Abstract
Objective
Functional neuroimaging studies have led to a significantly deeper understanding of the underlying neural correlates and the development of several mature models of depression in adults. In contrast, our current understanding of the underlying neural substrates of adolescent depression is very limited. Although numerous studies have consistently demonstrated a hyperactive amygdala in depressed adults, the few published pediatric studies have reported opposite results in the amygdala. Thus, the main purpose of this study was to further our knowledge of the underlying neural substrates of adolescent depression by examining the bilateral amygdala specifically and the whole brain in depressed adolescents compared to healthy controls.
Method
Twelve unmedicated adolescents diagnosed with current major depressive disorder without a comorbid psychiatric disorder and 12 well-matched controls ages 13 to 17 years performed a facial-emotion matching task during functional magnetic resonance imaging at 3 T.
Results
Region-of-interest analyses demonstrated: (1) significant bilateral amygdala activation in depressed and healthy adolescents, and (2) significantly greater left amygdala activation in depressed adolescents compared to controls. Whole-brain analysis revealed areas of significantly different brain activity in depressed adolescents compared to controls.
Conclusions
These results suggest that (1) depressed adolescents without a comorbid psychiatric disorder exhibit an abnormally hyperactive amygdala compared to healthy controls; (2) models of adult depression might be extended to include depressed adolescents; and (3) neuropsychiatric interventions that have been developed in depressed adults should be further examined in adolescents. J. Am. Acad. Child Adolesc.
Keywords: functional magnetic resonance imaging, amygdala, neuroimaging, anterior cingulate cortex, major depressive disorder
Findings from epidemiologic studies have demonstrated that up to 8.3% of US adolescents have major depressive disorder (MDD) or dysthymia.1 A series of studies have documented a strong relation between adolescent age and risk for depression; these studies have demonstrated a clear increase in the absolute risk for MDD during the adolescent years.2,3 The psychological, social, and physical alterations occurring during adolescence make this interval of life a significant risk for the occurrence of depression.4
Functional neuroimaging studies have begun to elucidate the underlying neural correlates of depression in adults. However, few such studies have been done in adolescents to understand the neurobiology of depression. The adult studies have supported the hypothesis that the amygdala is abnormally hyperactive in depressed patients. Four separate positron emission tomographic studies by Drevets et al.5 have repeatedly shown that amygdala activity is significantly increased in adults with MDD. Similar to the positron emission tomographic findings, several functional magnetic resonance imaging (fMRI) studies in depressed adults have demonstrated that the amygdala is hyperactive compared to controls.6,7 Based upon the results of the adult functional neuroimaging studies, several models of adult depression involving the amygdala have been proposed by researchers such as Drevets8 and Mayberg.9
Although adult neural models of depression are quite advanced, our current understanding of the underlying neural substrates of adolescent depression is limited. In contrast to the large and consistent adult functional neuroimaging literature, the few published pediatric fMRI depression studies of the amygdala have reported opposite findings. Although several of the published pediatric fMRI depression studies of the amygdala have reported a hyperactive amygdala in depressed youths compared to controls10–13 and in high-risk offspring of parents with MDD,14 the first published fMRI study15 and a more recent study10 in this area have found a decreased amygdala response in youths with MDD.
Due to the sparseness of fMRI studies and our limited knowledge of the neural substrates in adolescent depression, the primary aim of the present study was to help further elucidate our understanding of the underlying neural correlates of adolescent depression. To accomplish this aim, a 3-T fMRI study was conducted to examine functional brain activity using both a region of interest (ROI) focused on the bilateral amygdala and whole-brain analysis approach in a group of unmedicated adolescents with MDD without a comorbid psychiatric disorder compared to a group of well-matched healthy controls. To this end, we used blood oxygenation level-dependent (BOLD) fMRI in combination with a facial-emotion matching paradigm that has been shown to reliably activate the amygdala in healthy adolescents at high-field strengths.16 Based upon our published fMRI findings in typical adolescents,16 we hypothesized that our paradigm would activate the bilateral amygdala in the adolescents. Furthermore, based on the relatively large number of functional neuroimaging studies in adults6,7,17,18 and the few pediatric fMRI studies,10–14 we hypothesized a priori that amygdala activation would be greater in the adolescents with MDD compared to the healthy controls.
