Abstract
Umbilical cord blood (UCB) has been used successfully as a source of hematopoietic stem cells (HSCs) for allogeneic transplantation in children and adults in the treatment of hematologic diseases. However, compared with marrow or mobilized peripheral blood stem cell grafts from adult donors, significant delays in the rates and kinetics of neutrophil and platelet engraftment are noted after UCB transplant. These differences relate in part to the reduced numbers of HSCs in UCB grafts. To improve the rates and kinetics of engraftment of UCB HSC, several strategies have been proposed, including ex vivo expansion of UCB HSCs, addition of third-party mesenchymal cells, intrabone delivery of HSCs, modulation of CD26 expression, and infusion of two UCB grafts. This article will focus on ex vivo expansion of UCB HSCs and strategies to enhance UCB homing as potential solutions to overcome the problem of low stem cell numbers in a UCB graft.
Keywords: cord blood transplantation, CXCR4, engraftment, ex vivo expansion, hematopoietic stem cell, Notch, SDF-1, stem cell homing, umbilical cord blood
Umbilical cord blood as an alternative source of hematopoietic stem cells for hematopoietic transplants
With more than 12,000 umbilical cord blood (UCB) transplantations performed since 1988 for the treatment of hematologic malignancies and selected non-malignant disorders, UCB has emerged as an alternative source of hematopoietic stem cells (HSCs) for transplantation. This is especially important for minority patients and patients of mixed ethnicity, where UCB is a particularly attractive alternative donor stem cell source because it is readily available with no donor attrition and allows reduced stringency in HLA matching without an increase in graft-versus-host disease (GVHD). Furthermore, several unique properties of UCB have been identified. Compared with bone marrow (BM) cells, CD34+/CD38− UCB cells proliferate more rapidly and generate larger numbers of progeny cells [1,2]. In addition, longer telomere lengths of UCB cells have been proposed as a possible explanation for the greater proliferative capacity of UCB [3,4]. Despite this, the outcomes in adults undergoing HSC transplant with an UCB graft are significantly influenced by the low cell dose of the graft. Numerous clinical studies have consistently demonstrated that the total nucleated cell (TNC) and CD34+ cell doses in cord blood grafts are highly correlated with the rate of neutrophil and platelet engraftment, as well as the incidence of graft failure and early transplant-related complication [5–13]. Based on these studies, critical cell-dose thresholds have been established and outcomes for patients receiving less than the generally accepted threshold of over 2.5 × 107 TNC/kg are significantly inferior in terms of engraftment, transplant-related mortality and overall survival. For adult patients and children weighing more than 35–40 kg, obtaining an adequate cell dose from a single UCB unit is challenging. Thus, in order to realize the full treatment potential of UCB in adult patients, it is essential to pursue strategies that will enhance the kinetics and incidence of engraftment of UCB HSCs. Strategies to increase the efficiency of homing/engraftment of UCB HSCs and the development of novel ex vivo expansion methodologies to overcome the low HSC numbers are two such approaches to be addressed in this article.
Strategies to enhance UCB HSCs homing/engraftment
Role of CXCR4–SDF-1 axis
Stromal-derived factor (SDF)-1 binds to G-protein-coupled seven-transmembrane span CXCR4. Murine knockout data demonstrate that SDF-1 is secreted by BM stromal cells and is a critical factor for the colonization of fetal BM by fetal liver-derived HSCs. Furthermore, during adult life it functions in the retention/homing of HSCs in the marrow microenvironment. Recent data from our group and others suggest that responsiveness of HSCs to an SDF-1 gradient may be positively modulated/primed/enhanced by several factors: for example, C3 complement cleavage fragments (C3a and desArgC3a), fibronectin, fibrinogen and hyaluronic acid [14–18]. Thus, responsiveness of HSCs to an SDF-1 gradient may influence the final outcome of a hematopoietic transplant. Unfortunately, this crucial parameter is not employed on a routine basis in the clinic to evaluate graft quality. More importantly, because responsiveness of UCB HSCs to an SDF-1 gradient may be enhanced by employing priming strategies, this phenomenon may be of clinical importance.
Routine assays to evaluate the quality of the hematopoietic graft
Harvested UCB, similar to cells harvested from adult marrow or mobilized PBSC, are evaluated for the number of UCB mono-nuclear cells and CD34+ cells, as well as cell viability, by employing a 0.4% trypan blue exclusion test. Generally, performing in vitro clonogeneic assays checks graft quality, but results of these assays are not available to clinicians at the time of UCB thaw and infusion. These tests give some important clues about the number of HSCs and their proliferative potential. However, they do not evaluate the functional homing responsiveness of UCB graft HSCs and T-cells to chemokines signals: for example, responsiveness of UCB mononuclear cells to an SDF-1 gradient may be crucial for the homing/engraftment of HSCs after transplantation. Our group and others have conducted studies to optimize ex vivo UCB HSC migration measurements and priming strategies that allow for potentially more efficient engraftment of UCB HSCs. Laboratory studies by our group and others indicate that these priming strategies may allow for better homing/seeding efficiency of transplanted cells and, more importantly, accelerate hematopoietic recovery after transplantation. Improving engraftment of a limited number of UCB HSCs and accelerating hematopoietic recovery, which is usually delayed after UCB transplants, are both crucial for effective UCB transplantation utility in adult patients.
