripping buffer (Pierce Pharmaceuticals) for 5 min at room temperature. Membranes were reblocked in blocking buffer (5% dry-milk TBS-Tween) for 1 h. Membranes were then washed in TBS-Tween buffer (4 × 5 min) and incubated with apoE antibody (Calibiochem; raised in goat; 1 : 4,000) for 12 h at 4 °C. Membranes were washed in TBS-tween buffer (4 × 5 min) and incubated in secondary antibody, antigoat-HRP (raised in donkey, 1 μg/mL, Santa Cruz Biotechnology). Pixel densities of the β-actin and apoE bands were determined using FluorChem Q software. Background levels were automatically determined by the software using upper- and lower-edge interpolation. Semi quantification using densitometry for the β-actin and apoE bands were measured for each sample. The results are presented as a ratio between the apoE and β-actin bands.
1.4. Statistical analysis
Analysis of variance (ANOVA) was used to assess the effects of genotype and age on performance in the behavioral tests. A repeated measure ANOVA was used to assess learning and memory in the water maze training sessions and probe trials (repeated factor: session and probe trial; Fisher's LSD post-hoc test used when appropriate). Duncan's post-hoc tests were performed to compare performance between genotypes and age groups when appropriate. Pearson and Spearman's correlations were used to assess potential correlations between measures of anxiety in the elevated zero maze and elevated plus maze and measures of learning and memory in the Morris water maze and the passive avoidance tests. For the semiquantification of immunoblots, results for each brain area were analyzed using one-way ANOVAs and Bonferroni's post-hoc tests. p < 0.05 was considered significant for all tests.
2. Results
2.1. Impaired spatial learning of middle-aged and old mice and impaired spatial memory of old mice in the Morris water maze
During the visible platform training sessions (days 1–2), middle-aged apoE4 mice swam faster than middle-aged apoE3 mice and old apoE4 mice swam faster than both old apoE2 and apoE3 mice (effect of genotype (F2,85 = 7.09, p < 0.01) on average swim speed and an age × genotype interaction (F4,85 = 2.62, p = 0.04)). The cumulative distance to the platform decreased over the visible training sessions (main effect of session on cumulative distance to the platform; F1,85 = 119.23, p < 0.001), suggesting that the mice learned the task. Across all sessions and age groups, apoE4 mice showed a lower cumulative distance to the visible platform than apoE2 and apoE3 mice (main effect of genotype; F2,85 = 17.10, p < 0.001). Across all sessions and genotypes, young mice showed a lower cumulative distance to the visible platform than both middle-aged and old mice (main effect of age; F2,85 = 8.20, p < 0.01). There was no interaction between genotype and age for the visible platform training sessions. As swim speed can influence cumulative distance to the platform during water maze training, average swim speed during the visible platform training was used as a covariate in the statistical analysis. The effects of genotype and age did not change when the average swim speed during the visible session was included as a covariate (Fig. 1A, covariate adjusted means ± SEM). The results for time to reach the platform (latency) during the visible training sessions were similar to those for cumulative distance to the platform.
Fig. 1.
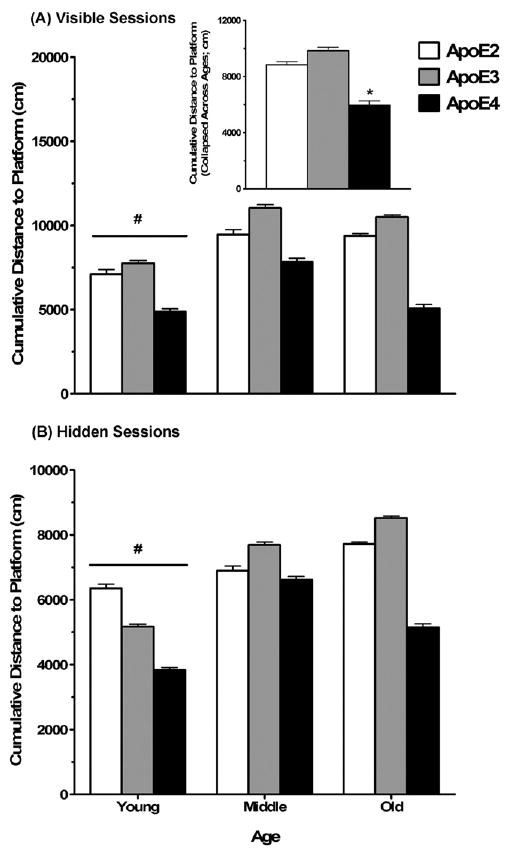
Increased task learning in the Morris water maze in young female mice and apoE4 female mice. (A) Young mice showed a lower cumulative distance to the platform in the visible platform training sessions compared with middle-aged and old mice. ApoE4 mice showed a lower cumulative distance to the visible platform compared with apoE2 and apoE3 mice. The inset shows cumulative distance to the visible platform collapsed across age, as there was no interaction between age and genotype. (B) Young mice showed a lower cumulative distance to the hidden platform compared with middle-aged and old mice. Note: the graphs represent visible session swim speed covariate-adjusted means ± SEM #p < 0.05 versus middle-aged and old mice. *p < 0.05 versus apoE2 and apoE3 mice. n = 6–19 mice per genotype per age.
During the hidden training sessions (days 3–5), the cumulative distance to the platform decreased over the sessions (main effect of session on cumulative distance to the platform; F1,85 = 54.66, p < 0.001), indicating that the mice learned the hidden task as well. Across all sessions and across all age groups, apoE4 mice showed a lower cumulative distance to the hidden platform than apoE2 and apoE3 mice (main effect of genotype; F2,85 = 4.43, p = 0.02). Across all hidden sessions and across all genotypes, young mice showed a lower cumulative distance to the hidden platform than middle-aged and old mice (main effect of age; F2,85 = 5.19, p < 0.01), but there was no genotype × age interaction. When average visible session swim speed was included as a covariate, the effect of age remained, with young mice showing a lower cumulative distance to the platform compared with middle-aged and old mice (Fig. 1B, covariate adjusted means ± SEM). The effect of genotype, however, became nonsignificant (F2,84 = 3.05, p > 0.05; Fig. 1B, covariate adjusted means ± SEM), suggesting that the lower cumulative distance to the platform of apoE4 mice was due to their faster swim speeds. During the hidden training sessions, the effect of age on the latency to reach the platform was similar to those found for cumulative distance to the platform. In contrast to the effects of genotype on cumulative distance to the platform, the effect of genotype on latency to reach the platform remained significant when average swim speed during the visible sessions was included as a covariate in the analysis.
