Abstract
Electrospinning is a long-known polymer processing technique that has received more interest and attention in recent years due to its versatility and potential use in the field of biomedical research. The fabrication of three-dimensional (3D) electrospun matrices for drug delivery and tissue engineering is of particular interest. In the present study, we identified optimal conditions to generate novel electrospun polymeric scaffolds composed of poly-d/l-lactide and poly-l-lactide in the ratio 50:50. Scanning electron microscopic analyses revealed that the generated poly(d/l-lactide-co-l-lactide) electrospun hybrid microfibers possessed a unique porous high surface area mimicking native extracellular matrix (ECM). To assess cytocompatibility, we isolated dermal fibroblasts from human skin biopsies. After 5 days of in vitro culture, the fibroblasts adhered, migrated and proliferated on the newly created 3D scaffolds. Our data demonstrate the applicability of electrospun poly(d/l-lactide-co-l-lactide) scaffolds to serve as substrates for regenerative medicine applications with special focus on skin tissue engineering.
Introduction
The process to form synthetic fibers between 3 nm and 5 μm in diameter by drawing material out of a fine nozzle using electrostatic forces, designated as electrospinning, has been known for many decades [1, 2]. In the last 10 years the interest in electrospinning as a potential polymer processing technique for applications in drug delivery and tissue engineering increased tremendously [2–5]. The multidisciplinary field of tissue engineering applies the principles of engineering and life sciences with the aim to generate biological substitutes that restore, maintain or improve tissue and organ function [6]. Tissue engineering approaches utilize (I) living cells or cell substitutes to replace damaged cells; (II) the delivery of tissue-inducing substances, such as growth and differentiation factors, to targeted locations; and (III) scaffold-based concepts that involve the combination of viable cells, biomolecules and three-dimensional (3D) scaffolds [6–12]. However, the applicability of either isolated cells or tissue-inducing substances is limited to defects that are small in size or that are well contained. Therefore, the main interest lies in the third approach—the use of cell-seeded 3D scaffolds.
Requirements for an optimal scaffold are the ability to allow cells to proliferate and migrate, to synthesize their own extracellular matrix (ECM) and to ultimately facilitate the formation of a functional tissue or organ [5, 8, 13]. As a complex microenvironment, the natural ECM consists of fibrous and soluble proteins as well as other bioactive molecules and displays a 3D topography [5, 14]. The electrospinning process permits the generation of fibrous scaffolds that exhibit a 3D nano- or microtopography with a high porosity similar to the natural ECM [1, 8, 11, 13, 15, 16]. Due to their flexibility in material selection, as well as the ability to easily control scaffold properties, electrospun substrates can be employed in various tissue engineering applications. One of the most important substrate properties is to support cell adhesion and growth. Several studies have shown that cells including epidermal fibroblasts, keratinocytes, chondrocytes, smooth muscle cells, cardiomyocytes and adult stem cells adhered and proliferated well when cultured on electrospun scaffolds [17–20].
Due to their excellent biocompatibility and biodegradability, polylactides, polyglycolides and their copolymers are used in a wide range of biomedical applications. Specifically, polylactides derived from lactic acid will degrade in the human body via a hydrolytic reaction, first producing oligomers and then lactic acids, of which the l-lactic acid is a natural intermediate in metabolism [21, 22]. Of particular interest when focusing on a clinical application, pure poly(l-lactide) and copolymers such as poly(d/l-lactide-co-l-lactide), among few other synthetic polymers, are presently approved by the Food and Drug Administration for clinical applications [23–25].