METHOD
Subjects
Twenty-four depressed and healthy adolescents participated. Twelve adolescents (mean ± SD, 15.9 ± 1.4 years; range, 14.08–17.25 years; five girls, seven boys) with a current DSM-IV diagnosis of MDD were compared to a carefully matched group of 12 typical adolescents (mean ± SD, 15.4 ± 1.7 years; range, 12.75–17.58 years; five girls, seven boys). All subjects were right-handed. Four of the healthy controls have been reported elsewhere.16
Healthy adolescents were recruited from all regions of San Diego by the Internet, flyers, and electronic mail. Depressed adolescents were recruited from mental health and primary care clinics in San Diego when they sought treatment.
Healthy adolescents were screened using the computerized Diagnostic Interview Schedule for Children 4.019 and the Diagnostic Predictive Scale20 to screen for the presence of any Axis I diagnoses.
Psychiatric diagnosis of the potentially depressed adolescents was determined through the administration of the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version21 to the adolescent and parent(s) by experienced child and adolescent psychiatrists. Using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version, all adolescents met full criteria for current MDD. None of the adolescents with MDD had a comorbid psychiatric disorder.
All subjects were administered the following items: Wechsler Abbreviated Scale of Intelligence,22 Customary Drinking and Drug Use Record,23 Standard Snellen Eye Chart, Ishihara Color Plates Test (8 plates, 2005 edition), Family Interview for Genetics Studies,24 Edinburgh Handedness Inventory,25 Children’s Depression Rating Scale-Revised (CDRS-R),26,27 and Children’s Global Assessment Scale (CGAS).28 The CDRS-R and CGAS were administered by a board-certified child and adolescent psychiatrist. In addition, all participants completed the following self-administered questionnaires: Tanner stage,29 demographics questionnaire, medical and developmental history form, and Multidimensional Anxiety Scale for Children.30
Male and female adolescents 13 to 17 years old with MDD from all ethnicities were allowed to enter. All depressed adolescents were medication free and symptomatic at the time of their fMRI scan. Exclusionary criteria for the adolescents with MDD included:
Having a full IQ score lower than 80 on the Wechsler Abbreviated Scale of Intelligence.
Being color blind or having less than 20/40 correctable vision as determined by the Ishihara Color Plates Test and Snellen Eye Chart.
Any contraindication to MRI imaging (ferrometallic implants, braces, claustrophobia).
Any history of neurologic disorder (e.g., meningitis, migraine, human immunodeficiency virus), head trauma with loss of consciousness longer than 2 minutes, a learning disability, a serious medical health problem, or a complicated or premature birth before 33 weeks of gestation (exclusionary due to potentially abnormal neurodevelopment).
Being pregnant or suspect being pregnant.
Any evidence of illicit drug use, misuse of prescription drugs, or more than 2 alcohol drinks per week currently or within the previous month as determined by the Customary Drinking and Drug Use Record.
Left-handedness.
Prepubertal status (Tanner stage 1 or 2).
Inability to fully understand and cooperate with the study procedures.
A psychiatric diagnosis other than MDD.
Use of medication with CNS effects within the previous 2 weeks before scanning.
CDRS-R T score lower than 55.
Typical adolescents were excluded from the study for any of the items listed for the adolescents with MDD and for any of these additional reasons: any current or lifetime DSM-IV Axis I psychiatric disorders as determined by the Diagnostic Interview Schedule for Children and Diagnostic Predictive Scale; any family history of mood or psychotic disorders in first-or second-degree relatives as assessed by the Family Interview for Genetics Studies; and a CDRS-R T score higher than 54.
The MDD and typical adolescent groups were matched for estimated IQ (Wechsler Abbreviated Scale of Intelligence), socioeconomic status, pubertal developmental stage (Tanner stage), age, gender, ethnicity, and state anxiety (Multidimensional Anxiety Scale for Children scores).
This research was approved by the University of California at San Diego and Rady Children’s Hospital institutional review boards. All adolescents gave written assent, and their parents/legal guardians gave written informed consent to participate. All participants received financial compensation for their time.
