Abstract
Endovenous laser ablation (EVLA) produces boiling bubbles emerging from pores within the hot fiber tip and traveling over a distal length of about 20 mm before condensing. This evaporation-condensation mechanism makes the vein act like a heat pipe, where very efficient heat transport maintains a constant temperature, the saturation temperature of 100°C, over the volume where these non-condensing bubbles exist. During EVLA the above-mentioned observations indicate that a venous cylindrical volume with a length of about 20 mm is kept at 100°C. Pullback velocities of a few mm/s then cause at least the upper part of the treated vein wall to remain close to 100°C for a time sufficient to cause irreversible injury. In conclusion, we propose that the mechanism of action of boiling bubbles during EVLA is an efficient heat-pipe resembling way of heating of the vein wall.
Keywords: Phlebology, Endovenous laser ablation, Boiling bubbles, Heat pipe function of treated vein
Endovenous laser ablation (EVLA) for the management of saphenous varicosities has a very high success rate with minimal complications [1]. Nevertheless, the exact mechanism of action is still under debate. Four EVLA mechanisms to injure the vein wall have been proposed. First, the optical-thermal response to scattered laser light [2]. Second, the response to heat diffused from the hot fiber tip [3]. These tips may reach temperatures of over 800°C [4, 5], a consequence of the strongly absorbing thin layer of black carbonized blood that is deposited on the fiber tip during EVLA [6]. Third, direct contact with the fiber tip [7, 8], contributing to vein wall perforations [9]. Fourth, the response to condensing boiling bubbles [7, 8, 10]. This mechanism was proposed [10] but not proven to be an essential constituent of EVLA. In this Brief Report we aim to give a tutorial description of the proposed mechanism of action of boiling bubbles during EVLA.
Boiling bubbles are created close to the hot fiber tip when the temperature of the blood exceeds the threshold for boiling. Most likely, these bubbles originate in tiny pores in these layers, comparable to the pores existing in walls of heated tubes, so-called heterogeneous nucleation [11]. These bubbles have been observed to travel over about 20 mm at constant volume before condensation sets in (Fig. 1). During their travel, the bubbles cause additional motion in the fluid, so-called micro-convection, which promotes heat transfer and temperature homogenization [12]. Mechanistically, the combination of creation, transport, and condensation of boiling vapor bubbles in EVLA-treated veins closely resembles the processes occurring in a so-called heat pipe [13]. Heat pipes were developed in the 1940s, are renowned for their efficiency of heat transport [14], and occur in many varieties in modern process technology [15]. Each fluid-flow and heat-transfer process in which evaporation takes place in one part and condensation in another part exhibits the main characteristics of a heat pipe.
Fig. 1.
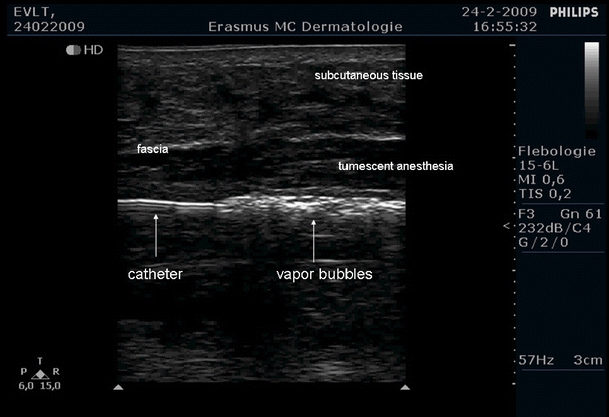
Boiling bubbles (with vertical shadows) still visible 20 mm distal from the fiber tip during EVLA with a 1,470-nm diode laser (Ceralas E, Biolitec) at 5 W, 0.6-mm-diameter fiber, about 1 mm/s pullback velocity, in a 3-mm-diameter catheter
The physics of heat-pipe function is based on the fact that the boiling bubbles are in local thermodynamic equilibrium with their surroundings [16]. First, suppose theoretically that the bubble content would be superheated, i.e., have a temperature above the saturation temperature. Then, heat transfer to the surrounding liquid would occur quickly. Second, suppose that the temperature of part of the bubble would sink below the saturation temperature. Then, this part of the vapor would condense immediately. The bubble would shrink or even collapse [17, 18]. Thus, when two phases of the same component, i.e., vapor and liquid, are cohabiting the vapor phase and its immediate surroundings must have a temperature exactly equal to the saturation temperature.
Measurements in our laboratory (not shown) identified that the bubbles created during EVLA contain mainly steam. As these bubbles are non-condensing over 20 mm, the volume where they move must be at 100°C. Since bubbles move to upper parts of their enclosure, at least the upper part of the vein wall is in contact with these bubbles. Typical pullback velocities of a few mm/s cause these parts of the treated vein wall to remain close to 100°C for at least several seconds. Then, because thermal rate process theory suggests irreversible injury if a threshold temperature of 75°C occurs during 1 s, or 70°C during 10 s [19], this warrants the conclusion that the vein wall will be irreversibly injured. Figure 2 shows a cartoon of the interactions.
Fig. 2.