In the present study, we aimed to generate novel electrospun polymeric scaffolds that can ultimately serve either as in vitro skin model for wound dressing applications [26] or as a synthetic dermal graft. Since it had been previously shown that porous scaffolds composed of either poly-d/l-lactide or poly-l-lactide provide favorable growth and proliferation conditions for various cells [27], we generated a suitable copolymer made of these polymers for our experiments. The properties of this kind of polymeric biomaterial can differ in a wide range according to the ratio of the used d-, l- and d/l-lactide for polymerization and their resulting molecular weights. It has been shown that varying compositions of lactides have an impact on the biodegradability as well as on the physical and mechanical properties of a biomaterial [28]. Accordingly, pure poly-l-lactides show a high crystallinity and mechanical stability while having a low degradability rate. To enhance their degradability by hydrolysis, d/l-lactides can be introduced into the polymer, increasing the presence of amorphous regions accompanied with a lower crystallinity. Here we selected a suitable linear copolymer, showing a sufficient balance between degradation properties and mechanical stability, which could be used in the electrospinning process for the creation of non-woven biomechanically stable and cytocompatible scaffolds. The particular random poly(d/l-lactide-co-l-lactide) employed in this study had a 50:50 l-lactide:d/l-lactide mole ratio with a molecular weight of approximately 102,000. To produce microfibers of the described specific polymer via electrospinning, chloroform was determined as the most appropriate solvent. To achieve an optimal balance between fiber strength, permeability and cell adhesion, porous fibers with a diameter of 4–5 μm were produced, correlating with the diameter of collagen fibers found in a range of tissues in vivo [29]. To test cytocompatibility of the electrospun poly(d/l-lactide-co-l-lactide) scaffolds, we seeded the constructs with dermal fibroblasts that had been isolated from human skin biopsies. After 5 days in vitro, we evaluated cell viability, proliferation and morphology.
The results of our experiments demonstrate the feasibility to create biomimetic electrospun scaffolds composed of poly(d/l-lactide-co-l-lactide) that may potentially serve as a substrate for various soft tissue engineering and regenerative medicine applications.
Materials and methods
Poly(d/l-lactide-co-l-lactide
A linear poly(d/l-lactide-co-l-lactide) (50:50) copolymer (Mw = 102,000) was prepared according to a previously established protocol [30, 31]. Briefly, appropriate amounts of l-lactide, d/l-lactide (Boehringer KG, Ingelheim, Germany) and the starter molecule 1,8-octanediol (Sigma–Aldrich Chemie GmbH, Munich, Germany) were placed into a reaction flask and the mixture was heated under an inert nitrogen gas atmosphere to 130°C. After complete melting, stannous 2-ethylhexanoate (0.05 wt% based on total lactide content; Sigma–Aldrich), which had been dissolved in dry toluene, was added. The reaction mixture was stirred until it became solid. After cooling to room temperature, the polymer was dissolved in dichloromethane (Sigma–Aldrich), precipitated in methanol and dried in vacuum. Preliminary cytotoxicity experiments using standard 3T3 mouse embryonic fibroblasts (LGC Standards GmbH, Wesel, Germany) indicated the cytocompatibility of the poly(d/l-lactide-co-l-lactide) material.
Electrospun fibrous matrices
Tetrahydrofuran, acetone, dichloromethane, hexafluoroisopropyl alcohol and chloroform (all Sigma–Aldrich) were tested for their suitability to dissolve poly(d/l-lactide-l-lactide) for its subsequent use for electrospinning. We determined that a 10% (w/w) solution was essential to spin the copolymer under the conditions described: A homogeneous solution was prepared by slow stirring 1.0 g of the copolymer in 6.1 ml of the solvent at room temperature for 3 h using a magnetic stirrer at 250 rpm. The obtained clear and viscous solution was directly transferred into a 5 ml plastic syringe, which was connected to a 0.40 × 25 mm-gauge blunt ended stainless-steel needle (= spinning nuzzle). The copolymer solution was then exposed to the electrospinning process using a custom designed electrospinning apparatus consisting of an adjustable high-voltage power supply with a limited current of 200 μA (ESV-100; Ingenieurbüro G. Fuhrmann, Leverkusen, Germany). The glass surface of a mirror (20 × 20 cm2; glass thickness 3 mm) was selected as collector plate for collecting the electrospun fibers. The needle and the metallic side of the mirror were connected to the ESV-100. The syringe was mounted vertically against the collector and the sample solution was fed at a constant rate through the syringe to the needle tip. The distance between the needle tip and the mirror was maintained at 20 cm. The voltage applied to the needle was adjusted to 15 kV. The flow rate of the solution was controlled between 0.5 to 0.7 ml h−1. Fiber diameters were determined by software analysis of microscopic images (Image-Pro Plus 5.0, Media Cybernetics, Inc., Silver Spring, MD, USA).