Experimental Task
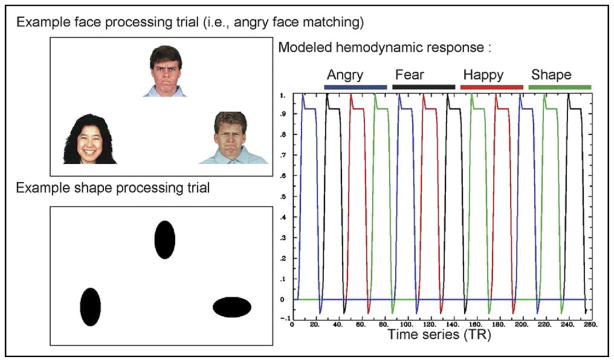
All participants were trained to perform the facial-emotion matching task before fMRI scanning. During the scan, each participant was shown a modified31 version of the task by Hariri et al.32,33 (Figure 1). For each trial, a target face located at the top of the screen and 2 probe faces located at the bottom of the screen were presented. The participants were asked to match the target face with one of the two probe faces that had the same emotional expression by pressing the left or right button on a Current Designs (Philadelphia, PA) response box. Each block contained six consecutive 5-second trials where the target was a fearful, happy, or angry face. The order of blocks was pseudo-randomized to reduce any order effects and was kept consistent across all subjects. Ten seconds of fixation and 2 seconds of instructions were placed between the blocks. The sensorimotor control task consisted of 5-second trials of tall or wide circles or ovals in a similar configuration to the facial-expression task. Analogous to the facial-expression task, participants were told to match the shape of the target to one of the two probes in the control task. The task had a total of 72 trials (18 trials per condition; three face conditions, and one shape condition) and was 512 seconds in duration. Additional details of this task have been published elsewhere.7,16,31 The percentages of correct and incorrect button presses and reaction times for the correct and incorrect responses were computed for all subjects.
FIGURE 1.
Facial-emotion matching task. Interspersed blocks of fixation crosses with varying time lengths were inserted between the face (angry, happy, fearful) and shape (oval/circle) blocks. Note: TR = repetition time.
Image Acquisition
Images were acquired on a 3-T GE scanner (General Electric, Milwaukee, WI) with Twin Speed gradients using the GE 8-channel head coil. Each session consisted of a 3-plane scout scan (10 seconds), a high-resolution anatomical scan, a series of T2*-weighted echoplanar imaging scans to measure the BOLD response, and echoplanar imaging-based field maps to correct for susceptibility-induced geometric distortions. Functional scans covering the entire brain were acquired parallel to the anterior and posterior commissure (T2*-weighted echoplanar imaging; repetition time, 2,000 ms; echo time, 32 ms; 64 × 64 matrix; 30 2.6-mm oblique slices with a 1.4-mm gap; 256 repetitions). During the same experimental session, a T1-weighted image with an inversion time of 450 ms to null the CSF (fast spoiled gradient echo sequence; repetition time, 8.0 ms; echo time, 3.1 ms; flip angle, 12°; field of view, 22 cm; matrix, 256 × 256; 0.98- × 0.98- × 1.0-mm3 voxels) was collected in the sagittal plane for anatomical reference. These sequences were optimized for the amygdala.
Statistical Analysis of Imaging Data
All functional and structural image processing and analyses were conducted with the Analysis of Functional NeuroImages (AFNI) software.34 Additional details of the following neuroimaging analysis pathway have been published elsewhere.16 To minimize motion artifact, an AFNI three-dimensional coregistration algorithm (3dvolreg) was used to realign all echoplanar images. Data were time corrected for slice acquisition order, and the time series data for each individual were analyzed using a multiple regression model (3dDeconvolve). Three motion parameters (roll, pitch, yaw) were used as nuisance regressors to account for motion artifacts. The angry, fearful, happy, and shape (circle/oval) sensorimotor conditions served as the four orthogonal regressors of interest. A modified γ variate function was convolved with these four regressors to account for the dispersion brain response and delay of the BOLD-fMRI signal due to hemodynamics.35,36 Two additional regressors modeled residual motion in the baseline and linear trends. The AFNI program, 3dDeconvolve, determined the estimated voxel-wise response amplitude. To account for individual variations in the anatomical landmarks, a Gaussian filter with a full-width half-maximum of 4 mm was applied to the voxel-wise percent signal change data.
After smoothing, imaging data for each subject were normalized to stereotaxic Talairach coordinates.37 Task effect was calculated by performing a paired t test for the all-faces (fearful + angry + happy faces) matching minus all-shapes (circles + ovals) matching contrast in the combined MDD and healthy adolescents. The group effect was calculated by performing a two-sample t test between the depressed and control groups for the all-faces matching minus all-shapes matching contrast. Using the identical anatomical masks that we published in our prior study of adults with MDD,7 an a priori ROI analysis was performed on the bilateral extended amygdala. For these ROIs, it was found through Monte-Carlo simulations using the AFNI program AlphaSim that a voxel-wise a priori probability of .05 would result in a corrected cluster-wise activation probability of .05 if a minimum volume of 192 μL and three connected voxels was used for the amygdala. The corrected voxel-wise probability in the ROI corresponded to p = .0027. The ROI masks were superimposed on each adolescent’s voxel-wise percent signal change brain image. Activation clusters located inside these ROIs that met the voxel and volume connection criteria were then extracted in the MDD and healthy adolescents for the all-faces versus all-shapes condition. Each condition (fearful versus shapes, angry versus shapes, happy versus shapes) was extracted from this cluster of activation that was defined for the all-faces versus all-shapes condition and used for further analyses.