SDF-1–CXCR4 axis is a pivotal factor in homing/engraftment of HSCs
SDF-1 regulates the trafficking of CD34+ HSCs, pre-B lymphocytes and T lymphocytes. SDF-1 is the ligand for CXCR4, which had been considered for many years to be its only receptor. Thus, the SDF-1–CXCR4 axis has a unique and important biological role [19–25]. Support for this notion comes from murine knockout data showing that SDF-1 secreted by BM stromal cells is critical for the colonization of BM by fetal liver-derived HSCs during embryogenesis. Furthermore, during adult life, SDF-1 is needed for homing/retention of HSCs in the BM [26] and HSCs engraft in the BM by following an SDF-1 gradient that is upregulated in the BM after conditioning for transplantation (e.g., total body irradiation, myeloablative chemotherapy) [27–30]. Thus, two parameters are of special importance in the engraftment of HSCs in BM: responsiveness of CXCR4 on HSCs to an SDF-1 gradient; and an effective level of SDF-1 expression in the BM environment.
Other biological effects of the SDF-1–CXCR4 axis
The major biological effects of SDF-1 are related to the ability of this chemokine to induce motility, chemotactic responses, adhesion and secretion of matrix metalloproteinases (MMPs) and angiopoietic factors (e.g., VEGF) in cells bearing cognate CXCR4. All of these processes are pivotal for HSC engraftment. SDF-1 also increases adhesion of early hematopoietic cells to VCAM-1, ICAM-1, fibronectin and fibrinogen by activating/modulating the function of several cell surface integrins. Aside from regulating cell trafficking, there is some controversy over whether SDF-1 also directly affects cell proliferation and survival. In our studies, SDF-1 does not affect the survival/proliferation of primary HSCs or any of several established hemato-lymphopoietic cell lines [31]. However, our evidence of SDF-1 not directly affecting the proliferation/survival of human HSCs in vitro does not rule out the possibility of an indirect effect. For example, by increasing the retention of early HSCs in BM, SDF-1 could affect HSC survival by promoting the interaction of these cells with adhesion molecules (AMs) expressed in the BM microenvironment. It is well-known that signals generated from AMs prevent cells from undergoing apoptosis in a mechanism described as ‘anoikis’ [32]. Lending support to this idea, HSCs from SDF-1 transgenic mice display both enhanced survival in vitro in response to growth factor withdrawal and enhanced myelopoiesis in vivo [33]. CXCR4 expressed on UCB CD4+ T cells may play an important role in their homing after transplantation and allogeneic engraftment [34–37]. Thus, the SDF-1–CXCR4 axis plays an important role in mediating engraftment of allogeneic UCB T cells that must overcome recipient immune-mediated graft rejection.
Negative & positive modulators of the SDF-1–CXCR4 axis
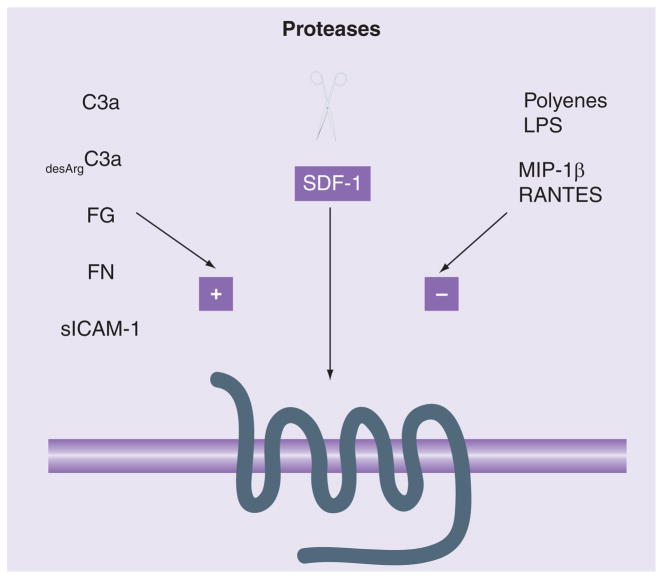
It is evident that the function of the SDF-1–CXCR4 axis must be tightly regulated in vivo by various biological mechanisms, a fact that we are only now beginning to fully understand. Our group and others have recently identified several factors that may positively affect the sensitivity/responsiveness of CXCR4+ cells to an SDF-1 gradient (Figure 1). The following factors were found to significantly increase the chemotaxis of HSCs to low/threshold doses of SDF-1 [38]: anaphylatoxin C3a (C3 complement protein cleavage fragment); desArgC3a (product of C3a degradation by carboxypeptidase); platelet-derived membrane microvesicles; hyaluronic acid; and sphingosine-1 phosphate. Similarly, we found that several other molecules, such as fibronectin, fibrinogen, thrombin, soluble uPAR and VCAM-1, also sensitize and/or increase the chemotactic responses of cells to low doses of SDF-1 [16]. These observations imply that the SDF-1–CXCR4 axis may be modulated by various molecules related to inflammation (e.g., C3a anaphylatoxin, desArgC3a, fibronectin, hyaluronic acid), coagulation (e.g., fibrinogen, uPAR, thrombin) or cell activation (e.g., s-VCAM-1, s-ICAM-1, membrane-derived vesicles) [16,17,38,39]. Interestingly, all these molecules are constituents of leukapheresis products collected from G-CSF-mobilized patients. We recently found that supernatants from these leukapheresis products increase the chemotactic responses of HSCs to SDF-1 and significantly enhance the homing of human UCB and BM-derived CD34+ cells in a nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mouse transplant model [16]. This increased responsiveness of CXCR4 to SDF-1 may be related to its incorporation into membrane lipid rafts.