In vitro degradation assays
To determine the in vitro degradation of the electrospun poly(d/l-lactide-l-lactide), we used 80 mg of the electrospun material and the same amount of smooth, solid and non-porous poly(d/l-lactide-l-lactide)-composed controls. These controls had a thickness of approximately 50–60 μm and were generated using the same material (poly(d/l-lactide-l-lactide)–chloroform (50:50) solution) as used for the electrospinning. All samples were exposed to 5 ml of simulated body fluid (SBF) medium of pH 7.4 in a thermostatic incubator at 37°C over 18 weeks. Every week 1 ml of the SBF was withdrawn and the SBF in the vessel was replenished up to 5 ml. The degree of degradation was monitored by the l-lactate sample concentration using an enzymatic l-lactate assay (R-Biopharm, Darmstadt, Germany) according to the manufacturer’s protocol.
Thermogravimetric analysis
In order to confirm that all solvent was evaporated from the electrospun poly(d/l-lactide-l-lactide), we performed thermogravimetric analyses at 20–200°C with a heating rate of 20°C/min at room temperature as described previously [32] using a STA 449C Jupiter (Netzsch-Gerätebau GmbH, Selb, Germany).
Primary cell isolation and culture
Primary human fibroblasts were isolated from foreskin by enzymatic treatment. Dermal and epidermal layers were separated by a 16 h digest in a 2 U/ml dispase solution in phosphate-buffered saline (PBS; Invitrogen GmbH, Darmstadt, Germany) at 4°C. The dermal layer was cut and treated for 45 min with 0.45 U/ml collagenase solution (Invitrogen). Cells were seeded in T-25 culture flasks in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen). The medium was changed every 2–3 days. Fibroblasts were used at passage 2–5.
Cell seeding of the electrospun matrix
After confirming that all solvent was removed from the electrospun material, the matrices were cut to 1.5 × 1.5 cm2. All samples were then sterilized using 70% ethanol solution for 20 min, followed by two washing steps with PBS. For cell seeding experiments, the electrospun matrices were placed in a petri dish with a 3.2 cm diameter. On each electrospun scaffold, 250,000 fibroblasts were seeded in DMEM and cultured for a maximum of 5 days at 37°C.
Cell proliferation assays
After a culture time of 17 h and 5 days, proliferation was measured using the WST-1 assay (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. Briefly, the WST-1 reagent is a water-soluble tetrazolium salt that can be used for cell proliferation or cell viability assays. The rate of WST-1 cleavage by mitochondrial dehydrogenases correlates with the number of viable cells in the culture. Therefore the assay primarily detects total enzymatic activities that are able to reduce the WST-1 substrate. Accordingly, the values obtained using this assay are a direct measure of cell number and proliferation, provided that cytotoxic effects and reductions in metabolism can be excluded. Fibroblasts that had been cultured on 3.2 cm diameter tissue culture polystyrene (TCPS) petri dishes served as control. The absorbance of the samples was measured at a wavelength of 450 nm employing a microtiter plate reader (Model 550; Bio-Rad Laboratories GmbH, München, Germany). All data are displayed in arbitrary units (a.u.) ± SD.
Live/dead staining of cells
To assess cytocompatibility, fibroblasts were cultured for 5 days on the electrospun scaffolds, followed by live/dead staining for 3 min using a solution consisting of 0.005% (w/v) fluorescein diacetate (FDA, Sigma–Aldrich) (stock solution: 0.5% (w/v, in acetone), 0.009% (w/v) propidium iodide (PI, Sigma–Aldrich) (stock solution: 0.1% (w/v) in PBS) in PBS. Cells were immediately visualized by fluorescence microscopy using a Zeiss Axiovert 200 M ApoTome equipped with the appropriate filter sets detecting FITC and rhodamine (Carl Zeiss Microimaging GmbH, Jena, Germany). Images were processed with Adobe Photoshop CS3.3 (Adobe Systems Inc., San Jose, CA, USA).
Scanning electron microscopy
All samples were fixed using 2% glutaraldehyde (Sigma–Aldrich) for 45 min at room temperature, dehydrated with a series of graded alcohols and air-dried overnight. The dried samples were sputter coated with platinum and scanning electron microscopic (SEM) micrographs were taken with a Zeiss Leo 1520 VP (Carl Zeiss).
Fiber diameters in the electrospun scaffolds were measured using scanning electron micrographs. The average fiber diameter was determined from the measurements taken as described before in more detail [13].
Statistical analyses
Statistical significance was assessed by Student’s t test. P-values less than 0.05 were defined as statistically significant.