For the whole-brain analysis, the AFNI program 3dttest was used to examine differences in brain activation between the depressed and healthy adolescents for the all-faces minus all-shapes contrast. A threshold/cluster method was then applied. This threshold adjustment method was based on Monte-Carlo simulations and was used to guard against identifying false-positive areas of activation using a 4-mm full-width half-maximum Gaussian filter. On the basis of these simulations, it was determined that a minimum volume of 704 μL of adjacent voxels was necessary to ensure a corrected cluster-wise activation probability of .05 for the whole-brain analysis.
All statistical analyses of behavioral and clinical scales data were carried out with SPSS 14.0.38 Correlation analyses of the task-related activation in the left amygdala with the CDRS-R and CGAS scores were conducted for both groups combined (MDD plus controls) and the adolescents with MDD alone.
RESULTS
Sociodemographic and Clinical
The MDD and healthy adolescents did not significantly differ in estimated IQ (t22 = −0.81, p = .43), socioeconomic status (χ26 [n = 24] = 7.17, p = .31), pubertal developmental stage (Tanner stage, χ26 [n = 24] = 6.60, p = .37), age (t22 = 0.88, p = .39), gender (χ21 [n = 24] = 0.00, p = 1.00), ethnicity (χ23 [n = 24] = 0.00, p = 1.00), and anxiety (Multidimensional Anxiety Scale for Children scores, t22 = 1.42, p = .17). The adolescents with MDD had a mean CDRS-R T score of 76 (SD, 9), signifying a depressive disorder is very likely to be confirmed and further evaluation should be pursued. In contrast, the healthy adolescents had a mean CDRS-R T score of 32 (SD, 4), signifying that a depressive disorder is very unlikely to be confirmed.
Behavioral Data
Depressed (MDD) and healthy control adolescents responded to the task with similar reaction times for faces (MDD: mean reaction time = 1.74 seconds, SD = 0.40; control: mean reaction time = 1.57 seconds, SD = 0.30; t22 = 1.20, p = .24) and shapes (MDD: mean = 1.24 seconds, SD = 0.23; control: mean = 1.12 seconds, SD = 0.26; t22 = 1.18, p = .25). The groups also did not significantly differ on performance as indexed by percent correct selection for faces (MDD: mean = 95%, SD = 0.09; control: mean = 96%, SD = 0.04; t22 = −0.57, p = .58) or shapes (MDD: mean = 94%, SD = 0.07; control: mean = 95%, SD = 0.07; t22 = −0.56, p = .58).
Functional Neuroimaging
Task Effect
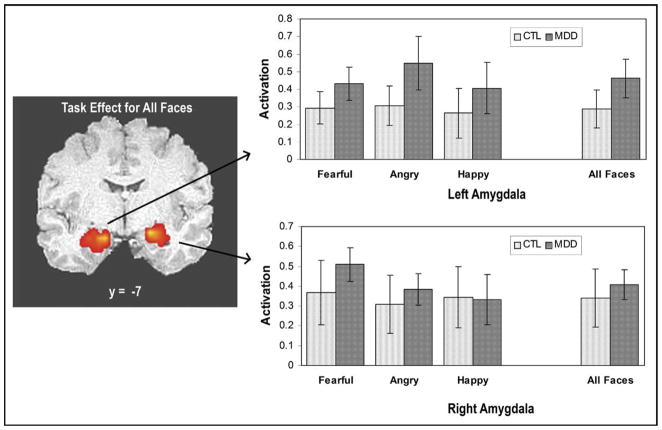
Compared to the shape (circle/oval) sensorimotor condition, fearful, angry, happy, and all faces (fearful + angry + happy) were associated with significantly increased bilateral amygdala activation in the MDD and healthy adolescents (Figure 2). The bar graphs adjacent to the coronal images in Figure 2 show that, for each of the examined contrast conditions, a positive BOLD percent signal change was observed in the left and right amygdala.
FIGURE 2.
Coronal image showing left and right amygdala activation to the fearful, angry, happy, and all-faces versus all-shapes condition for the healthy control (CTL) and depressed (MDD) adolescents. The analyses were conducted within all (MDD plus CTL) participants and then graphed separately. Each individual task condition (fearful, angry, happy) was extracted from the cluster of activation that was defined for the all-faces versus all-shapes contrast. Bar graphs depict the percent signal change in the left and right amygdala for the CTL and adolescents with MDD. Error bars represent SE.