When spatial memory retention was assessed in the probe trials (no platform, days 3–5), there was a probe trial × genotype × age interaction (F8,162 = 2.71, p < 0.01). Thus, the effects of genotype and age were explored for each probe trial separately. Young and middle-aged mice showed a lower cumulative distance to the platform location (during the hidden training sessions) compared with old mice in probe trial 1 (main effect of age; F2,81 = 7.14, p < 0.01; Fig. 2A) and in probe trial 2 (main effect of age; F2,85 = 4.03, p = 0.02; Fig. 2B). In probe trial 3, young, but not middle-aged, mice showed a lower cumulative distance to the platform location compared with old mice (main effect of age; F2,85 = 6.15, p < 0.01; Fig. 2C). There was no interaction between genotype and age for any of the probe trials. Importantly, there were no genotype differences in any of the probe trials.
Fig. 2.
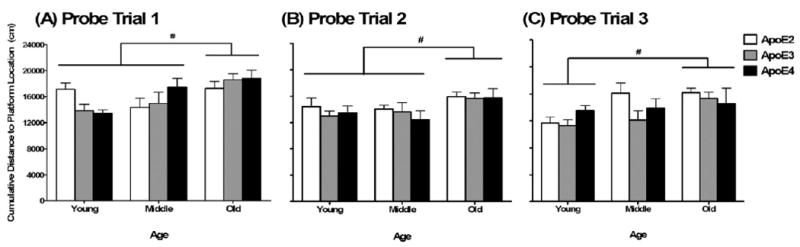
Impaired spatial memory retention in the Morris water maze probe trials in old female mice. (A and B) Old mice showed an increased cumulative distance to the platform location during the hidden trials compared with middle-aged and young mice in probe trials 1 and 2. (C) Old mice showed an increased cumulative distance to the platform location during the hidden trials compared with young mice in probe trial 3. #p < 0.05 versus other groups. n = 6–17 mice per genotype per age.
2.2. Enhanced passive avoidance learning in apoE4 mice and impaired passive avoidance memory retention in old apoE2 mice
One animal was removed from the passive avoidance analysis due to a failure to enter the dark compartment during the first passive avoidance learning trial. For passive avoidance learning (trials to criterion), there was a genotype × age interaction (F4,104 = 4.17, p < 0.01). Thus, the effect of genotype on trials to criterion was explored within each age group using separate one-way ANOVAs. In the young mice, apoE2 and apoE4 mice required fewer trials to reach criterion than apoE3 mice (main effect of genotype; F2,24 = 5.85, p < 0.01; Fig. 3A). In the middle-aged mice, apoE3 and apoE4 mice required fewer trials to reach criterion than apoE2 mice (main effect of genotype; F2,21 = 6.94, p < 0.01; Fig. 3A). In the old mice, apoE4 mice required fewer trials to reach criterion than both apoE2 and apoE3 mice, while apoE3 mice required fewer trials to reach criterion than apoE2 mice (main effect of genotype; F2,59 = 16.14, p < 0.001).
Fig. 3.
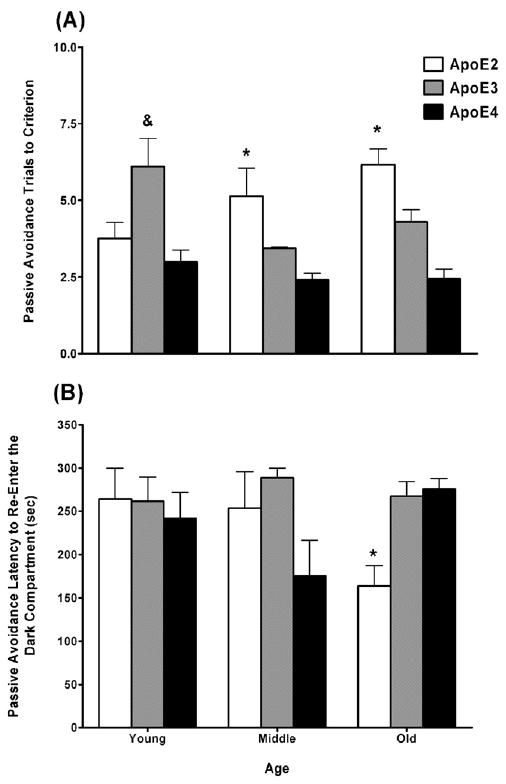
Enhanced passive avoidance learning in apoE4 female mice and impaired passive avoidance retention in old apoE2 female mice. (A) Young apoE3 mice and middle-aged and old apoE2 mice required more trials to reach criterion during passive avoidance training. (B) Old apoE2 mice showed reduced latency to re-enter the dark compartment where they previously received an aversive stimulus 24 h after training. *p < 0.05 versus apoE3 and apoE4 mice. &p < 0.05 versus apoE2 and apoE4 mice. n = 7–25 mice per genotype per age.
For passive avoidance memory retention (latency to reenter the dark compartment), there was a genotype × age interaction (F4,106 = 4.03, p < 0.01). Thus, the effect of genotype on test duration was explored within each age group using separate one-way ANOVAs. In both the young and middle-aged mice, there was no effect of genotype on latency to re-enter the dark compartment. However, in the old mice, apoE2 mice showed a lower latency to re-enter the dark compartment than apoE3 and apoE4 mice (main effect of genotype; F2,60 = 10.58, p < 0.001; Fig. 3B).
2.3. Decreased activity of apoE4 mice in the open field
As exploratory behavior and measures of anxiety can contribute to performance in cognitive tests, we assessed whether there are genotype differences in exploratory behavior in the open field or measures of anxiety in the elevated zero maze or elevated plus maze. Across all age groups, apoE4 mice moved less in the open field than apoE2 and apoE3 mice (main effect of genotype; F2,108 = 17.66, p < 0.001; Fig. 4). Middle-aged and old mice moved less in the open field than young mice across all genotypes (main effect of age; F2,108 = 16.36, p < 0.001; Fig. 4), but there was no genotype × age interaction.
Fig. 4.