Figure 1. Schematic of SDF-1–CXCR4 axis modulation/priming by various factors.
The SDF-1–CXCR4 axis is modulated by various external factors. In one regard, these target SDF-1 or the N-terminus of CXCR4, which are both cleaved by leukocyte-derived proteases or matrix metalloproteinases. Conversely, they target the SDF-1–CXCR4 axis, which can be primed positively (e.g., by platelet-derived membrane microvesicles, C3a, desArgC3a, uPAR, fibrinogen, fibronectin, hyaluronic acid, sICAM1 and cVCAM1) or negatively (e.g., by polyene antibiotics). Several of these molecules can also be found in tissues affected by inflammation and modulate the responsiveness of CXCR4+ cells to an SDF-1 gradient.
LPS: Lipopolysaccharide.
Based on all of these investigations, a new and more complex picture of the mechanisms that regulate the activity of the SDF-1–CXCR4 axis is emerging. The functionality of the CXCR4 receptor on HSCs and allogeneic UCB graft CD4+ T cells is modulated by factors such as (Figure 1):
Level of receptor expression on the cell surface
Sulfation status of its N-terminus
Expression and biological availability of SDF-1 in tissues
Cleavage of the CXCR4 N-terminus on the cells and SDF-1 in the extracellular space by serine proteases and MMPs
Heterologous desensitization by the CCR5 receptor
Modulation of CXCR4 incorporation into membrane lipid rafts positively by molecules related to inflammation/tissue remodeling and negatively by polyene antibiotics
Summary of strategies to improve homing/engraftment of UCB HSCs
In summary, the early stages of HSC seeding, which precede proliferation/differentiation are collectively termed ‘homing’. HSC homing is considered to take place in several overlapping steps. Cells infused into peripheral blood respond to a chemotactic SDF-1 gradient from the BM, attach to the BM endothelium, transmigrate through the basal membrane in a methylprednisolone-dependent manner, and finally home to a niche where they can survive, expand and proliferate, or engraft. Thus, the regeneration of normal marrow after transplant is a function of proper engraftment of transplanted cells. Likewise, the proper function of BM after transplantation depends on the crucial engraftment of the most primitive long-term repopulating HSCs. Improving the speed of engraftment via strategies to improve homing of HSCs to the marrow might improve the overall results of UCB transplantation, especially in adult cord blood transplant recipients. Recently, two new strategies have been proposed. The first is based on inhibition of human analog of the murine-identified HSC-expressed dipeptidyl-peptidase (CD26) [40]. The second, developed by our group, is based on ex vivo priming of HSCs before transplantation with small molecules, such as C3 complement fragments, fibrinogen, fibronectin and hyaluronic acid [16,17,38,39,41,42].
Ex vivo expansion of HSCs
Substantial effort has focused on the exogenous signals that may be used to favor stem cell self-renewal versus differentiation in order to develop optimal conditions for the ex vivo expansion of stem cells. The effect of cytokines that support hematopoietic cell survival, proliferation and differentiation has been extensively studied in vitro, but a significant role for these cytokines in enhancing self-renewal has not been shown. Consequently, a stochastic model of cellular determination has been suggested in which the fate of hematopoietic precursors is not instructed by soluble cytokines [43,44], but rather by specific interactions between stem and other cells within a particular microenvironment or stem cell niche. These interactions are mediated by extrinsic regulators of stem cell fate and are likely to play a key role in maintaining the numbers of stem cells by regulating their self-renewal and differentiation, now demonstrated by the work of several groups [45–47]. Thus, more recent studies are aimed at identifying intrinsic and extrinsic factors that regulate HSC fate. Clinical trials, which have also mainly evaluated cytokine-driven expansion systems, have not yet provided definitive evidence for stem cell expansion, but have demonstrated the feasibility and safety of ex vivo culturing of stem cells. However, a newer generation of clinical trials, as discussed later, is currently underway evaluating the use of extrinsic regulators of stem cell fate (Notch) and co-culture systems utilizing nonhematopoietic components (mesenchymal stromal cells) of the stem cell niche. Here, we address studies of ex vivo HSC expansion in animals and humans, as well as promising approaches under development.