Results and discussion
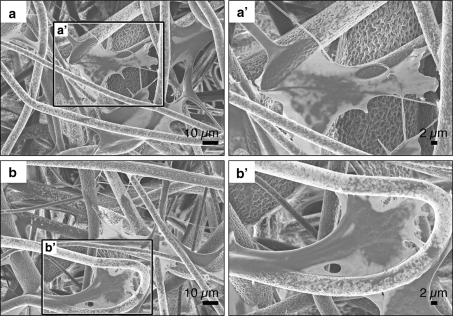
Solvents such as tetrahydrofuran and acetone, as well as halogenated hydrocarbons, including dichloromethane, chloroform and hexafluoroisopropyl alcohol, were tested for their suitability to dissolve poly(d/l-lactide-l-lactide) (50:50) for electrospinning. Chloroform was selected as the most appropriate solvent due to its superior evaporation property, resulting in an optimal drying rate during the spinning process, which prevented that the fibers stuck together (Fig. 1a, b). An increase fiber surface was achieved while obtaining a unique porous fiber surface structure (Fig. 1b), which is a known phenomenon induced by phase separation resulting from the rapid evaporation of the solvent during the electrospinning process [33]. The average dimension of the obtained electrospun fiber matrices was approximately 10 cm2. The scaffold thickness ranged between 50 and 100 μm. Scanning electron microscopy revealed that the generated poly(d/l-lactide-co-l-lactide) hybrid microfibers had an average diameter of 4.26 ± 1.48 μm.
Fig. 1.
SEM images of poly(d/l-lactide-co-l-lactide)-composed scaffolds. a Overview of the generated electrospun poly(d/l-lactide-co-l-lactide) material. b The electrospun poly(d/l-lactide-co-l-lactide) fibers have a unique porous surface. c SEM image of smooth, non-porous and non-fibrous poly(d/l-lactide-l-lactide)-composed controls
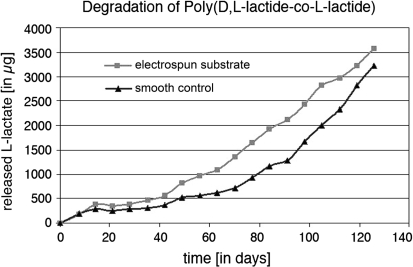
Degradation of polylactides under physiological conditions depends on structural parameters such as molecular weight, monomer composition (l-, d- and meso-lactide), degree of branching, but foremost their surface properties [34]. In order to assess the degradation rate of dense biomaterials the mass loss is measured over time; however, this method is not suitable to determine the degradation of electrospun materials with a low mass and a high surface area. To date, there are only few data available describing the hydrolytic degradation of electrospun matrices [22]. No data are available concerning the degradation of highly porous poly(d/l-lactide-l-lactide) fibers. In this study, we attempted to address this problem capturing small amounts of the degradation products of poly(d/l-lactide-l-lactide)—l-lactate—using an enzymatic l-lactate assay. To assess the impact of the topography of the material on degradation by hydrolysis, enzymatic assays were performed simultaneously using the poly(d/l-lactide-co-l-lactide) electrospun matrices as well as poly(d/l-lactide-l-lactide)-composed smooth, solid, non-porous controls (Fig. 1c). The results of the degradation experiments are summarized in Fig. 2. In contrast to the controls, electrospun matrices exhibited a larger surface area, which led to the assumption that the electrospun material would demonstrate a faster degradation in aqueous solution. Indeed, we observed that after 18 weeks, 3.5 mg l-lactate was released from the electrospun samples compared to 3.2 mg lactate released from the controls; however, this effect was not statistically significant (P = 0.79) and also much lower than expected. The rate of degradation was only slightly enhanced by the increased surface. Nevertheless, our data showed that based on their degradation profile, electrospun poly(d/l-lactide-co-l-lactide) scaffolds are suitable materials for biomedical applications.
Fig. 2.