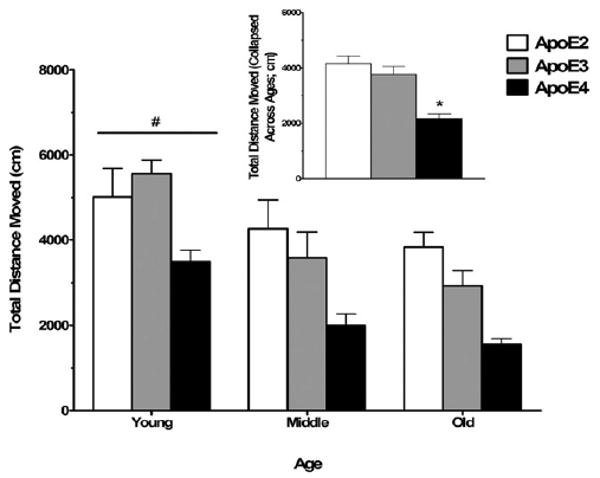
Reduced activity levels in the open field among middle-aged and old female mice and apoE4 female mice. Middle-aged and old mice moved less distance in the open field compared with young mice. ApoE4 mice moved less distance than apoE2 and apoE3 mice. The inset shows total distance moved collapsed across ages, as there was no interaction between age and genotype. #p < 0.05 versus middle-aged and old mice. *p < 0.05 versus apoE2 and apoE3 mice. n = 7–27 mice per genotype per age.
2.4. Increased measures of anxiety in apoE4 mice in the elevated zero maze and elevated plus maze
Across all age groups, apoE4 mice spent less percent time in the open areas of the elevated zero maze than apoE2 and apoE3 mice (main effect of genotype; F2,103 = 18.09, p < 0.001; Fig. 5). Young mice spent less percent time in the open areas of the elevated zero maze than middle-aged and old mice across all genotypes (main effect of age; F = (2, 103) = 4.83, p = 0.01; Fig. 5). ApoE4 mice also spent less percent time in the open arms of the elevated plus maze than apoE2 and apoE3 mice, while apoE2 mice spent less percent time in the open arms of the elevated plus maze than apoE3 mice (main effect of genotype; F2,109 = 6.90, p < 0.01; Fig. 6). Middle-aged mice spent less percent time in the open arms of the elevated plus maze than young and old mice across all genotypes (main effect of age; F2,109 = 3.61, p = 0.03; Fig. 3), but there were no interactions between genotype and age for either test. As apoE4 mice were less active in the open field, we reanalyzed the data using the total distance moved as a covariate to exclude the possibility that differences in overall activity levels contributed to the genotype differences in measures of anxiety. The effects of genotype and age remained significant for both the elevated zero maze and plus maze when total distance moved in each maze was included as a covariate in each analysis.
Fig. 5.
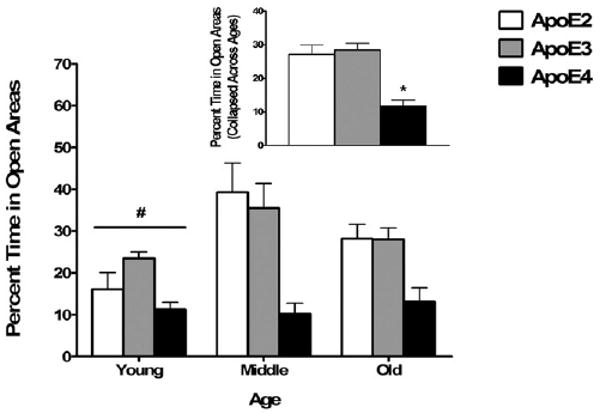
Increased measures of anxiety in the elevated zero maze among young female mice and apoE4 female mice. Young mice spent less time in the open areas of the zero maze compared with middle-aged and old mice. ApoE4 mice spent less time in the open areas of the zero maze compared with apoE2 and apoE3 mice. The inset shows the percentage of time spent in the open areas collapsed across ages, as there was no interaction between age and genotype. #p < 0.05 versus middle-aged and old mice. *p < 0.05 versus apoE2 and apoE3 mice. n = 6–25 mice per genotype per age.
Fig. 6.
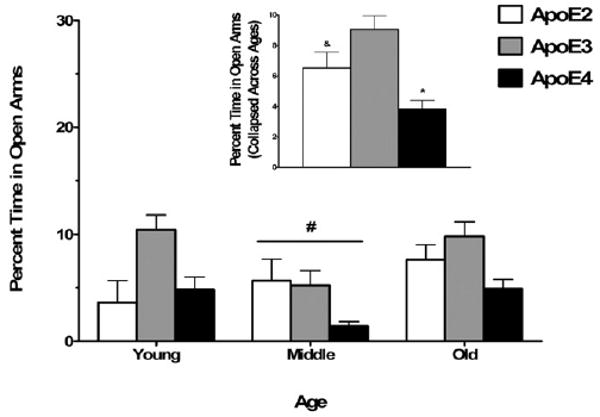
Increased measures of anxiety in the elevated plus maze among middle-aged female mice and apoE4 female mice. Middle-aged mice spent less time in the open arms of the plus maze compared with young and old mice. ApoE4 mice spent less time in the open arms of the plus maze compared with both apoE2 and apoE3 mice, while apoE2 mice spent less time in the open arms compared with apoE3 mice. The inset shows the percentage of time spent in the open arms collapsed across ages, as there was no interaction between age and genotype. #p < 0.05 versus young and old mice. *p < 0.05 versus apoE2 and apoE3 mice. &p < 0.05 versus apoE3 mice. n = 7–26 mice per genotype per age.
2.5. Association between measures of anxiety and learning and memory
To determine whether measures of anxiety might have contributed to performance on the cognitive tests, we assessed potential correlations between measures of anxiety (the percent time spent in the open areas of the elevated zero maze and open arms of the elevated plus maze) with measures of learning and memory in the Morris water maze and the passive avoidance tests. During the visible training sessions of the water maze, the mice with higher measures of anxiety showed better performance. Percent time in the open areas of the elevated zero maze correlated with cumulative distance to the platform (r = 0.29, p < 0.01; Fig. 7A) and latency to reach the platform (r = 0.32, p < 0.01; Fig. 7B) during the visible training sessions of the water maze. There were trends of percent time in the open arms of the elevated plus maze correlating with cumulative distance to the platform and latency to reach the platform during the visible training sessions of the water maze, but they did not reach significance (r = 0.21, p = 0.06 and r = 0.21, p = 0.05, respectively). Measures of anxiety did not correlate with spatial learning during the hidden sessions of the water maze or spatial memory retention in the water maze probe trials.