Ex vivo HSC expansion using extrinsic & intrinsic regulators of stem cell fate
Optimization of cytokine-driven expansion systems has not led to clinically significant HSC expansion, perhaps due to a predominantly permissive, rather than directed, role in determining stem cell fate. The search for extrinsic and intrinsic regulators that act directly on human HSCs in regulating cell fate and self-renewal has suggested a role for regulatory molecules active in early development that are important in HSC maintenance and regulation. More recent studies have focused on the regulation of intrinsic signaling pathways by retrovirus-mediated transduction of HSCs (e.g., expression of homeobox genes). However, culture of cells for clinical application requires use of extrinsic regulators of cell development, including bone morphogenic proteins (BMPs), angiopoietin-like proteins, Shh, Wnts and Notch ligands.
Retroviral-mediated overexpression of Hox transcription factors, in particular HoxB4, has led to extensive ex vivo HSC expansion (3 logs over control cultures) without loss of full in vivo lymphomyeloid repopulating ability [48–51]. Although methods to alter homeobox gene expression in the absence of transducing cells are not available, the self-renewal induced by HoxB4 has suggested the exploration of extrinsic regulators of cell-fate involved in embryonic development, such as BMP-4, a member of the TGF-β superfamily, and Shh of the Hedgehog family of proteins, both of which have been implicated in early hematopoietic development [52,53]. In ex vivo expansion of human cord blood, soluble human BMP-4 has been shown to increase the survival of repopulating blood cells in culture [52], while Shh has been shown to induce a few-fold increase in repopulating cells via mechanisms that are dependent on downstream BMP signals [54]. Wnt proteins, which are involved in the growth and differentiation of a variety of primitive tissues, have also been implicated in the regulation of hematopoiesis, possibly exerting their effects through stromal cells [55], and have been shown to stimulate the proliferation of hematopoietic precursor cells. [56] There are also data supporting the presence of an interactive relationship between the various signaling pathways. For example, Notch1 receptors have been shown to be upregulated in response to Wnt signaling in HSCs [57].
Notch signaling in hematopoietic stem/progenitor cell development
A role for Notch in hematopoiesis was initially suggested by the detection of the human Notch1 gene in CD34+ or CD34+lin− human hematopoietic precursors [58]. Subsequently, ligands that activate the Notch pathway have become the most extensively studied and successfully utilized extrinsic regulators, with growing data supporting a role of Notch signaling in maintenance and/or self-renewal of HSCs. All four Notch receptors (Notch 1, 2, 3 and 4) identified in vertebrates have been detected in hematopoietic cells [59], and several investigators have reported the expression of Notch-1 and -2 in human CD34+ or CD34+Lin− precursors [59–61], and the expression of the Notch ligands, Delta-1 and Jagged-1 in human BM stromal cells and human hematopoietic precursors [62–65]. Moreover, expression of a constitutively active, truncated form of Notch-1 in murine hematopoietic precursors inhibited differentiation and enhanced self-renewal, leading to the establishment of an immortal cell line that phenotypically resembles primitive hematopoietic precursors [66]. This cell line, depending upon the cytokine context, can differentiate along the lymphoid or myeloid lineage. More recently, expression of constitutively active Notch in murine precursors transplanted in vivo led to increased stem cell numbers that were evident in secondary transplantation studies [67]. While these findings point to a role for Notch signaling in the regulation of stem cell self-renewal, expression of activated Notch-1 in human CD34+ cord blood precursors induced only a modest increase in the number of progenitors [68].
In order to affect nontransduced cells, exogenous Notch ligands have been used to induce endogenous Notch signaling in HSCs. Initial studies in mice and humans using soluble or cell-bound Notch ligand revealed limited increases in precursor cell numbers [63,64,68]. However, Varnum-Finney et al. and others have demonstrated a requirement for ligand immobilization to induce Notch signaling [66,69]. Studies performed with hematopoietic precursors cultured in the presence of immobilized Delta1 demonstrated profound effects on the differentiation of isolated murine marrow precursors with a multi-log increase in the number of Sca-1+Gr-1− cells with short-term lymphoid and myeloid repopulating ability [70]. Similarly, studies with human cord blood CD34+CD38− precursors cultured in serum-free conditions with immobilized ligand and cytokines resulted in an approximately 100–200-fold increase in the number of CD34+ cells generated compared with control cultures, and included cells capable of repopulating immunodeficient mice [71,72]. Moreover, more recent studies with both murine and human hematopoietic progenitors have demonstrated density-dependent effects of Delta1 on fate decisions of hematopoietic progenitors, and suggest that optimal ligand densities are required to promote expansion of cells that retain repopulating ability [71,73]. Other investigators have shown similar outcomes. Kertesz et al. demonstrated that culture of murine lineage-negative hematopoietic progenitor cells with immobilized Jagged1 resulted in expansion of serially transplantable HSCs, with superior expansion dependent on the combinatorial effect of Notch and cytokine-induced signaling pathways [74]. These data indicate that manipulation of Notch signaling can enhance stem cell self-renewal ex vivo and thereby increase HSC numbers for transplantation.