Summary of the results of the degradation assays of electrospun poly(d/l-lactide-co-l-lactide) scaffolds in comparison with solid, poly(d/l-lactide-co-l-lactide)-composed controls
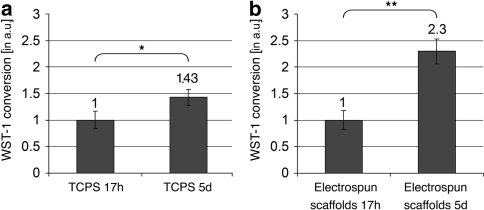
In order to assess the proliferation behavior of the fibroblasts we used the WST-1 cell proliferation assay. The results of the proliferation tests are summarized in Fig. 3. Briefly, fibroblasts were cultured for either 17 h or 5 days on smooth control TCPS petri dishes (Fig. 3a) or on the electrospun poly(d/l-lactide-co-l-lactide) scaffolds (Fig. 3b). The enzymatic activity of fibroblasts assessed after 17 h was determined as the starting point of the experiment and was set to 1 in each sample group. We found in both the 5-day-TCPS and the 5-day-electrospun scaffold groups a significant increase of the WST-1 conversion when compared to the cultures after 17 h. Although our data do not allow the calculation of division rates, they clearly indicate that an expansion of human dermal fibroblasts is possible when cultured on the electrospun poly(d/l-lactide-co-l-lactide) matrices. Moreover, the 3D substrate obviously allowed prolonged increase in cell numbers whereas contact inhibition started earlier at the TCPS control. Thus, our electrospun substrate composed of the novel biodegradable polymer poly(d/l-lactide-co-l-lactide) represents a suitable scaffold material for the cultivation of primary skin fibroblasts.
Fig. 3.
WST-1 proliferation tests reveal that fibroblasts that were grown for 5 days on either TCPS culture ware (a) or on the electrospun poly(d/l-lactide-co-l-lactide) scaffolds (b) show a significant increase in overall activity to reduce WST-1 reagent reflecting increase in cell numbers by proliferation. All data are displayed in arbitrary units (a.u.) ± SD. * P < 0.05 17 h vs. 5 days on TCPS; ** P < 0.05 17 h vs. 5 days on electrospun scaffolds
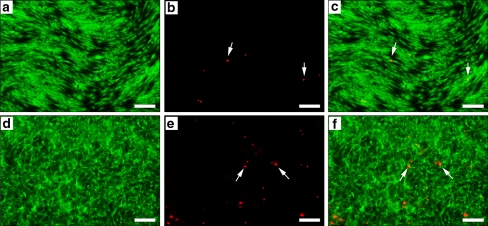
To further assess the biocompatibility of the electrospun poly(d/l-lactide-co-l-lactide) hybrid material we performed live/dead staining. Viable cells were labeled using fluorescein diacetate, whereas dead cells were identified by propidium iodide uptake as displayed in Fig. 4. On both TCPS, serving here again as control substrate, and electrospun poly(d/l-lactide-co-l-lactide) scaffolds almost all cells were viable, as depicted by the green fluorescent cells (Fig. 4a, d; overlay: Fig. 4c, f). Only a few propidium iodide-labeled dead cells (Fig. 4b, e; overlay: Fig. 4c, f) could be noted. Although there was no significance difference between the two substrates in regards to cell viability, we observed a difference in the morphological growth pattern of the fibroblasts. On TCPS, the cells were closely aligned with an elongated morphology. In contrast, fibroblasts grown on the 3D electrospun poly(d/l-lactide-co-l-lactide) matrices displayed a branched morphology with a more physiological adherence pattern, previously noted as the typical shape of migrating fibroblasts in 3D fibrous scaffolds or matrices [35, 36]. These observations confirm the results of the WST-1 proliferation tests in which fibroblasts expanded significantly on the electrospun scaffolds from 17 h to 5 days of culture.
Fig. 4.
Viability assessment of primary human fibroblasts cultured on TCPS (a–c) and electrospun poly(d/l-lactide-co-l-lactide) (d–f) scaffolds after 5 days of culture. A high number of viable green fluorescent cells was observed on both, TCPS (a, c) and the electrospun poly(d/l-lactide-co-l-lactide) matrix (c, f). In contrast, only a small number of red fluorescent nuclei depicting dead cells (see white arrows) were detected on either TCPS (b, c) or the electrospun poly(d/l-lactide-co-l-lactide) substrates (e, f). Of note, the morphological growth pattern of the fibroblasts cultured on TCPS (a) differs from the 3D scaffolds (d). Cells are branched compared to the flat elongated morphology on TCPS. All scale bars equal 200 μm. (Color figure online)
The interaction of the fibroblasts with the electrospun poly(d/l-lactide-co-l-lactide) scaffolds was further examined by SEM (Fig. 5). Dermal human fibroblasts were found evenly distributed throughout the entire scaffold. SEM images further revealed that the fibroblasts interacted directly with several fibers as shown by the formation of long cytoplasmic branches, a phenomenon that was also observed by F. Grinnell in 2008 [37]. These numerous long branches help cells monitor their extracellular environment and may also be involved in the migration of cells through the electrospun scaffold.
