Abstract
The cilia and the cytoplasm are separated by a region called the transition zone, where wedge-shaped structures link the microtubule doublets of the axoneme to the ciliary membrane, thereby forming a ciliary “gate.” In this issue, Craige et al. (J. Cell Biol. doi:10.1083/jcb.201006105) demonstrate in Chlamydomonas reinhardtii that Nphp6/cep290, which is mutated in nephronophthisis (NPHP), is an integral component of these connectors and maintains the structural integrity of this gate.
Cilia, tiny hairlike organelles that protrude from the cell surface, are located on almost all polarized cell types of the human body. Although the basic structures of different types of cilia are similar, they exert various tissue-specific functions during development, tissue morphogenesis, and homeostasis. Their prevalence and involvement in various cellular functions could explain why cilia-related disorders (ciliopathies) can affect many organ systems. Ciliopathies can either involve single organs, such as cystic kidney disease, or can occur as multisystemic disorders, such as Bardet Biedl syndrome and nephronophthisis (NPHP)-related disorders with phenotypically variable and overlapping disease manifestations (Badano et al., 2006; Fliegauf et al., 2007). Among syndromic forms of cystic kidney diseases, NPHP is the most common and complex disorder in childhood. NPHP comprises a genetically heterogenous group of renal cystic disorders with an autosomal recessive inheritance pattern. NPHP can cause end-stage renal disease in early infancy, childhood, and adolescence, as well as in adulthood, and can be associated with extra-renal disease manifestations such as ocular motor apraxia (Cogan syndrome), retinitis pigmentosa, Leber congenital amaurosis, coloboma of the optic nerve, cerebellar vermis aplasia (Joubert syndrome), liver fibrosis, cranioectodermal dysplasia, cone-shaped epiphyses, asphyxiating thoracic dysplasia (Jeune’s syndrome), Ellis-van Creveld syndrome, and, rarely, situs inversus (Omran and Ermisch-Omran, 2008). In addition, it has been shown that NPHP mutations can cause Meckel syndrome, a perinatal lethal disease characterized by congenital cystic kidney disease and encephalocele.
Several genes responsible for NPHP have been identified (summarized in Omran and Ermisch-Omran, 2008), and many of the encoded proteins, such as NPHP1, NPHP2 (inversin), NPHP3, NPHP4 (nephroretinin), NPHP6, and NPHP8, have been found to interact with each other (Olbrich et al., 2003; Mollet et al., 2005; Delous et al., 2007; Bergmann et al., 2008). Although important mechanistic insights in the pathogenesis of NPHP have been established, such as perturbed Wnt signaling, the exact functional role of NPHP proteins still remained enigmatic (Simons et al., 2005; Bergmann et al., 2008). In this issue, Craige et al. shed new light in the function of NPHP6. They demonstrate that NPHP6 is a structural component of the champagne glass–shaped structures that link the microtubular doublets of the axoneme to the ciliary necklace, a distinct portion of the ciliary membrane first described almost 40 yr ago (Gilula and Satir, 1972) . Up to now, nothing was known about the protein composition of this unique structure at the ciliary base.
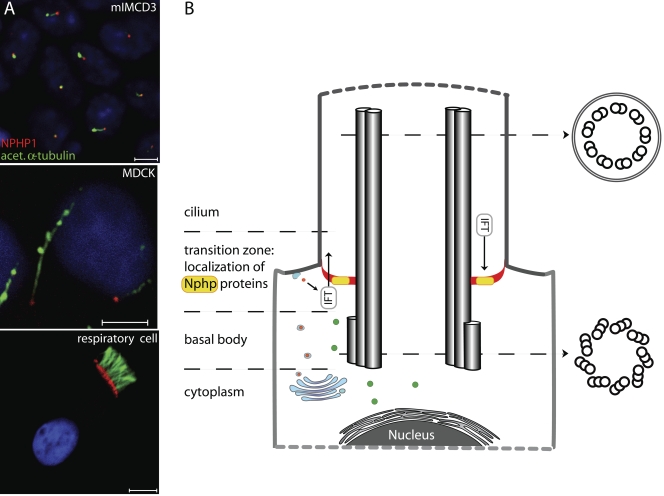
The ciliary compartment including the ciliary membrane is equipped with a distinct composition of proteins, and the compartment border is located at the transition zone, where intraflagellar transport (IFT) particles are involved in active transport of cargoes from and to the ciliary compartment across the compartment border driven by two kinesin-2 family members: the heterotrimeric KIF3A–KIF3B–KAP complex and the homodimeric KIF17 motor (Fig. 1). Interestingly, several studies demonstrated that NPHP proteins sublocalize to the ciliary base of primary cilia (NPHP1, NPHP4, NPHP6, NPHP8, NPHP9, and NPHP11) as well as to the connecting cilium of the photoreceptor (NPHP1, NPHP5, and NPHP6), which is considered to be the orthologous structure of the transition zone (Olbrich et al., 2003; Mollet et al., 2005; Otto et al., 2005; Sayer et al., 2006; Delous et al., 2007; Bergmann et al., 2008; Otto et al., 2008; Valente et al., 2010). Detailed analyses of proteins such as NPHP1 revealed specific and exclusive localization at the transition zone (Fig. 1 A), which suggests a possible gatekeeper-like functional role of NPHP proteins at the ciliary compartment border to control delivery and exit of proteins to and from the cilium, respectively (Fliegauf et al., 2006). During ciliogenesis, NPHP1 becomes immediately recruited to the transition zone, which indicates that NPHP proteins may also be important for formation of this organelle. Interestingly, localization of these proteins to the transition zone has been evolutionary conserved and is also observed in Caenorhabditis elegans (Jauregui et al., 2008).