Adult versus UCB HSCs
As discussed earlier, several lines of evidence suggest that UCB contains a higher frequency of primitive hematopoietic progenitor cells and early committed progenitors than adult BM or peripheral blood [75,76]. There is also increasing evidence that the stem cells isolated from UCB survive longer in culture [58] and may be less mature and have greater proliferative capacity [77–80]. Phenotypic analyses of UCB have shown that the more primitive cell population, which expresses the CD34 antigen, but not the CD38 antigen, is fourfold more prevalent than in BM or peripheral blood progenitor cells (PBPC), and that this subpopulation has a higher in vitro cloning efficiency than the same population isolated from adult BM [81]. These findings correlate with data from studies using in vitro colony-forming assays to show that UCB contains greater numbers of immature colony-forming cells compared with BM or PBPC [82–84]. Secondly, there are numerous reports of the increased proliferative potential of UCB cells in response to cytokine stimulation. For example, using IL-11, stem cell factor (SCF) and GCSF or GM-CSF, Cairo et al. demonstrated an 80-fold increase after a 14-day expansion of UCB versus adult BM [85]. Moreover, compared with adult BM, UCB has been shown to have increased serial in vitro replating efficiency [78] and increased culture lifespan with increased progenitor cell production [82,86]. Finally, in vivo assays of UCB versus BM have shown that HSCs from UCB, but not adult BM, can engraft NOD/SCID mice without the use of exogenous cytokines [87]. More recently, Rosler et al. demonstrated that expanded cord blood had a competitive repopulating advantage as compared with expanded adult BM using an in vivo assay with NOD/SCID mice [88].
A potential contribution to the differences observed between UCB and adult HSCs may arise from the differential response of HSCs from different sources in cytokine-driven expansion systems, leading to variations in cell cycle status and homing ability of the expanded cells. Other possible sources of stem cells may have even greater proliferative potential. For example, murine fetal liver cells have greater proliferation and repopulation potential than HSCs isolated from adult murine BM or peripheral blood [89,90]. Overall, these results suggest that UCB progenitor cells are functionally superior to adult BM, with greater proliferative potential and possibly greater self-renewal capacity. Thus, UCB may represent a more viable target for ex vivo stem cell expansion, a possibility that has led to several clinical studies on the expansion of cord blood cells to augment conventional UCB transplantation.
Clinical ex vivo expansion trials with UCB
UCB expansion trials were undertaken to determine whether the delayed engraftment associated with UCB transplantation could be overcome if a portion of the cells from the UCB unit were expanded ex vivo [91–95]. Like the trials using PBPCs and BM, these initial trials utilized cytokine-based expansion systems as well as newer automated perfusion systems. The choice of exogenous cytokines used for culture has varied, reflecting the still undefined optimal conditions for expansion of the stem/progenitor cell. In all of the initial studies, only a portion of the single cord blood graft was used for expansion, and the expanded cells were infused in addition to unmanipulated cells because there is a lack of definitive data to suggest that cultured cells retain HSC properties and do not differentiate. There have been no adverse toxicities associated with infusion of the expanded cell product, nor has there been any change in engraftment kinetics. However, only two of the studies have enrolled more than a handful of patients, making it difficult to draw any definitive conclusions. More recently, novel culture systems for the ex vivo expansion of UCB progenitors have begun patient accrual, including a Notch-mediated ex vivo expansion trial at the Fred Hutchinson Cancer Research Center and a trial utilizing a copper chelating agent. These will be discussed in the following sections.
Cytokine-based ex vivo expansion
In one of the larger studies to date, Jaroscek et al. reported preliminary data from a Phase I trial at Duke University Medical Center undertaken to assess augmentation of UCB transplantation with cells expanded ex vivo in the AastromReplicell® Cell Production System [91]. This trial included 28 patients with both malignant and inherited disorders who were conditioned with one of three regimens, depending on diagnosis. On day 0, a portion of a single unrelated UCB unit was expanded ex vivo in medium supplemented with fetal bovine serum, horse serum and cytokines (EPO, PIXY321 and Flt3L) for 12 days. The expanded cells were then infused to augment the conventional transplant on day +12. Although expansion of TNC and colony-forming cells occurred in vitro in all cases, no increase in CD34+lin− cell number was achieved. In vivo, significant effects on engraftment kinetics were not observed with median time to neutrophil engraftment (absolute neutrophil count [ANC] >500) of 22 days (range: 13–40 days). No adverse reactions were observed due to infusion of the cultured cells. This Phase I trial was an important contribution to the concept and development of ex vivo expansion of UCB CD34+ cells for use in the clinical setting because it demonstrated both the safety of reserving an aliquot of an already small cord blood unit for expansion and the feasibility of expansion in a clinical setting. It is also possible that, although the numbers of CD34+lin− and CD3+ cells were not expanded with this culture system and may have explained the failure to improve engraftment, the delayed infusion (day +10 to +12) of expanded cells may have masked or prevented the benefit of a more rapid engraftment. Newer generation clinical cord blood expansion trials (discussed later) are currently underway based on a double cord blood model, in which one unit is infused unmanipulated on the day of transplant with a second unit that has been expanded ex vivo.