Fig. 5.
SEM images of primary human fibroblasts cultured for 5 days on electrospun poly(d/l-lactide-co-l-lactide) scaffolds. Images (a) and (b) depict cells interacting with several fibers in the electrospun matrix, exhibiting a branched morphology. Scale bars equal 10 μm (a and b) and 2 μm (a′ and b′)
Conclusions
In this study, poly(d/l-lactide-co-l-lactide) hybrid microfibers of lower molecular weight and high porosity were successfully prepared by electrospinning. We have shown that our novel electrospun poly(d/l-lactide-l-lactide) substrates are appropriate matrices to culture and expand human dermal fibroblasts. The engineered fibrous network offers room for the migration of fibroblasts, which is also an important property of the extracellular space of these cells within their physiological environment. We provided evidence for cell migration and proliferation, and showed biological acceptability of the substrate indicated by a branched, more physiological morphology of the fibroblasts. The results of our long-term degradation studies suggest that the electrospun non-woven matrices could be potentially used for applications in skin tissue engineering.
Acknowledgments
The authors kindly thank Monika Riedl, Patricia Jaaks and Julia Pistor for their technical support. The authors are grateful for the financial support by the Fraunhofer-Gesellschaft Internal Programs (Grant No. Attract 692263 (to KS-L)).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Murugan R, Ramakrishna S. Nano-featured scaffolds for tissue engineering: a review of spinning methodologies. Tissue Eng. 2006;12(3):435–447. doi: 10.1089/ten.2006.12.435. [DOI] [PubMed] [Google Scholar]
- 2.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Chew SY, Wen Y, Dzenis Y, Leong KW. The role of electrospinning in the emerging field of nanomedicine. Curr Pharm Des. 2006;12(36):4751–4770. doi: 10.2174/138161206779026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngiam M, Ramakrishna S, Raghunath M, Chan CK. Nanofiber patent landscape. Recent Pat Nanotechnol. 2007;1(2):137–144. doi: 10.2174/187221007780859645. [DOI] [PubMed] [Google Scholar]
- 5.Schenke-Layland K. Non-invasive multiphoton imaging of extracellular matrix structures. J Biophotonics. 2008;1(6):451–462. doi: 10.1002/jbio.200810045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 7.Lareu RR, Arsianti I, Subramhanya HK, Yanxian P, Raghunath M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: a preliminary study. Tissue Eng. 2007;13(2):385–391. doi: 10.1089/ten.2006.0224. [DOI] [PubMed] [Google Scholar]
- 8.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 9.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mironov V, Kasyanov V, Markwald RR. Nanotechnology in vascular tissue engineering: from nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008;26(6):338–344. doi: 10.1016/j.tibtech.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Schenke-Layland K, Rofail F, Heydarkhan S, et al. The use of three-dimensional nanostructures to instruct cells to produce extracellular matrix for regenerative medicine strategies. Biomaterials. 2009;30(27):4665–4675. doi: 10.1016/j.biomaterials.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visconti RP, Kasyanov V, Gentile C, Zhang J, Markwald RR, Mironov V. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin Biol Ther. 2010;10(3):409–420. doi: 10.1517/14712590903563352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, et al. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29(19):2907–2914. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Votteler M, Kluger P, Walles W, Schenke-Layland K. Macromol Biosci. 2010 (in press). [DOI] [PubMed]
- 15.Isenberg B, Wong JY. Building structure into engineered tissues. Mater Today. 2006;9(12):54–60. doi: 10.1016/S1369-7021(06)71743-6. [DOI] [Google Scholar]
- 16.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11(1–2):101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 17.Baker SC, Atkin N, Gunning PA, et al. Characterisation of electrospun polystyrene scaffolds for three-dimensional in vitro biological studies. Biomaterials. 2006;27(16):3136–3146. doi: 10.1016/j.biomaterials.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Park KE, Kang HK, Lee SJ, Min BM, Park WH. Biomimetic nanofibrous scaffolds: preparation and characterization of PGA/chitin blend nanofibers. Biomacromolecules. 2006;7(2):635–643. doi: 10.1021/bm0509265. [DOI] [PubMed] [Google Scholar]
