Abstract
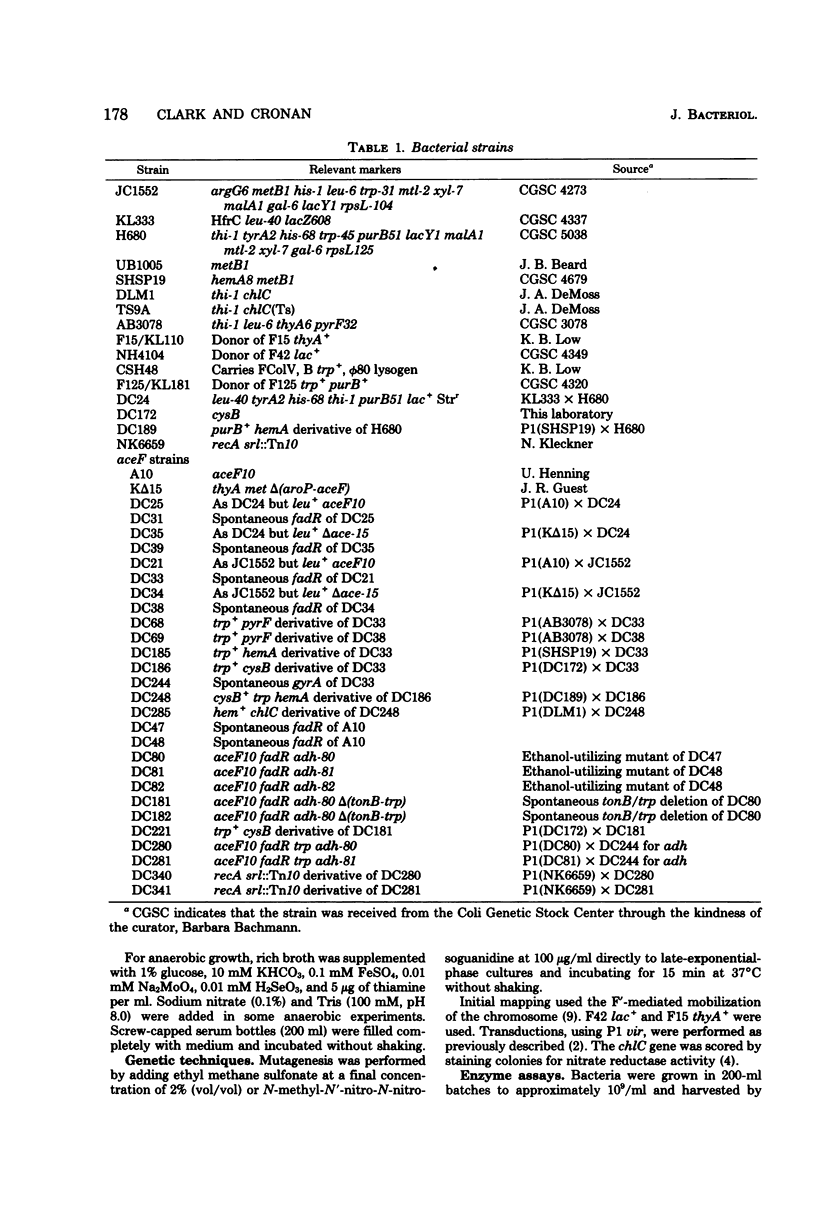
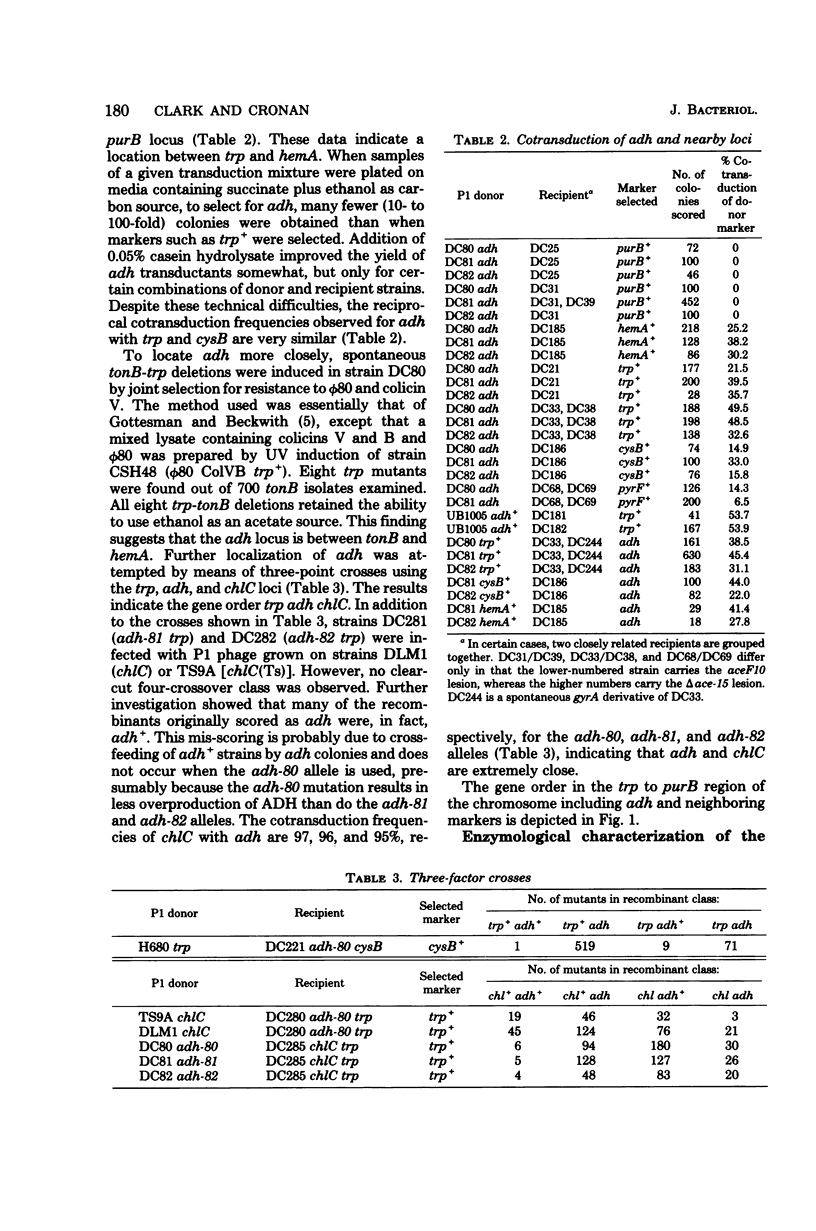
Mutants of Escherichia coli which overproduce alcohol dehydrogenase were obtained by selection for the ability to use ethanol as an acetate source in a strain auxotrophic for acetate. A mutant having a 20-fold overproduction of alcohol dehydrogenase was able to use ethanol only to fulfill its acetate requirement, whereas two mutants with a 60-fold overproduction were able to use ethanol as a sole carbon source. The latter two mutants produced only 25% of the wild-type level of nitrate reductase, when grown under anaerobic conditions. Alcohol dehydrogenase production was largely unaffected by catabolite repression but was repressed by nitrate under both aerobic and anaerobic conditions. The genetic locus responsible for alcohol dehydrogenase overproduction was located at min 27 on the E. coli genetic map; the gene order, as determined by transduction, was trp tonB adh chlC hemA. The possible relationship of alcohol dehydrogenase to anaerobic redox systems such as formate-nitrate reductase is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cronan J. E., Jr, Silbert D. F., Wulff D. L. Mapping of the fabA locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1972 Oct;112(1):206–211. doi: 10.1128/jb.112.1.206-211.