Shpall and colleagues are currently conducting a number of cord blood expansion trials at the MD Anderson Cancer Center (TX, USA): a cytokine-mediated expansion trial and a tetraethylenepentamine (TEPA)-based ex vivo expansion trial. The cytokine-mediated expansion trial is the successor trial to an earlier published trial performed at the University of Colorado (CO, USA) [94], with some important changes. In this trial, patients were randomized to receive either two unmanipulated cord blood units or one unmanipulated unit and one ex vivo expanded unit following either myeloablative or nonmyeloablative preparative regimens. All patients had high-risk hematologic malignancies, with 36 patients receiving two unmanipulated units and 35 patients receiving one unmanipulated unit and one expanded unit. For those patients receiving expanded cells, the smaller of the two units underwent CD133-selection using the CliniMACS® device on day 14 prior to transplant. In addition, the ‘negative’ fraction containing the T cells from the unit was cryopreserved. The CD133+ cells were then placed in culture with media supplemented with cytokines (SCF, G-CSF and thrombopoietin) for 14 days. Patients received the unmanipulated unit, the T-cell containing ‘negative’ fraction and the expanded cells on the day of transplant.
The median time to neutrophil engraftment in patients receiving a reduced intensity regimen was 14 (range 5–32 days; n = 12) and 7 days (range: 4–15 days; n = 14) for patients receiving two unmanipulated units and those that received an unmanipulated unit and ex vivo-expanded unit, respectively. Development of acute grade 2–4 GVHD was 43% in both groups and overall survival at 2 years was 50% in the expanded group and 20% in the unmanipulated group.
Ex vivo expansion of UCB HSCs using TEPA, a copper chelator
Based on preclinical data in which Peled et al. demonstrated enhanced expansion of CD34+ UCB progenitors [96], an additional ex vivo clinical expansion trial is being conducted at the MD Anderson Cancer Center using the copper chelator, TEPA. Similar to the cytokine-mediated expansion trial, patients with hematologic malignancies are eligible; however, all patients in this trial must have identified a single unit of cord blood that has been cryopreserved in two fractions. The smaller of the two UCB fractions is CD133-selected and ex vivo expanded in the presence of cytokines and the copper chelator TEPA starting 21 days prior to infusion. The results of ten patients with high-risk malignancies (nine beyond first remission) were recently reported [97]. Eight of the ten units identified for these patients were less than 2 × 107 TNC/kg pre-expansion. The average fold expansion of TNC placed in culture was 219 (range: 2–620) and the median time to engraftment was 30 days for neutrophils (range: 16–46 days) and 48 days for platelets (range: 35–105 days). Four out of nine engrafting patients experienced acute GVHD, all grade II, with skin involvement, and four out of eight patients surviving more than 100 days developed chronic GVHD. All cases were resolved with steroid therapy. All but one patient had sustained donor engraftment. Three patients were surviving at 21, 22 and 31 months post-transplant.
Notch-mediated ex vivo expansion
At the Fred Hutchinson Cancer Research Center, a Phase I trial assessing the potential efficacy of cord blood progenitors cultured in the presence of an engineered form of the Notch ligand, Delta1, in contributing to rapid early engraftment in patients undergoing a high-dose UCB transplant is currently accruing patients. As discussed earlier, there is accumulating evidence on the role of the Notch signaling pathway in hematopoietic cell fate decisions. To date, ten patients with high-risk acute leukemia in morphologic remission at the time of transplant have been enrolled with a median age of 27.5 years (range: 3–43 years) and median weight of 61.5 kg (range: 16–79 kg). Based on pioneering studies by Wagner and colleagues at the University of Minnesota (MN, USA) demonstrating safety of double cord blood unit infusion, patients receive a myeloablative preparative regimen (1320 cGy total body irradiation, 120 mg/kg cytoxan and 75 mg/m2 fludarabine) followed by infusion of one non-manipulated and one ex vivo expanded cord blood graft. All patients received prophylaxis for GVHD consisting of cyclosporine and mycophenolate mofetil beginning on day 3.