Fig. 7.
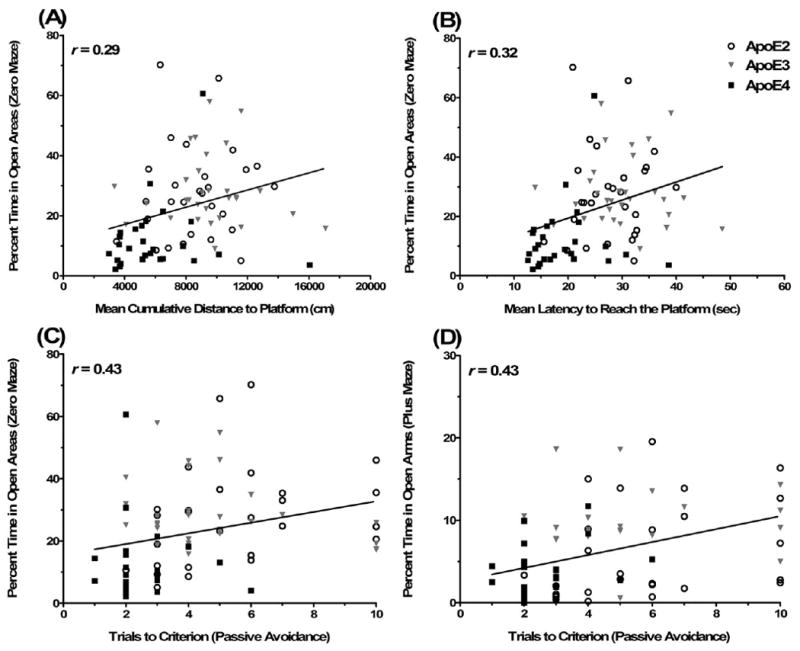
Measures of anxiety were correlated with learning and memory in the Morris water maze and passive avoidance tests. (A) Percent time in the open areas of the elevated zero maze correlated with the mean cumulative distance to the platform in the visible platform training sessions of the Morris water maze. (B) Percent time in the open areas of the elevated zero maze correlated with the mean latency to reach the platform in the visible platform training sessions of the Morris water maze. (C) Percent time in the open areas of the elevated zero maze correlated with the number of trials to reach criterion in passive avoidance training. (D) Percent time in the open arms of the elevated plus maze correlated with the number of trials to reach criterion in passive avoidance training.
During passive avoidance learning, the mice with higher measures of anxiety showed better performance as well. Percent time in the open areas of the elevated zero maze correlated with trials to criterion during passive avoidance training (r = 0.43, p < 0.001; Fig. 7C). Similarly, percent time in the open arms of the elevated plus maze correlated with trials to criterion during passive avoidance training (r = 0.42, p < 0.001; Fig. 7D). Percent time in the open areas of the elevated zero maze or open arms of the plus maze did not correlate with latency to re-enter the dark compartment 24 h after passive avoidance training.
2.6. Comparison between targeted replacement and GFAP apoE3 and apoE4 mice in the water maze
We compared the direction and magnitude of the genotype effect of the young TR mice in the water maze with that of age-matched GFAP-apoE mice. While there was no difference in the ability of apoE3 TR mice and GFAP-apoE3 mice to locate the platform in the water maze, apoE4 TR mice outperformed GFAP-apoE4 mice (F1,15 = 5.02, p = 0.04; Fig. 8A). In contrast to the water maze learning curves, when spatial memory retention was assessed in the probe trial following the last day of hidden platform training, there were no line differences between the cumulative distance to the target location in either apoE3 or apoE4 mice (Fig. 8B). ApoE4 mice showed less spatial memory retention than apoE3 mice and showed a higher cumulative distance to the target location.
Fig. 8.
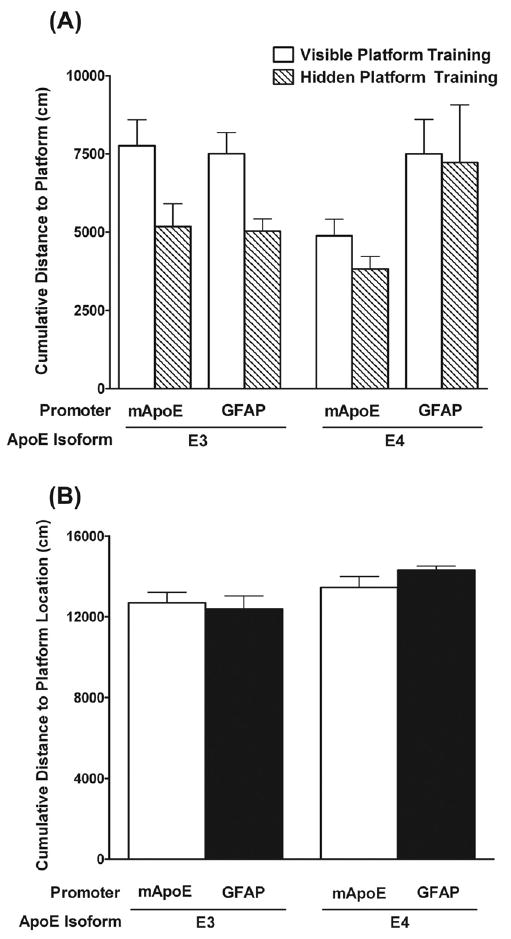
Comparison of water maze performance between young apoE targeted replacement and GFAP-apoE mice. (A) GFAP-apoE4 mice showed poorer water maze learning (increased cumulative distance to the target platform) than apoE4 targeted replacement mice. This was not seen in the two lines of apoE3 mice. (B) GFAP-apoE and apoE targeted replacement mice showed similar genotype differences in spatial memory retention in the probe trial following the last day of hidden platform training with apoE4 mice showing worse spatial memory retention than apoE3 mice. n = 6–19 mice per genotype.
2.7. ApoE expression levels in the amygdala, hippocampus, and cortex of targeted replacement and GFAP apoE mice
Next, we determined whether the genotype differences in measures of anxiety and cognitive performance are associated with genotype differences in apoE expression in the amygdala, hippocampus, or cortex. As shown in the inset of Fig. 9, immunoreactivity of apoE was specific, as no protein was detected in brain tissue of an Apoe−/− mouse. There were no significant differences between apoE expression in the amygdala or hippocampus of young and old apoE2, apoE3, or apoE4 TR mice (Fig. 9). However, in the cortex of apoE2 mice, young mice had significantly more apoE than old mice (F5,15 = 3.7; p < 0.03). Such an age effect was not seen in the cortex of apoE3 or apoE4 mice.