- 19.Rockwood DN, Akins RE, Jr, Parrag IC, Woodhouse KA, Rabolt JF. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials. 2008;29(36):4783–4791. doi: 10.1016/j.biomaterials.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li WJ, Jr Cooper JA, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2(4):377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Califano V, Bloisi F, Vicari LRM, Yunos DM, Chatzistavrou X, Boccaccini AR, et al. Matrix assisted pulsed laser evaporation (MAPLE) of poly(d,l lactide) (PDLLA) on three dimensional bioglass (R) structures. Adv Eng Mater. 2009;11(8):685–689. doi: 10.1002/adem.200900092. [DOI] [Google Scholar]
- 22.Kim K, Yu M, Zong X, et al. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24(27):4977–4985. doi: 10.1016/S0142-9612(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 23.Al-Sukhun J, Tornwall J, Lindqvist C, Kontio R. Bioresorbable poly-l/dl-lactide (P[L/DL]LA 70/30) plates are reliable for repairing large inferior orbital wall bony defects: a pilot study. J Oral Maxillofac Surg. 2006;64(1):47–55. doi: 10.1016/j.joms.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Ma PX. The nanofibrous architecture of poly(l-lactic acid)-based functional copolymers. Biomaterials. 2010;31(2):259–269. doi: 10.1016/j.biomaterials.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ylikontiola L, Sundqvuist K, Sandor GK, Tormala P, Ashammakhi N. Self-reinforced bioresorbable poly-l/dl-lactide [SR-P(L/DL)LA] 70/30 miniplates and miniscrews are reliable for fixation of anterior mandibular fractures: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(3):312–317. doi: 10.1016/j.tripleo.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 26.MacNeil S. Biomaterials for tissue engineering of skin. Mater Today. 2008;11(5):26–35. doi: 10.1016/S1369-7021(08)70087-7. [DOI] [Google Scholar]
- 27.Grad S, Zhou L, Gogolewski S, Alini M. Chondrocytes seeded onto poly(l/dl-lactide) 80%/20% porous scaffolds: a biochemical evaluation. J Biomed Mater Res A. 2003;66(3):571–579. doi: 10.1002/jbm.a.10007. [DOI] [PubMed] [Google Scholar]
- 28.Essawy HA, Helaly FM, Shabana MA. Synthesis of poly(lactide) blends by melt/solid polycondensation. J Elastomers Plast. 2007;39(4):303–316. doi: 10.1177/0095244307076498. [DOI] [Google Scholar]
- 29.Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol. 2002;65(2):109–126. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- 30.Kricheldorf HR, Kreiser-Saunders I, Boettcher C. Polylactones: 31. Sn(II)octoate-initiated polymerization of l-lactide: a mechanistic study. Polymer. 1995;36(6):1253–1259. doi: 10.1016/0032-3861(95)93928-F. [DOI] [Google Scholar]
- 31.Schnabelrauch M, Vogt S, Larcher Y, Wilke I. Biodegradable polymer networks based on oligolactide macromers: synthesis, properties and biomedical applications. Biomol Eng. 2002;19(2–6):295–298. doi: 10.1016/S1389-0344(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 32.Choi S, Lee SG, Joo CW, Im SS, Kim SH. Formation of interfiber bonding in electrospun poly(etherimide) nanofiber web. J Mater Sci. 2004;39(4):1511–1513. doi: 10.1023/B:JMSC.0000013931.84760.b0. [DOI] [Google Scholar]
- 33.Bognitzki M, Czado W, Frese T, et al. Nanostructured fibers via electrospinning. Adv Mater. 2001;13(1):70–72. doi: 10.1002/1521-4095(200101)13:1<70::AID-ADMA70>3.0.CO;2-H. [DOI] [Google Scholar]
- 34.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–2346. doi: 10.1016/S0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 35.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13(5):264–269. doi: 10.1016/S0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 36.Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev. 2007;59(13):1299–1305. doi: 10.1016/j.addr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grinnell F. Fibroblast mechanics in three-dimensional collagen matrices. J Bodyw Mov Ther. 2008;12(3):191–193. doi: 10.1016/j.jbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]