The unit selected for ex vivo expansion was thawed, CD34 cells selected using the Isolex 300i and cultures initiated in the presence of the Notch ligand Delta1 and serum-free media supplemented with SCF, thrombopoietin, FLT3L, IL-3 and IL-6. After 16 days in culture, cells are harvested and prepared for infusion, which takes place 4 h after infusion of the noncultured unrelated donor graft. There was an average fold expansion of CD34+ cells of 164 (±48 standard error of measurement [s.e.m.]; range: 41–471) and an average fold expansion of total cell numbers of 562 (±134 s.e.m., range: 146–1496). The infused CD34+ cell dose derived from the expanded cord blood graft averaged 6 × 106 CD34/kg (range: 0.93–13 × 106) versus 0.24 × 106 CD34/kg (range: 0.06–0.54 × 106; p = 0.0004) from the non-manipulated cord blood graft. There was no significant difference in the average TNC dose/kg dose infused between the non-manipulated and expanded cell grafts.
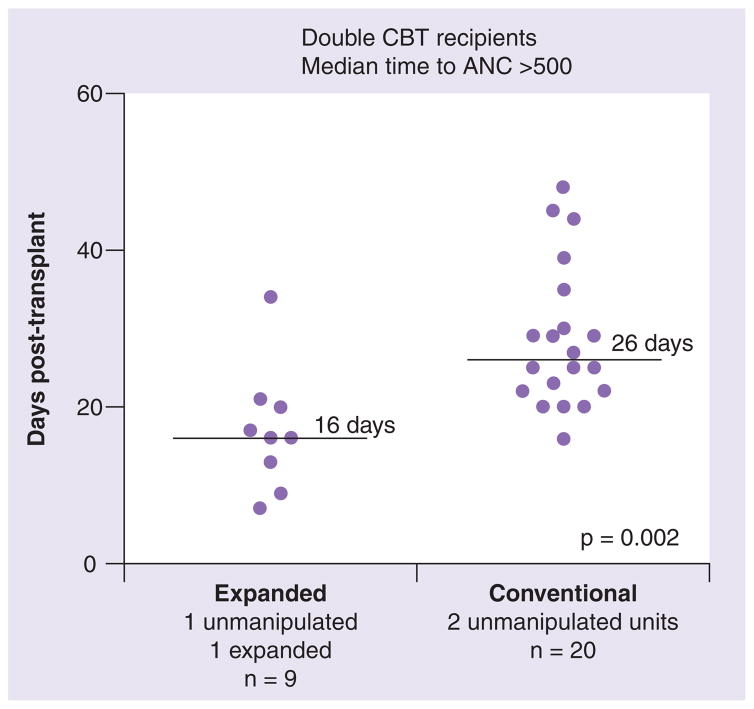
Time to ANC of at least 500/μl was evaluable in nine of the first ten patients. In these nine patients, time to achieve an ANC of at least 500/μl has been shortened significantly, with a median time of 16 days (range: 7–34 days). This compares quite favorably with a median time of 26 days (range: 16–48 days; p = 0.002) in a concurrent cohort of 20 patients undergoing double cord blood transplantation at our institution with an identical conditioning and post-transplant immune suppression regimen (Figure 2). In addition, this cohort did not differ significantly in age, weight, diagnosis or infused cell doses as provided by the non-manipulated units. One patient did experience primary graft rejection. No infusional toxicities or other safety concerns have been observed. Average follow-up time for this set of ten patients is currently 354 days (range: 77–806 days), and seven out of ten patients remain alive with no evidence of disease, and sustained complete donor engraftment. Acute grade II GVHD has been observed in all evaluable patients, except for one who had overall grade III acute GVHD. All patients responded to therapy. No chronic extensive GVHD has been observed and three patients have been diagnosed with chronic limited GVHD.
Figure 2. Culture of cord blood progenitors with Delta1ext-IgG results in more rapid neutrophil recovery in a myeloablative double cord blood transplantation setting.
The individual and median times (horizontal line) to ANC of at least 500/μl for patients receiving double unit cord blood transplantations with two non-manipulated units (conventional) versus with one ex vivo expanded unit and one non-manipulated unit (expanded) is presented.
ANC: Absolute neutrophil count; CBT: Cord blood transplantation.
Summary of strategies to enhance UCB stem cell engraftment in adult patients
Umbilical cord blood HSC research has revolutionized the unrelated allogeneic field of medicine, now comprising 30% of all unrelated marrow and stem cell transplant procedures performed in the USA annually (Center for International Blood and Marrow Transplant Research [201]: ‘Sources of Cells for Transplant’). Little is known about the cellular mechanisms underlying successful donor HSC and T-cell homing and engraftment in vivo despite infusion of UCB graft CD34 HSC doses ranging generally 1 log less than that of BM or mobilized peripheral cells from adult donors [98–106]. Use of double cord blood units has been shown to improve the rates and kinetics of UCB engraftment in adult patients [107–109]. The mechanisms underlying engraftment of the one ‘winning’ UCB unit in the two-unit setting, however, are poorly understood [110–112]. With the rapid emergence in the use of UCB as an alternative allogeneic stem cell source in the unrelated setting since 1993, clinical reports to date have been primarily retrospective, and multi-institutional Phase II prospective studies are at early stages within National Heart, Lung and Blood Institute Blood and Marrow Transplant Clinical Trials Network multi-institutional collaborating sites. Therefore, innovative pilot Phase I clinical trials (as summarized earlier) developed from hypothesis-driven laboratory and translational studies are needed to overcome the current obstacles to UCB allogeneic engraftment, ultimately to move the field forward for the benefit of hematology patients.