Fig. 9.
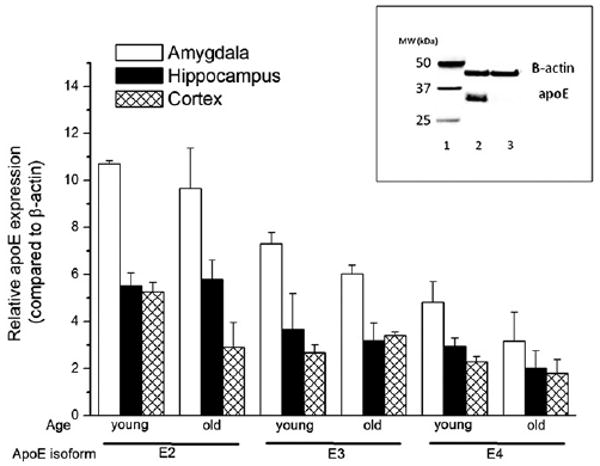
ApoE expression levels in the amygdala, hippocampus, and cortex of young and old apoE targeted replacement mice. ApoE levels are expressed relative to β-actin levels. The inset shows representative β-actin and apoE levels in brain tissue of a human apoE targeted replacement mouse and an Apoe−/− mouse. Lane 1 shows the protein ladder, lane 2 shows the β-actin and apoE levels in brain the tissue of a targeted replacement mouse, and lane 3 shows the β-actin levels in brain tissue of an Apoe−/− mouse. n = 3 mice per genotype per time point.
In the amygdala (F5,15 = 6.2; p = 0.009), hippocampus (F5,15 = 4.2; p = 0.026), and cortex (F5,15 = 3.7; p = 0.033), apoE levels were higher in apoE2 than apoE4 mice (Fig. 9). In the amygdala, there was a trend towards higher apoE levels in apoE3 than apoE4 mice and lower apoE levels in apoE3 than apoE2 levels but that did not reach significance (p = 0.1). To determine whether potential differences in apoE expression could have contributed to the divergent water maze learning curves of TR apoE4 and GFAP-apoE4 mice, we compared apoE expression in these two models. There was no difference in apoE expression in the amygdala or hippocampus of TR apoE4 and GFAP-apoE4 mice (Fig. 10; p = 1.0). However, apoE4 levels were lower in the cortex of GFAP-apoE4 than apoE4 TR mice (F7,18 = 8.3; p < 0.01; Fig. 10). In contrast to apoE4 mice, apoE expression levels were lower in the amygdala (F7,18 = 8.3; p = 0.001), hippocampus (F7,18 = 3.9; p = 0.008), and cortex (F7,18 = 6.9; p = 0.01) of GFAP-apoE3 mice than apoE3 TR mice (Fig. 10). In contrast to the TR mice, apoE expression levels were lower in the amygdala (p = 0.002) and hippocampus (p = 0.001), with a trend towards lower apoE levels in the cortex (p = 0.09) of the GFAP-apoE3 mice than GFAP-apoE4 mice.
Fig. 10.
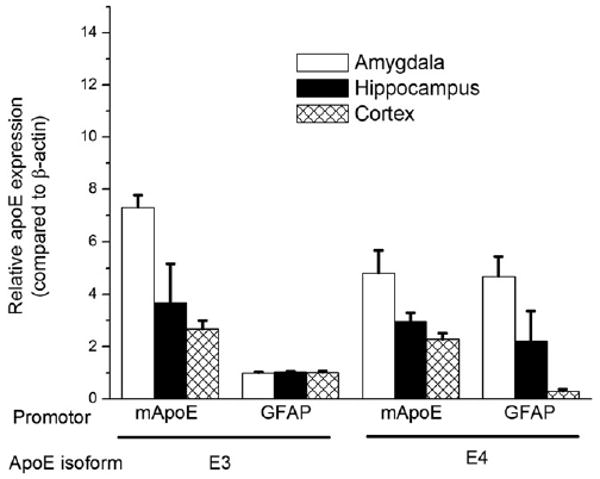
ApoE expression levels in the amygdala, hippocampus, and cortex of young apoE targeted replacement and GFAP-apoE mice. ApoE levels are expressed relative to β-actin levels. n = 15 mice.
3. Discussion
While apoE4 modulates measures of anxiety in probable AD patients (Robertson et al., 2005) and the risk to develop AD and cognitive impairments in nondemented healthy elderly (Berteau-Pavy et al., 2007; Farrer et al., 1997; Greenwood et al., 2005a; Greenwood et al., 2005b; Saunders et al., 1993), little is known about the potential relationship between apoE4, measures of anxiety, and cognitive performance across ages. In the present study, we behaviorally analyzed female apoE TR mice expressing apoE under control of the mouse apoE promoter. ApoE4 mice showed decreased activity levels and higher measures of anxiety than mice expressing apoE2 or apoE3 across all ages. The increased measures of anxiety of the apoE4 mice may have contributed to their enhanced performance in the water maze and passive avoidance tests. In addition, middle-aged and old mice showed impaired task learning during the visible sessions and impaired spatial learning during the hidden sessions of the water maze compared with young mice. In the water maze probe trials, only old mice showed impaired spatial memory retention compared with young mice. These findings show a consistent age-related decline in learning and memory across all genotypes.
In contrast to learning and memory, there were no consistent age-related effects on measures of anxiety. In the elevated zero maze, young mice showed the highest measures of anxiety, whereas in the elevated plus maze, middle-aged mice showed the highest measures of anxiety. However, in both tests old female mice of all genotypes showed lower measures of anxiety than younger mice. These findings are consistent with observations in humans, where healthy aging is generally associated with a decrease in anxiety levels (for a review, see Jorm, 2000).