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A. Role of the chlC gene in formation of the formate-nitrate reductase pathway in Escherichia coli. J Bacteriol. 1978 Feb;133(2):626–630. doi: 10.1128/jb.133.2.626-630.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Beckwith J. R. Directed transposition of the arabinose operon: a technique for the isolation of specialized transducing bacteriophages for any Escherichia coli gene. J Mol Biol. 1969 Aug 28;44(1):117–127. doi: 10.1016/0022-2836(69)90408-2. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Biochemical and genetic studies with nitrate reductase C-gene mutants of Escherichia coli. Mol Gen Genet. 1969;105(4):285–297. doi: 10.1007/BF00277583. [DOI] [PubMed] [Google Scholar]
- Hacking A. J., Lin E. C. Disruption of the fucose pathway as a consequence of genetic adaptation to propanediol as a carbon source in Escherichia coli. J Bacteriol. 1976 Jun;126(3):1166–1172. doi: 10.1128/jb.126.3.1166-1172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Pauli G., Overath P. ato Operon: a highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur J Biochem. 1972 Sep 25;29(3):553–562. doi: 10.1111/j.1432-1033.1972.tb02021.x. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still J. L. Alcohol enzyme of Bact. coli. Biochem J. 1940 Sep;34(8-9):1177–1182. doi: 10.1042/bj0341177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]