Depressed Versus Healthy Adolescents
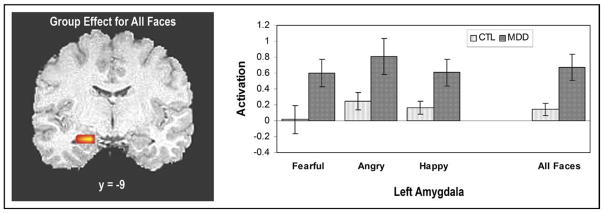
A voxel-based two-sample t test was performed comparing the depressed to the healthy adolescents for the all-faces matching minus all-shapes matching contrast. A significant cluster of activation survived the ROI analysis in the left amygdala (x = −22.0, y = −9.0, z = −16.0; cluster volume = 192 μL; t22 = 2.95). In contrast, no significant differences between the two groups were observed in the right amygdala (Figure 3). A repeated-measures analysis of variance (ANOVA) was performed on the percent signal change in the left amygdala with task condition (fearful, angry, happy) as a within-subject factor and group (MDD, controls) as a between-subjects factor. A significant group effect was found (F1,22 = 8.68, p = .007). No significant group-by-condition effect was observed (F1,22 = 0.37, p = .55).
FIGURE 3.
Coronal image demonstrating greater activation in the left amygdala for the depressed (MDD) adolescents compared to the healthy controls (CTL). Each task condition (fearful, angry, happy) was extracted from the cluster of activation that was defined for the all-faces versus all-shapes contrast. Bar graphs show the extracted percent signal change in the left amygdala for each of the task conditions (fearful, angry, happy) and the combined all-faces versus all-shapes contrast. Error bars represent SE.
Whole-Brain Analyses
The whole-brain analyses revealed several brain regions that were significantly different in activation between the MDD and healthy adolescents for the all-faces versus shapes contrast. Compared to controls, adolescents with MDD showed greater activation in the left parahippocampal gyrus [Brodmann Area (BA) 35; x = −26, y = −46, z = 6; cluster volume = 2,048 μL; t22 = 4.42], left anterior cingulate (BA 24/32; x = −17, y = 13, z = 23; cluster volume = 1,728 μL; t22 = 3.80), left cingulate gyrus (BA 31; x = −23, y = −38, z = 33; cluster volume = 1,344 μL; t22 = 4.21), right anterior cingulate (BA 24/32; x = 16, y = 28, z = 17; cluster volume = 832 μL; t22 = 3.71), left cingulate gyrus (BA 24/32; x = −11, y = 5, z = 37; cluster volume = 704 μL; t22 = 3.78). Compared to controls, adolescents with MDD demonstrated less activation in the bilateral cuneus (BA 7; x = −1, y = −71, z = 32; cluster volume = 768 μL; t22 = 3.83). All x, y, and z values are Talairach coordinates and represent the center of the observed cluster. The whole-brain findings of greater left and right anterior cingulate cortex (ACC) activation in the adolescents with MDD compared to healthy controls were confirmed in each of the face-emotion types. A repeated-measures ANOVA was performed on the percent signal change in the left ACC with task condition (fearful, angry, happy) as a within-subject factor and group (MDD, controls) as a between-subjects factor. A significant group effect was observed (F1,22 = 14.41, p = .001). No significant group-by-condition effect was found (F1,22 = 0.912, p = .35). A similar analysis was done on the right ACC. A significant group effect was seen (F1,22 = 13.73, p = .001), and no significant group-by-condition effect was discovered (F1,22 = 0.16, p = .69).
Brain-Behavior Correlation Analyses
Increased left amygdala activation was significantly correlated with higher depression scores on the CDRS-R for the all-faces versus shapes (Spearman ρ = 0.48, p = .018, two-tailed) and fearful versus shapes (Spearman ρ = 0.46, p = .023, two-tailed) conditions in the combined MDD and healthy adolescents. A trend was observed between left amygdala activation and CGAS scores (Spearman ρ = −0.38, p = .077, two-tailed) in the fearful versus shapes condition for the combined groups. Potentially due to homogeneity of the MDD group and small sample (n = 12), no significant associations were found between left amygdala activation and CDRS-R or CGAS scores in the adolescents with MDD alone (p >.1).