ApoE4 female TR mice showed enhanced performance during water maze and passive avoidance training. As apoE4 female mice showed both enhanced task learning during the visible platform sessions and enhanced spatial learning during the hidden sessions, we cannot exclude the possibility that enhanced ability to learn the task contributed to the enhanced spatial learning in the mice. These cognitive data are consistent with our earlier studies in apoE4 TR female mice (Villasana et al., 2006; Villasana et al., 2008) but inconsistent with the impaired cognitive function in apoE4 TR mice reported by Mathis and co-workers (Bour et al., 2008; Grootendorst et al., 2005). Differences in environmental conditions, such as environmental stress, might have contributed to these divergent findings. ApoE4 is only a risk factor to develop ACD and cognitive impairments following environmental challenges. Consistent with this scenario, we found that apoE4 female mice are more susceptible to the detrimental effects of 137Cs cranial irradiation on water maze performance than apoE2 female mice and on passive avoidance learning than apoE2 and apoE3 female mice (Villasana et al., 2008).
Based on the enhanced water maze learning curves of the apoE4 TR mice, we compared the water maze performance of TR and GFAP-apoE mice. There was a striking similarity between the ability of apoE3 TR mice and GFAP-apoE3 mice to locate the platform in the water maze and spatial memory retention in the probe trial. This was particularly remarkable considering the relative large line differences in apoE expression in the amygdala, hippocampus, and cortex, with much lower levels of apoE expression in GFAP-apoE3 than apoE3 TR mice. In contrast to the apoE3 mice, TR apoE4 mice showed much better water maze learning than GFAP-apoE4 mice but no difference in spatial memory retention in the probe trial following the last day of hidden training. In addition, apoE expression in the amygdala and hippocampus was similar in the apoE4 TR and GFAP-apoE4 mice. Only in the cortex apoE4 levels were lower in GFAP than TR mice. Based on the lower cortical apoE levels and reduced water maze performance of GFAP-apoE4 than apoE4 TR mice and the trend towards lower apoE levels in apoE4 than apoE3 TR mice, one might conclude that it is beneficial to increase apoE4 levels. However, the fact that GFAP-apoE4 mice show worse spatial memory retention than mice lacking apoE (van Meer et al., 2007) indicates that this might not be the case.
ApoE levels were higher in the amygdala, hippocampus, and cortex of apoE2 than apoE4 TR female mice. These results are consistent with the reported trend towards higher apoE levels in the hippocampus, frontal cortex, and cerebellum of 3–5 mo old TR male mice (Sullivan et al., 2004). In young mice, there was a trend towards higher apoE levels in the amygdala of apoE3 than apoE4 TR mice which was not seen in the hippocampus or cortex. Such a trend was seen in the hippocampus and cortex of the old TR mice. These results are consistent with the lack of genotype differences in the hippocampus, frontal cortex, and cerebellum of 3–5 mo old TR male mice (Sullivan et al., 2004) but at odds with the higher apoE levels in the hippocampus and cortex of 5 mo old apoE3 than apoE4 TR female mice (Ramaswamy et al., 2005). Differences in the age of the mice and in environmental conditions potentially affecting apoE transcriptional and translational regulation might have contributed to these slightly divergent findings.
ApoE isoforms may differentially modulate anxiety levels by acting in the amygdala, which plays an important role in the regulation of anxiety (Amaral, 2002; Davidson, 2002; Davis, 1992). Compared with wild type and NSE-apoE3 male mice, NSE-apoE4 male mice show lower levels of microtubule-associated protein 2-positive neuronal dendrites in the amygdala (Robertson et al., 2005). Interestingly, in AD there is amygdala atrophy and in nondemented individuals amygdala atrophy is more pronounced in ε4 homozygotes than heterozygotes (den Heijer et al., 2002; Lehtovirta et al., 1996). Thus, apoE isoforms may differentially affect amygdalar integrity and volume, and as a consequence have differential effects on measures of anxiety. Further research is warranted to ascertain the extent of amygdalar integrity and atrophy in TR apoE mice.
Measures of anxiety in the elevated zero maze and plus maze correlated with the cognitive measures of cumulative distance to the visible platform and latency to reach the platform in the water maze and with trials to reach criterion during passive avoidance learning. Therefore, in apoE4 mice, increased anxiety levels may contribute to their superior performance in parts of the water maze and passive avoidance tests. These findings are consistent with those from previous studies where mice that show increased measures of anxiety also show better cognitive performance in the Morris water maze training sessions (Acevedo et al., 2006; Villasana et al., 2006) and the passive avoidance test (Acevedo et al., 2006; Acevedo et al., 2006; Acevedo et al., 2008). However, not all studies in mice show similar associations. Although NSE-apoE4 and Apoe−/− male mice show higher measures of anxiety compared with wild type and NSE-apoE3 male mice (Robertson et al., 2005), there was no genotype difference in cognitive performance in these mice at 6 (Raber et al., 1998) or 18 mo of age (Raber et al., 2000b). Similarly, while NSE-apoE4 female mice show poorer cognitive performance than NSE-apoE3, wild type, or Apoe−/− female mice, there was no genotype difference in measures of anxiety (unpublished observations). In contrast to what is seen in the TR mice in the current study, in mice lacking histamine H3 receptors, decreased anxiety levels were associated with improved learning and memory in the Barnes maze (Rizk et al., 2004). Similarly, in healthy human apoE4 carriers, increased anxiety levels were associated with impaired performance on the Wisconsin Card Sorting Test (Caselli et al., 2004). Elevated anxiety levels are associated with elevated levels of glucocorticoids and epinephrine, and in healthy participants, elevated glucocorticoids and epinephrine levels are associated with increased long-term memory for emotional faces and visual slides, respectively (Cahill and Alkire, 2003; Putman et al., 2004). This complex relationship might involve an inverted-U shape relationship, with moderate levels of cortisol facilitating memory and cognition while higher levels impair memory and cognition (Abrari et al., 2009; Andreano and Cahill, 2006; Domes et al., 2005). Thus the relationship between measures of anxiety and cognitive performance is complex and the direction of the relationship might depend on the anxiety and cognitive tests used, how much anxiety is increased, and genetic factors. Nonetheless, our results highlight the importance of measuring anxiety levels when assessing cognitive function.
We observed impaired passive avoidance memory retention in old apoE2 female mice, as indicated by a decreased latency to re-enter the dark compartment. This result contrasts with the superior cognitive function in elderly women carrying the ε2 allele compared with those carrying ε3 or ε4 alleles (Kang et al., 2005). To determine whether decreased anxiety levels in the apoE2 mice might have contributed to their decreased passive avoidance memory retention, we assessed measures of anxiety and the correlation between measures of anxiety and latency to re-enter the dark compartment during the passive avoidance test only in the old mice in an exploratory analysis. Old apoE2 female mice showed decreased anxiety levels compared with old apoE4 mice (data not shown). However, percent time in the open areas/arms of the elevated zero maze and plus maze did not correlate with latency to re-enter the dark compartment (data not shown), suggesting that decreased anxiety levels did not contribute to poorer passive avoidance memory retention in the old apoE2 mice. The impaired passive avoidance retention in old apoE2 mice may be due to their vulnerability to develop atherosclerosis compared with apoE3 and apoE4 mice (Knouff et al., 1999).
