Abstract
Gene fusions have a critical role in cancer progression. Mechanisms associated with the genesis and cell type specificity of these fusions are not well understood. A prototypical gene fusion, TMPRSS2-ERG, involves the 5’ untranslated region of the androgen-regulated gene TMPRSS2 with the ERG gene, and is the most common gene fusion associated with prostate cancer. We demonstrate that androgen signaling induces chromosomal proximity between TMPRSS2 and ERG loci, and facilitates the formation of the TMPRSS2-ERG gene fusion when subjected to an agent that causes DNA double strand breaks. These results provide a conceptual framework for the genesis of gene fusions and may provide suggestions as to the general etiology of human prostate cancer.
Gene fusions are a hallmark of cancer development (1), but the mechanisms underlying their genesis and cell type specificities are unclear (2).
Fusions of the TMPRSS2 and ERG genes, which are located 3 Mb apart on human chromosome 21q22.2, are found in about 50% of prostate cancers and consist of the 5’ untranslated region of TMPRSS2, an androgen-regulated gene, fused to the protein-coding sequence of ERG, which encodes an erythroblast transformation-specific (ETS) transcription factor (2). Recently, estrogen was shown to induce rapid chromosomal movements that bring together estrogen receptor α-bound genes on different chromosomes (3). Given the broad similarities between estrogen and androgen signaling, we hypothesized that androgen might likewise induce chromosomal movements and bring together the TMPRSS2 and ERG genes, thereby facilitating the formation of gene fusions.
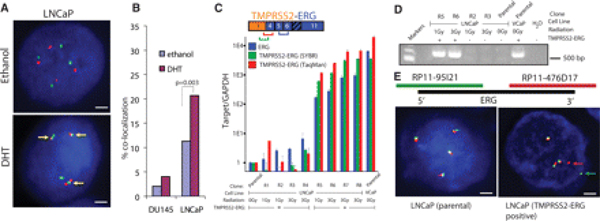
To examine the effects of androgen signaling on the formation of TMPRSS2-ERG, we studied LNCaP prostate cancer cells, which are androgen sensitive but lack this fusion gene (4) (fig. S1). We treated these cells with the androgen receptor TMPRSS2 and ERG genomic loci (34/300 and 62/300 nuclei for ethanol and DHT, respectively, P = 0.003, χ2 test) (Fig. 1, A and B, and fig. S2). This effect was dependent on AR (fig. S3). Importantly, androgen did not induce proximity between the TMPRSS2 and ERG loci in DU145 human prostate cancer cells, which are androgen insensitive (4/197 and 8/197 nucleai for ethanol and DHT, respectively, P = 0.038, χ2 test).
Figure 1.
(A) FISH based evaluation of induced proximity between TMPRSS2 (green) and ERG (red) on stimulation with 100 nM DHT in LNCaP cells. Colocalization is indicated by yellow arrows. Scale bar indicate 2 µm. (B) Induced proximity between TMPRSS2 and ERG in DU145 and LNCaP cells is quantified and represented as the percentage of nuclei exhibiting colocalization signals. GAPDH, glyceraldehydes-3-phosphate dehydrogenase. (C) SYBR Green (Moleculr Probes, Eugene, Orgeon) and TaqMan (applied Biosystems, Foster City, California) quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of the TMPRSS2-ERG fusion transcript. (D) Gel based RT-PCR analysis with primers spanning the first exon of TMPRSS2 and sixth exon of ERG for representative clones. (E) FISH analysis of the ERG locus. Split signals representing ERG rearrangement are highlighted by arrows.
To determine whether this induced proximity facilitates the formation of gene fusions, we treated LNCaP cells with DHT for 12 hours and then irradiated the cells to induce DNA double-strand breaks. After irradiation [1 or 3 grays (Gy)], single cells were seeded in multiple 96-well plates by using flow cytometry and were clonally expanded. TMPRSS2-ERG fusion transcripts were detected in 25% (3/12) of the clones treated with 3-Gy irradiation but in only 2.3% (1/43) of those treated with 1 Gy (Fig. 1, C and D, fig S4, and table S1). The expression of TMPRSS2-ERG transcripts in positive LNCaP clones was similar to that in VCaP prostate cancer cells (3), which endogenously harbor the gene fusion. Furthermore, TMPRSS2-ERG-expressing LNCaP cells showed evidence consistent with chromosomal aberrations at the ERG locus (Fig. 1E and fig. S5). Parental LNCaP cells have four copies of chromosome 21, generating four yellow signals. LNCaP cells that harbor TMPRSS2-ERG transcripts generate three yellow signals and a pair of red and green signals, suggestive of a rearrangement of the ERG locus.
Our results build on earlier work documenting the role of chromosomal proximity in gene fusions (5) and, in so doing, provide a conceptual framework for the genesis of gene fusions in hormone-regulated epithelial cancers. Androgen-induced proximity between TMPRSS2 and ERG could help explain why TMPRSS2-ERG fusions are restricted to the prostate, which is uniquely dependent on androgen signaling. We speculate that androgen signaling colocalizes the 5’ and 3’ gene fusion partners, thereby increasing the probability of a gene fusion when subjected to agents that cause DNA double-strand breaks.
Supplementary Material
References and Notes
- 1.Rowley JD. Annu Rev Genet. 1998;32:495. doi: 10.1146/annurev.genet.32.1.495. [DOI] [PubMed] [Google Scholar]
- 2.Tomlins SA, et al. Science. 2005;310:644. [Google Scholar]
- 3.Hu Q, et al. Proc Natl Acad Sci U S A. 2008;105:19199. doi: 10.1073/pnas.0810634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Materials and methods are available as supporting material on Science Online.
- 5.Nikiforova MN, et al. Science. 2000;290:138. doi: 10.1126/science.290.5489.138. [DOI] [PubMed] [Google Scholar]
- 6.This work was supported by NIH grants P50CA69568, R01CA132874, and DOD W81XWH-09-2-0014. A.M.C. and S.A.T. are named as co-inventors on a patent related to gene fusions in prostate cancer that is licensed to Gen-Probe, Incorporated.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.