Comparison of Adolescent and Adult MDD
Using the adolescent data from the present study and adult data from our prior study,7 two repeated-measures ANOVAs (left and right amygdala) were performed to determine whether there were any age (adult versus adolescent) by depression (MDD versus HC) by condition (fearful, angry, happy) interactions. Data for the largest task-related activation in each amygdala determined from each cohort separately (to decrease biasing toward one sample) were extracted. There were no significant differences in the response profiles of depression in the two groups when the age-by-group-by-condition interaction was examined in the left (F2,50 = 0.755, p = .475) and right (F2,50 = 0.470, p = .628) amygdala. The results of the repeated-measures ANOVA where the current dataset is compared to a prior cohort of depressed adults scanned when performing the same task indicate that depression affects the response pattern to an emotional-face decision-making task in a similar way in adolescent and adult samples.
DISCUSSION
The present study is the first to use a facial-emotion matching task at high-field strengths to examine differences in functional brain activity between depressed adolescents without a comorbid psychiatric disorder and a group of well-matched healthy controls. This study yielded three main results. First, the ROI analysis showed that the facial-emotion matching paradigm activated the bilateral amygdala in the MDD and healthy adolescents. Second, the ROI analysis revealed that the left amygdala was significantly more active for the depressed adolescents without a concomitant psychiatric disorder compared to the matched controls. Third, the whole-brain analysis demonstrated that the ACC was significantly more active for the adolescents with MDD compared to controls.
The first result confirmed our original hypothesis and is consistent with published fMRI studies using the same facial-emotion matching paradigm and fMRI scanning parameters in adult7 and healthy adolescent16 populations.
The second result also confirmed our original a priori hypothesis that amygdala activation would be greater in the adolescents with MDD compared to controls. This finding is consistent with adult positron emission tomographic5 and fMRI studies.6,7,39,40 This result is also consistent with the majority of fMRI pediatric depression studies.10–14 However, in contrast to the finding of amygdala hyperactivity in several pediatric MDD studies including the present one, the first published study by Thomas et al.15 reported a reduction in the fMRI BOLD signal in the left amygdala.
There are several possible reasons for this difference in amygdala activation findings (i.e., hyperactivation versus hypoactivation). First, the studies varied in total sample size and gender composition. The first published fMRI study of pediatric MDD by Thomas et al.15 examined five girls and no boys, whereas all of the subsequent studies examined larger samples and included both genders.10–14 Second, Thomas et al.15 suggested that their findings might “reflect primarily increased baseline activation.”15 In their study, Thomas et al.15 compared fearful faces to fixation. None of the other studies,10–14 including the present one, used fixation as the baseline comparison condition. Furthermore, as shown by Canli et al.,41,42 the control condition (neutral baseline) can itself produce changes in activation as a function of the serotonin (5-HT) transporter (5-HTT) genotype. Using a fixation baseline condition, Canli et al.41,42 demonstrated that variation in the 5-HTT gene in adults is associated with differential activation to neutral, negative, and positive stimuli in the amygdala. Consistent with these adult findings, Lau et al.12 similarly reported differential amygdala activation to fearful faces depending on the adolescents’ 5-HTT genotype. Because the study by Lau et al.12 is the only pediatric MDD fMRI study to report on the subjects’ 5-HTT genotypes, it is unknown to what extent variations in the youths’ 5-HTT genotypes might have contributed to the differential findings of amygdala activation. Third, the studies varied in whether attention was constrained or unconstrained (passive viewing) during task performance. In the study by Thomas et al.,15 the subjects passively viewed the stimuli. In the present study, the participants were required to perform a facial-emotion matching task. The study by Beesdo et al.10 may provide significant insight because they examined amygdala activation under constrained and unconstrained conditions. They reported that the MDD and anxious adolescents demonstrated similar signs of amygdala hyperactivation to fearful faces when subjectively experienced fear was rated. However, of particular importance, the investigators also found that under the passive viewing condition, the adolescents with MDD (with or without a comorbid anxiety disorder) showed hypoactivation in the left amygdala compared to the healthy controls. Taken together, these results and the findings of the present study are consistent with the idea that differences in amygdala activation might be due to the differences in attentional condition under which the task is performed.