In conclusion, this study supports a role for apoE in regulating measures of anxiety. The apoE4 mice show the highest levels of anxiety across all ages examined. Measures of anxiety correlated with performance in the water maze and passive avoidance tests, and increased anxiety levels might have contributed to the superior performance of apoE4 mice in the water maze and passive avoidance tests. These findings demonstrate the importance of assessing measures of anxiety when assessing cognitive function.
Acknowledgments
The authors thank Summer Acevedo, Wendy McGinnis, and Anthony Bader for their help with the data analysis. This work was supported by IIRG-05-14021 (Alzheimer's Association), NNJ05HE63G (NASA) grants, and T32DA07262 (NIDA).
Footnotes
Disclosure statement
None of the authors has any actual or potential financial conflicts or conflict of interest related to this study.
References
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Post-training administration of corticosterone enhances consolidation of contextual fear memory and hippocampal long-term potentiation in rats. Neurobiol Learn Mem. 2009;91:260–265. doi: 10.1016/j.nlm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Piper B, Craytor MJ, Benice TS, Raber J. Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatr Res. 2010;67:293–299. doi: 10.1203/PDR.0b013e3181cb8e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo SE, McGinnis G, Raber J. Effects of 137Cs gamma irradiation on cognitive performance and measures of anxiety in Apoe−/− and wild-type female mice. Radiat Res. 2008;170:422–428. doi: 10.1667/rr1494.1. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Ohtsu H, Benice TS, Rizk-Jackson A, Raber J. Age-dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc−/−) mice. Brain Res. 2006;1071:113–123. doi: 10.1016/j.brainres.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Pfankuch T, Ohtsu H, Raber J. Anxiety and cognition in female histidine decarboxylase knockout (Hdc(−/−)) mice. Behav Brain Res. 2006;168:92–99. doi: 10.1016/j.bbr.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Alexander DM, Williams LM, Gatt JM, Dobson-Stone C, Kuan SA, Todd EG, Schofield PR, Cooper NJ, Gordon E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol. 2007;75:229–238. doi: 10.1016/j.biopsycho.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Arendt T, Schindler C, Bruckner MK, Eschrich K, Bigl V, Zedlick D, Marcova L. Plastic neuronal remodeling is impaired in patients with Alzheimer's disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17:516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay LL, Zemcov A, Blass JP, McDowell FH. Factors associated with duration of survival in Alzheimer's disease. Biol Psychiatry. 1985;20:86–93. doi: 10.1016/0006-3223(85)90139-8. [DOI] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE epsilon4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007;147:6–17. doi: 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Bongers G, Leurs R, Robertson J, Raber J. Role of H3 receptor-mediated signaling in anxiety and cognition in wild-type and Apoe−/− mice. Neuropsychopharmacology. 2004;29:441–449. doi: 10.1038/sj.npp.1300352. [DOI] [PubMed] [Google Scholar]
- Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Brichtova E, Kozak L. Apolipoprotein E genotype and traumatic brain injury in children – association with neurological outcome. Childs Nerv Syst. 2008;24:349–356. doi: 10.1007/s00381-007-0459-6. [DOI] [PubMed] [Google Scholar]
- Burvill PW, Hall WD, Stampfer HG, Emmerson JP. The prognosis of depression in old age. Br J Psychiatry. 1991;158:64–71. doi: 10.1192/bjp.158.1.64. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE. A distinctive interaction between chronic anxiety and problem solving in asymptomatic APOE e4 homozygotes. J Neuropsychiatr Clin Neurosci. 2004;16:320–329. doi: 10.1176/jnp.16.3.320. [DOI] [PubMed] [Google Scholar]
- Crawford FC, Vanderploeg RD, Freeman MJ, Singh S, Waisman M, Michaels L, Abdullah L, Warden D, Lipsky R, Salazar A, Mullan MJ. APOE genotype influences acquisition and recall following traumatic brain injury. Neurology. 2002;58:1115–1118. doi: 10.1212/wnl.58.7.1115. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- de Toledo M, Bermejo-Pareja F, Vega-Quiroga S, Munoz-Garcia D. Behavioural disorders in Alzheimer's disease. Data from a populational study. Rev Neurol. 2004;38:901–905. [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ. Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Domes G, Rothfischer J, Reichwald U, Hautzinger M. Inverted-U function between salivary Cortisol and retrieval of verbal memory after hydrocortisone treatment. Behav Neurosci. 2005;119:512–517. doi: 10.1037/0735-7044.119.2.512. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A metaanalysis. APOE and Alzheimer Disease Meta Analysis Consortium. J Am Med Assoc. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gilley DW, Wilson RS, Bennett DA, Bernard BA, Fox JH. Predictors of behavioral disturbance in Alzheimer's disease. J Gerontol. 1991;46:362–371. doi: 10.1093/geronj/46.6.p362. [DOI] [PubMed] [Google Scholar]
- Gochee PA, Powell EE, Purdie DM, Pandeya N, Kelemen L, Shorthouse C, Jonsson JR, Kelly B. Association between apolipoprotein E epsilon4 and neuropsychiatric symptoms during interferon alpha treatment for chronic hepatitis C. Psychosomatics. 2004;45:49–57. doi: 10.1176/appi.psy.45.1.49. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005a;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Putnam K, Levy J, Parasuraman R. Scaling of visuospatial attention undergoes differential longitudinal change as a function of APOE genotype prior to old age: results from the NIMH BIOCARD study. Neuropsychology. 2005b;19:830–840. doi: 10.1037/0894-4105.19.6.830. [DOI] [PubMed] [Google Scholar]
- Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Guangda X, Bangshun X, Xiujian L, Yangzhong H. Apovarepsilon(4) allele increases the risk for exercise-induced silent myocardial ischemia in non-insulin-dependent diabetes mellitus. Atherosclerosis. 1999;147:293–296. doi: 10.1016/s0021-9150(99)00198-7. [DOI] [PubMed] [Google Scholar]