Figure 1.
NPHP proteins function at the ciliary gate (transition zone). (A) Localization of nephrocystin (red, NPHP1) at the transition zone is shown in murine (mIMCD3) immotile renal cilia (top), immotile canine renal MDCK cilia (middle), and motile human respiratory cilia (bottom). The ciliary axoneme is stained with antibodies targeting acetylated α-tubulin (green). Bars, 5 µm. (B) The triplet microtubule structure of the basal body is converted into the axonemal doublet structure at the transition zone of primary cilia. Proximal transition y-shaped fibers (red) connect each outer microtubule doublet to the membrane and mark the border at which IFT proteins start to shuffle cargoes to and from the ciliary compartment. The ciliary compartment, including the ciliary membrane, is therefore equipped with a distinct composition of proteins such as polycystin-2 and BBS proteins (i.e., BBS4), which differs from the cytoplasm and the apical plasma membrane. NPHP6/CEP290 as well as other NPHP proteins (e.g., NPHP1) localize at the transition zone and probably function as gatekeepers that control access and exit of proteins to and from the ciliary compartment, respectively.
In this issue Craige et al. (2010) exploit the excellent genetic and biochemical tools available in Chlamydomonas reinhardtii to investigate the role of cep290/Nphp6 in the regulation of ciliary protein trafficking. Using immunoelectron microscopy, they show that cep290 localizes to the wedge-shaped structures that bridge and connect the flagellar membrane to the axonemal outer doublets within the transition zone. Further ultrastructural studies revealed defects of those structures in cep290 mutants, which indicates that cep290 is essential for integrity of the ciliary “gate” and an integral component of this poorly characterized structure. Detailed analyses of anterograde and retrograde IFT transport kinetics did not reveal gross alterations, which indicated that cep290 does not regulate IFT motor activity. Mass spectrometry analyses of flagella identified a complex pattern of abnormal protein composition. Biochemistry analyses of the flagella found increased amounts of IFT complex B proteins and BBS4, and decreased levels of the IFT complex A protein IFT139 as well as polycystin-2, which confirms that cep290 functions as a gatekeeper to control protein content of the flagella compartment. Alteration of polycystin-2 and BBS4 levels might even explain the complex clinical phenotype of cystic kidney disease and BBS-like findings present in children affected by CEP290/NPHP6 mutations (den Hollander et al., 2006; Sayer et al., 2006; Valente et al., 2006; Baala et al., 2007).
Craige et al. (2010) also make some interesting observations that could be relevant to somatic gene therapy. Using dikaryon rescue studies, they show that cep290 is a dynamic protein that shuttles between the cytoplasm and the transition zone and that can incorporate into preassembled mutant transition zones and restore function. These results could be applied toward targeted gene therapy in NPHP-related diseases, such as Leber congenital amaurosis, a retinal degeneration disease in which cep290 is frequently mutated. Expression of CEP290 by gene therapy vectors in photoreceptors of patients could restore ciliary function.
The cellular biological findings presented by Craige et al. (2010) are of major scientific interest because they open a new NPHP research field focusing on the ciliary compartment border. Future studies will address the roles of other interacting NPHP proteins for the integrity and/or function of the ciliary gate. Cell type–specific differences of the composition of the ciliary gate might account for the phenotypic differences observed in NPHP patients. Recent findings indicate similarities between the mechanisms regulating nuclear and ciliary import. Consistently, ciliary targeting of the IFT motor protein KIF17 has been shown to be regulated by a ciliary-cytoplasmic gradient of the small GTPase Ran, with high levels of GTP-bound Ran (RanGTP) in the cilium (Dishinger et al., 2010). Furthermore, KIF17 interacts with the nuclear import protein importin-β2 in a manner dependent on the ciliary localization signals and inhibited by RanGTP. Thus, the wedge-shaped fibers may function as the ciliary equivalent of the nuclear pore. Further work will shed light on the relationship between the different components of this interesting structure.
Acknowledgments
Special thanks to Anita Becker Heck, Heike Olbrich, Marouan Abouhamed, and Niki T Loges for literature research and figure preparation.
H. Omran is supported by grants from the Deutsche Forschungsgemeinschaft DFG Om 6/4 and 6/5, GRK1104, and the SFB592 as well as grants from the European Community (EU-CILIA and SYSCILIA).