Overall, the adolescent MDD fMRI studies performed to date are generally consistent in their findings of a hyperactive amygdala. Taken together, the present study’s results and the previously published adolescent fMRI findings10,12–14 suggest that this observation of a hyperactive amygdala is consistent across several domains. First, we found that the amygdala is hyperactive in the MDD compared to healthy adolescents for both stimulus classes (positive and negative). Consistent with our finding, Lau et al.12 reported that genotypic differences in amygdala response in depressed and anxious adolescents were seen in fearful and (to a weaker extent) happy faces. Second, a hyperactive amygdala is observed in adolescents with MDD,10,12,13 anxious adolescents,10 and adolescents at high risk for MDD (offspring of parents with MDD).14 Third, we found a hyperactive amygdala using a pseudorandom block design that involved a single-attention condition. Other studies have reported similar results using an event-related fMRI design involving several different attention conditions including a passive viewing condition.10,12–14 It should again be emphasized, however, that as shown by Beesdo et al.,10 the finding of a hyperactive or hypoactive amygdala seems to be dependent on the attentional condition during which the task is performed.
The present study examined a group of adolescents with MDD without a comorbid psychiatric disorder compared to a group of carefully matched controls. Our findings are consistent with the results of Beesdo et al.10 In their study, the investigators reported greater amygdala activation in the anxiety and MDD (with and without anxiety) adolescent groups compared to controls in the a priori-defined fearful-afraid versus fearful-passive contrast. Functional MRI studies have found a hyperactive amygdala in adolescents with an anxiety disorder.13,43,44 In an fMRI study of adolescents with generalized anxiety disorder, McClure et al.43 found greater amygdala activation to fearful faces while the subjects attended to their own subjective level of fear. Taken together, our results and the findings of these other fMRI studies suggest that adolescents with only an anxiety disorder or only MDD or both disorders appear to have a hyperactive amygdala compared to healthy controls. It is important to note, however, that the findings by Beesdo et al.10 show that amygdala activity in adolescents with anxiety or MDD appear to be both similar and different depending on the attentional condition under which the fMRI task is performed.
Our finding of significant differences in left amygdala activation between the MDD and healthy adolescents is consistent with the results of Thomas et al.,15 Roberson-Nay et al.,13 and Beesdo et al.10 However, Lau et al.12 found differences in the right amygdala, and Monk et al.14 and Forbes et al.11 found differences in the bilateral amygdala. Hence, although the published adolescent MDD studies suggest that differences in amygdala activation are often observed in the left side, they also demonstrate that the results are mixed with regard to laterality. Although the task of Hariri et al.45 tends to elicit greater right than left amygdala reactivity in adults, a large meta-analysis done on the adult fMRI studies to examine the question of laterality effect on emotional-faces processing found no support for the hypothesis of overall right lateralization of emotional processing.46 Therefore, because the number of published adolescent amygdala fMRI studies is far fewer than in adults and no similar meta-analysis of the adolescent findings has been performed, we suggest that it is premature to draw any conclusions regarding the laterality of our left amygdala result.
Although the focus of our study was the amygdala and not the ACC, we found greater ACC activity in the adolescents with MDD. To our knowledge, this study is the first one to report greater ACC activity in adolescents with MDD without a comorbid psychiatric disorder compared to a group of well-matched controls using a facial-emotion matching paradigm. Our results are consistent with the only other published adolescent depression fMRI study of the ACC.47 Of note, however, most models of adult depression have emphasized the importance of function in the subgenual cingulate (BA 25), which did not differ between groups in the present study. Future studies should use the same fMRI task and scanning parameters to determine how adolescents with MDD and adults with MDD are similar or different in their activation of the ACC.
The results from the present study have several important clinical implications. In a model describing the putative roles of the amygdala in organizing multiple aspects of emotional and stress responses, Drevets8 detailed how abnormally increased amygdala activity might explain several of the clinical symptoms seen in adults with MDD. In another model, Mayberg9 proposed that adult depression is a result of a “network dysfunction.” Among the key brain areas included in Mayberg’s network are the amygdala and ACC. The results of the present study showing greater amygdala and ACC activation in adolescents with MDD compared to controls suggest that perhaps Drevets’ and May-berg’s network dysfunction models of adult depression might be extended to apply to depressed adolescents. Because our results are consistent with the models of adult depression, they support the further exploration and possible therapeutic applications of neuropsychiatric interventions that have been developed based on the functional neuroimaging research in adults. However, because the potential consequences of how the interventions designed for adults might affect the maturing adolescent brain is unknown, future explorations and studies of these and other potential treatments in depressed adolescents must be pursued with great care and caution.