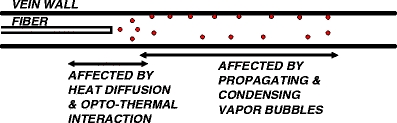
Cartoon of three EVLA heat-transfer mechanisms (excluding direct contact of hot fiber tip and vein wall), which are effective at different time points. The centered EVLA catheter typically has a 3-mm diameter and the tumescent anesthesia forces the vein wall to fold itself over the catheter. Heat diffusion from the hot tip and the optical-thermal interaction have their primary effect about perpendicular to the fiber near the fiber tip, over about 6 mm vein wall length (left arrow) [3]. Boiling bubbles (small spheres) reach distances of about 20 mm (right arrow)
During EVLA, the heat-pipe resembling function of the treated vein ensures that the boiling bubbles enhance the transport of heat from the hot fiber tip to the blood volume over a distal length of about 20 mm. We emphasize that heat-loss mechanisms such as thermal conduction and convection determine the gradient of the temperature at e.g., the vessel wall but not the temperature itself.
In conclusion, we propose the mechanism of action of boiling bubbles during EVLA is a heat-pipe resembling efficient way of heating of the vein wall.
Acknowledgments
We thank one of the reviewers for providing us with thought-provoking comments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Cees W. M. van der Geld, Email: C.W.M.v.d.Geld@tue.nl
Martin J. C. van Gemert, Email: m.j.vangemert@amc.uva.nl
References
- 1.Van den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230–239. doi: 10.1016/j.jvs.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Mordon SR, Wassmer B, Zemmouri J. Mathematical modeling of 980-nm and 1320-nm endovenous laser treatment. Lasers Surg Med. 2007;39:256–265. doi: 10.1002/lsm.20476. [DOI] [PubMed] [Google Scholar]
- 3.Van den Bos RR, Kockaert MA, Neumann HAM, Bremmer RH, Nijsten T, Van Gemert MJC. Heat conduction from the exceedingly hot fiber tip contributes to the endovenous laser ablation of varicose veins. Lasers Med Sci. 2009;24:247–251. doi: 10.1007/s10103-008-0639-y. [DOI] [PubMed] [Google Scholar]
- 4.Verdaasdonk RM, Holstege FC, Jansen ED, Borst C. Temperature along the surface of modified fiber tips for Nd:YAG laser angioplasty. Lasers Surg Med. 1991;11:213–222. doi: 10.1002/lsm.1900110304. [DOI] [PubMed] [Google Scholar]
- 5.Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28:56–61. doi: 10.1046/j.1524-4725.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- 6.Amzayyb M, van den Bos RR, Kodach VM, de Bruin DM, Nijsten T, Neumann HAM, van Gemert MJC. Carbonised blood deposited on fibers during 810, 940 and 1,470 nm endovenous laser ablation: thickness and absorption by optical coherence tomography. Lasers Med Sci. 2010;25:439–447. doi: 10.1007/s10103-009-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan C-M, Anderson RR. Endovenous laser ablation: mechanism of action. Phlebology. 2008;23:206–213. doi: 10.1258/phleb.2008.008049. [DOI] [PubMed] [Google Scholar]
- 8.Disselhoff BCVM, Rem AI, Verdaasdonk RM, der Kinderen DJ, Moll FL. Endovenous laser ablation: an experimental study on the mechanism of action. Phlebology. 2008;23:69–76. doi: 10.1258/phleb.2007.007038. [DOI] [PubMed] [Google Scholar]
- 9.Vuylsteke M, Van Dorpe J, Roelens J, De Bo T, Mordon S, Fourneau I (2010) Intraluminal fibre-tip centring can improve endovenous laser ablation: a histological study. Eur J Vasc Endovasc Surg 40:110–116 [DOI] [PubMed]
- 10.Proebstle TM, Lehr HA, Kargl A, Espinola-Klein C, Rother W, Bethge S, Knop J. Endovenous treatment of the greater saphenous vein with a 940-nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser-generated steam bubbles. J Vasc Surg. 2002;35:729–736. doi: 10.1067/mva.2002.121132. [DOI] [PubMed] [Google Scholar]
- 11.Najibi SH, Müller-Steinhagen H, Jamialahmadi M. Calcium sulphate scale formation during subcooled flow boiling. Chem Eng Sci. 1997;52:1265–1284. doi: 10.1016/S0009-2509(96)00505-2. [DOI] [Google Scholar]
- 12.van der Geld CWM. The dynamics of a boiling bubble before and after detachment. Heat Mass Transf. 2009;45:831–846. doi: 10.1007/s00231-007-0254-7. [DOI] [Google Scholar]
- 13.Faghri A (1995) Heat pipe science and technology. Taylor & Francis Group, Washington, DC
- 14.Grooten MHM, van der Geld CWM (2009) Predicting heat transfer in long R-134a filled thermosyphons. ASME J Heat Transfer 131:051501–1 to 051501–9
- 15.Hagens H, Ganzevles FLA, van der Geld CWM, Grooten MHM. Air heat exchangers with long heat pipes: experiments and predictions. Appl Therm Eng. 2007;27:2426–2434. doi: 10.1016/j.applthermaleng.2007.03.004. [DOI] [Google Scholar]
- 16.Collier J, Thome J. Convective boiling and condensation. Oxford: Clarendon Press; 1994. [Google Scholar]
- 17.Carey V. Liquid-vapor phase-change phenomena. Washington, DC: Hemisphere Publ. Corp; 1994. [Google Scholar]
- 18.Kreith F, Bohn M (1996) Principles of heat transfer. Harper and Row, New York
- 19.Pearce J, Thomsen S (1995) Rate process analysis of thermal damage. In: Welch AJ, van Gemert MJC (eds) Optical-thermal response of laser-irradiated tissue. Plenum Press, New York, p 569, Fig. 17.5.