- Gustavson A, Cummings J. Current Clinical Neurology, Alzheimer's Disease; A Physician's Guide for Pratical Management. Humana Press; Totowa, New Jersey: 2004. Assessment and treatment of neuropsychiatric Symptoms Internationaux Alzheimer's Diseases. [Google Scholar]
- Jorm AF. Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychol Med. 2000;30:11–22. doi: 10.1017/s0033291799001452. [DOI] [PubMed] [Google Scholar]
- Kang JH, Logroscino G, De Vivo I, Hunter D, Grodstein F. Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiol Aging. 2005;26:475–484. doi: 10.1016/j.neurobiolaging.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovirta M, Soininen H, Laakso MP, Partanen K, Helisalmi S, Mannermaa A, Ryynanen M, Kuikka J, Hartikainen P, Riekkinen PJ., Sr SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E epsilon 4 allele. J Neurol Neurosurg Psychiatry. 1996;60:644–649. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Gwyther LP, Whitehouse PJ. Special care unit research: ethical issues. Alzheimer Dis Assoc Disord. 1994;8:S360–S367. [PubMed] [Google Scholar]
- Morris CM, Massey HM, Benjamin R, Leake A, Broadbent C, Griffiths M, Lamb H, Brown A, Ince PG, Tyrer S, Thompson P, McKeith IG, Edwardson JA, Perry RH, Perry EK. Molecular biology of APO E alleles in Alzheimer's and non-Alzheimer's dementias. J Neural Transm Suppl. 1996;47:205–218. doi: 10.1007/978-3-7091-6892-9_14. [DOI] [PubMed] [Google Scholar]
- Nathoo N, Chetty R, van Dellen JR, Barnett GH. Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol. 2003;56:132–136. doi: 10.1136/mp.56.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia M, Prack MM, Williams DL. Differential regulation of apolipoprotein-E messenger RNA in zona fasciculata cells of rat adrenal gland determined by in situ hybridization. Mol Endocrinol. 1992;6:288–298. doi: 10.1210/mend.6.2.1373819. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Plomin R, McClearn GE, Smith DL, Skuder P, Vignetti S, Chorney MJ, Chorney K, Kasarda S, Thompson LA, Detterman DK, Petrill SA, Daniels J, Owen MJ, McGuffin P. Allelic associations between 100 DNA markers and high versus low IQ. Intelligence. 1995;21:31–48. [Google Scholar]
- Porter VR, Buxton WG, Fairbanks LA, Strickland T, O'Connor SM, Rosenberg-Thompson S, Cummings JL. Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. J Neuropsychiatr Clin Neurosci. 2003;15:180–186. doi: 10.1176/jnp.15.2.180. [DOI] [PubMed] [Google Scholar]
- Prack MM, Nicosia M, Williams DL, Gwynne J. Relationship between apolipoprotein E mRNA expression and tissue cholesterol content in rat adrenal gland. J Lipid Res. 1991;32:1611–1618. [PubMed] [Google Scholar]
- Pritchard AL, Harris J, Pritchard CW, Coates J, Haque S, Holder R, Bentham P, Lendon CL. The effect of the apolipoprotein E gene polymorphisms and haplotypes on behavioural and psychological symptoms in probable Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2007;78:123–126. doi: 10.1136/jnnp.2006.092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman P, Van Honk J, Kessels RP, Mulder M, Koppeschaar HP. Salivary Cortisol and short and long-term memory for emotional faces in healthy young women. Psychoneuroendocrinology. 2004;29:953–960. doi: 10.1016/j.psyneuen.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Raber J, Chen S, Mucke L, Feng L. Corticotropin-releasing factor and adrenocorticotrophic hormone identified as potential mediators of central OB effects. J Biol Chem. 1997;272:15057–15060. doi: 10.1074/jbc.272.24.15057. [DOI] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Akana SF, Bhatnagar S, Dallman MF, Wong D, Mucke L. Hypothalamic-pituitary-adrenal dysfunction in Apoe(−/−) mice: possible role in behavioral and metabolic alterations. J Neurosci. 2000a;20:2064–2071. doi: 10.1523/JNEUROSCI.20-05-02064.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000b;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. Androgens, apoE, and Alzheimer's disease. Sci Aging Knowl Environ. 2004;2004:re2. doi: 10.1126/sageke.2004.11.re2. [DOI] [PubMed] [Google Scholar]
- Raber J. Role of apolipoprotein E in anxiety. Neural Plast. 2007:91236. doi: 10.1155/2007/91236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabins PV, Mace NL, Lucas MJ. The impact of dementia on the family. J Am Med Assoc. 1982;248:333–335. [PubMed] [Google Scholar]
- Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. Effect of domain interaction on apolipoprotein E levels in mouse brain. J Neurosci. 2005;25:10658–10663. doi: 10.1523/JNEUROSCI.1922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3 receptor−/− mice. Eur J Neurosci. 2004;19:1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- Robertson J, Curley J, Kaye J, Quinn J, Pfankuch T, Raber J. apoE isoforms and measures of anxiety in probable AD patients and Apoe−/− mice. Neurobiol Aging. 2005;26:637–643. doi: 10.1016/j.neurobiolaging.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E affects the rate of Alzheimer disease expression: beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol. 1994;53:429–437. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E genotyping in the differential diagnosis, not prediction, of Alzheimer's disease. Ann Neurol. 1995;38:6–14. doi: 10.1002/ana.410380105. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mace BE, Maeda N, Schmechel DE. Marked regional differences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience. 2004;124:725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Turic D, Fisher PJ, Plomin R, Owen MJ. No association between apolipoprotein E polymorphisms and general cognitive ability in children. Neurosci Lett. 2001;299:97–100. doi: 10.1016/s0304-3940(00)01789-4. [DOI] [PubMed] [Google Scholar]
- van Meer P, Acevedo S, Raber J. Impairments in spatial memory retention of GFAP-apoE4 female mice. Behav Brain Res. 2007;176:372–375. doi: 10.1016/j.bbr.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- Villasana L, Poage C, van Meer P, Raber J. Passive avoidance learning and memory of 56Fe sham-irradiated and irradiated human apoE transgenic mice. Radiat Biol Radioecol. 2008;48:191–194. [PubMed] [Google Scholar]
- Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandez-Avila M. Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatr Review. 2003;54:819–825. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neurosci Lett. 2000;294:179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]