The present study’s results are tempered by several limitations. First, our study used an emotion-matching task that is not directly relevant to MDD theoretically and empirically. Unlike several prior adolescent MDD studies,10,12–14 we did not examine successful versus unsuccessful face encoding. We also did not find a behavioral group effect. We used a predictable, non-jittered, block design that reduced behavioral variance. This design may have resulted in our finding of no significant group behavioral effects. However, the absence of behavioral differences between the two groups is also advantageous because it removes any performance confound. In addition, this design was selected because of greater statistical power and an enduring history of this task being used in adults32,33 and adolescents.16 Second, another limitation of our task design was that adolescents were engaged for a significant amount of time in passive viewing of the stimuli after they had made their behavioral response. Because it is unknown what mental processes the adolescents were engaged in during passive viewing, our results must be moderated by this reality. Third, in contrast to our findings using an ROI approach, significant amygdala activity did not emerge from our whole-brain analyses. Although there is good scientific justification for using an ROI approach to examine the amygdala, our results suggest that there still exists room for future studies to develop improved fMRI tasks and pulse sequences to examine the adolescent amygdala.
In summary, our findings contribute to the field of adolescent depression by demonstrating that adolescents with MDD without a concomitant psychiatric disorder have greater left amygdala and bilateral ACC activity compared to a group of well-matched healthy controls. These results suggest that models of adult depression might be extended to include adolescents, and that therapeutic interventions developed based on the adult models of depression should be further examined as potential treatments for adolescents with depression.
Acknowledgments
This work was supported by grants from NIMH (K23MH70791), NARSAD, and Klingenstein Foundations to T.T.Y.
We acknowledge the invaluable help of Dr. Saul Levine, Dr. Donald Slymen, Dr. Rebecca Theilmann, Kevin Hahn, Poonam Manwani, Derrick Shen, and Sarah Jurick.
Footnotes
Disclosure: Drs. Yang, Simmons, Matthews, Tapert, Frank, Max, Bischoff-Grethe, Lansing, Brown, Strigo, and Paulus, and Mr. Wu report no biomedical financial interests or potential conflicts of interest.
This article is discussed in an editorial by Dr. David R. Rosenberg on page 7.
References
- 1.Birmaher B, Ryan ND, Williamson DE, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35(11):1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Feehan M, McGee R, Williams SM. Mental health disorders from age 15 to age 18 years. J Am Acad Child Adolesc Psychiatry. 1993;32(6):1118–1126. doi: 10.1097/00004583-199311000-00003. [DOI] [PubMed] [Google Scholar]
- 3.McGee R, Feehan M, Williams S, Anderson J. DSM-III disorders from age 11 to age 15 years. J Am Acad Child Adolesc Psychiatry. 1992;31(1):50–59. doi: 10.1097/00004583-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Cicchetti D, Toth SL. The development of depression in children and adolescents. Am Psychol. 1998;53(2):221–241. doi: 10.1037//0003-066x.53.2.221. [DOI] [PubMed] [Google Scholar]
- 5.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12(9):3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111(1):13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 9.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 10.Beesdo K, Lau JY, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes EE, Christopher May J, Siegle GJ, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau JY, Goldman D, Buzas B, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry. 2009;65(4):349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberson-Nay R, McClure EB, Monk CS, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an FMRI study. Biol Psychiatry. 2006;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 15.Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 16.Yang TT, Simmons AN, Matthews SC, et al. Increased amygdala activation is related to heart rate during emotion processing in adolescent subjects. Neurosci Lett. 2007;428(2–3):109–114. doi: 10.1016/j.neulet.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12(6):527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 18.Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull. 1992;28(3):261–274. [PubMed] [Google Scholar]
- 19.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Lucas C, Zhang H, Mroczek D. The DISC predictive scales: efficiently predicting DISC diagnoses. Presented at the 44th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Toronto. 1997. [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 23.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug. J Study Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell ME. Family Interview for Genetic Studies (FIGS): Manual for FIGS. Rockville, MD: National Institute of Mental Health (NIMH); 1992. [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Poznanski EO. Children’s Depression Rating Scale-Revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 27.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64(4):442–450. [PubMed] [Google Scholar]
- 28.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 29.Tanner JM. Growth and Adolescence. Oxford: Blackwell; 1962. [Google Scholar]
- 30.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62(3):282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 32.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 33.Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 34.Cox RW. Software for analysis and visualization of functional magnetic neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2(2):157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- 36.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 37.Talairach J, Tournoux P. Three-dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. Coplanar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 38.SPSS Base System User’s Guide [computer program] Chicago: SPSS; 1990. [Google Scholar]
- 39.Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17(8):843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- 40.Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;102(34):12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canli T, Qiu M, Omura K, et al. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 44.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 46.Fusar-Poli P, Placentino A, Carletti F, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neurosci Lett. 2009;452(3):262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 47.Yang TT, Simmons AN, Matthews SC, et al. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20(4):440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]