References
- Baala L., Audollent S., Martinovic J., Ozilou C., Babron M.C., Sivanandamoorthy S., Saunier S., Salomon R., Gonzales M., Rattenberry E., et al. 2007. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am. J. Hum. Genet. 81:170–179 10.1086/519494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano J.L., Mitsuma N., Beales P.L., Katsanis N. 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7:125–148 10.1146/annurev.genom.7.080505.115610 [DOI] [PubMed] [Google Scholar]
- Bergmann C., Fliegauf M., Brüchle N.O., Frank V., Olbrich H., Kirschner J., Schermer B., Schmedding I., Kispert A., Kränzlin B., et al. 2008. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am. J. Hum. Genet. 82:959–970 10.1016/j.ajhg.2008.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige B., Tsao C.-C., Diener D.R., Hou Y., Lechtreck K.-F., Rosenbaum J.L., Witman G.B. 2010. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190:927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M., Baala L., Salomon R., Laclef C., Vierkotten J., Tory K., Golzio C., Lacoste T., Besse L., Ozilou C., et al. 2007. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat. Genet. 39:875–881 10.1038/ng2039 [DOI] [PubMed] [Google Scholar]
- den Hollander A.I., Koenekoop R.K., Yzer S., Lopez I., Arends M.L., Voesenek K.E., Zonneveld M.N., Strom T.M., Meitinger T., Brunner H.G., et al. 2006. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 79:556–561 10.1086/507318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishinger J.F., Kee H.L., Jenkins P.M., Fan S., Hurd T.W., Hammond J.W., Truong Y.N., Margolis B., Martens J.R., Verhey K.J. 2010. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat. Cell Biol. 12:703–710 10.1038/ncb2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M., Horvath J., von Schnakenburg C., Olbrich H., Müller D., Thumfart J., Schermer B., Pazour G.J., Neumann H.P., Zentgraf H., et al. 2006. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J. Am. Soc. Nephrol. 17:2424–2433 10.1681/ASN.2005121351 [DOI] [PubMed] [Google Scholar]
- Fliegauf M., Benzing T., Omran H. 2007. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8:880–893 10.1038/nrm2278 [DOI] [PubMed] [Google Scholar]
- Gilula N.B., Satir P. 1972. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 53:494–509 10.1083/jcb.53.2.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui A.R., Nguyen K.C., Hall D.H., Barr M.M. 2008. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J. Cell Biol. 180:973–988 10.1083/jcb.200707090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet G., Silbermann F., Delous M., Salomon R., Antignac C., Saunier S. 2005. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum. Mol. Genet. 14:645–656 10.1093/hmg/ddi061 [DOI] [PubMed] [Google Scholar]
- Olbrich H., Fliegauf M., Hoefele J., Kispert A., Otto E., Volz A., Wolf M.T., Sasmaz G., Trauer U., Reinhardt R., et al. 2003. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat. Genet. 34:455–459 10.1038/ng1216 [DOI] [PubMed] [Google Scholar]
- Omran H., Ermisch-Omran B. 2008. Nephronophthisis and medullary cystic kidney disease. In Comprehensive Pediatric Nephrology. Geary D.F., Schaefer F., Mosby Elsevier, Philadelphia: 143–154 [Google Scholar]
- Otto E.A., Loeys B., Khanna H., Hellemans J., Sudbrak R., Fan S., Muerb U., O’Toole J.F., Helou J., Attanasio M., et al. 2005. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat. Genet. 37:282–288 10.1038/ng1520 [DOI] [PubMed] [Google Scholar]
- Otto E.A., Trapp M.L., Schultheiss U.T., Helou J., Quarmby L.M., Hildebrandt F. 2008. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J. Am. Soc. Nephrol. 19:587–592 10.1681/ASN.2007040490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer J.A., Otto E.A., O’Toole J.F., Nurnberg G., Kennedy M.A., Becker C., Hennies H.C., Helou J., Attanasio M., Fausett B.V., et al. 2006. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 38:674–681 10.1038/ng1786 [DOI] [PubMed] [Google Scholar]
- Simons M., Gloy J., Ganner A., Bullerkotte A., Bashkurov M., Krönig C., Schermer B., Benzing T., Cabello O.A., Jenny A., et al. 2005. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 37:537–543 10.1038/ng1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente E.M., Silhavy J.L., Brancati F., Barrano G., Krishnaswami S.R., Castori M., Lancaster M.A., Boltshauser E., Boccone L., Al-Gazali L., et al. 2006. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 38:623–625 10.1038/ng1805 [DOI] [PubMed] [Google Scholar]
- Valente E.M., Logan C.V., Mougou-Zerelli S., Lee J.H., Silhavy J.L., Brancati F., Iannicelli M., Travaglini L., Romani S., Illi B., et al. 2010. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat. Genet. 42:619–625 10.1038/ng.594 [DOI] [PMC free article] [PubMed] [Google Scholar]