Expert commentary
Umbilical cord blood transplantation, particularly for adult patients, has been limited by the small number of HSCs in each graft, resulting in delayed myeloid and lymphoid engraftment, contributing to infectious complications. Current strategies to overcome this UCB graft cell dose limitation include: ex vivo expansion, infusion of two UCB units, co-transplantation with hematopoietic or mesenchymal cells, and HSC priming to enhance homing and engraftment. These strategies can be applied individually or in combination to overcome delayed UCB engraftment in adult hematology patients.
Five-year view
Current and future strategies to overcome this UCB graft cell dose limitation include: ex vivo expansion, infusion of two UCB units, co-transplantation with hematopoietic or mesenchymal stem cells, and HSC priming to enhance homing and engraftment have shown efficacy in single institution early Phase I/II clinical studies. Initial efforts to expand UCB progenitors ex vivo have resulted in expansion of differentiated HSCs and no significant improvement in rates and kinetics of engraftment. The future of ex vivo expansion includes new strategies to preserve immature hematopoietic progenitor function employing both cytokines and stroma to maintain and expand the stem cell niche. Further novel ex vivo expansion strategies include manipulating newly discovered signaling pathways, such as Notch, to promote HSC expansion with less differentiation. UCB-derived CD34+ HSCs have been shown to express significantly lower levels of CD49e, CD49f and CXCR-4 than adult-derived mobilized peripheral blood and BM. Complement proteins, including C3a treatment, enhances ex vivo transmigration of CD34+ HSCs from UCB and BM by inducing expression of CXCR-4 and MMP-2/MMP-9. Short-term treatment priming of UCB-derived CD34+ HSCs to upregulate levels of the homing-related molecules with their noted increased ex vivo transmigratory and in vivo homing potential may overcome the delayed reconstitution after UCB.
Improved methods for ex vivo expansion, two-unit infusion, co-infusion with additional HSCs or mesenchymal stem cells, and HSC priming to enhance homing, either alone or in combination strategies, will make UCB available to more patients, decrease engraftment times and allow more rapid immune reconstitution post-transplant.
Key issues.
Umbilical cord blood (UCB) is now recognized as an alternative donor source of hematopoietic stem cells (HSCs) for allogeneic transplantation.
Compared with conventional stem cell sources (bone marrow and peripheral blood stem cell grafts), the use of cord blood stem cell grafts are associated with significantly delayed platelet and neutrophil engraftment.
Ex vivo expansion of UCB stem/progenitor cells and enhancing UCB homing are two potential strategies to improve the kinetics and incidence of engraftment in cord blood transplantation.
The CXCR4–SDF-1 axis plays a pivotal role in the homing/retention and engraftment of HSCs in the marrow microenvironment.
With the goal of enhancing homing of UCB HSCs, it is possible to manipulate the sensitivity/responsiveness of the CXCR4–SDF-1 axis with small molecules, such as C3a, hyaluronic acid, fibronectin and fibrinogen.
Pre-treatment (ex vivo priming) of a cord blood graft just prior to infusion with a small-molecule modulator of the CXCR4–SDF-1 axis (C3a) may result in improved homing of UCB HSCs and improved kinetics and incidence of engraftment.
Using the double unit cord blood transplant platform, ex vivo expansion of UCB HSCs is currently under investigation, with multiple clinical trials ongoing.
The preliminary findings in a few of these trials, namely approaches manipulating the Notch signaling pathway to enhance self-renewal of an early progenitor cell and co-culture of cord blood mononuclear cells and third-party donor MSCs, have demonstrated a significant decrease in the time to neutrophil engraftment.
Larger clinical trials utilizing the methods described in this review are necessary to better understand the efficacy of these approaches.
In addition, insights as to the mechanisms underlying the emergence of single donor dominance in the double cord blood approach is required to better understand the obstacles to improving engraftment in cord blood transplantation.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Mary Laughlin has received funding from the NIH (5RC1HL099447-02). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Bordeaux-Rego P, Luzo AC, Costa FF, Saad ST, Crosara Alberto DP. Both IL-3 and IL-6 are necessary for better ex vivo expansion of CD133+ cells from umbilical cord blood. Stem Cells Dev. 2010;19(3):413–422. doi: 10.1089/scd.2009.0098. [DOI] [PubMed] [Google Scholar]
- 2.Kadereit S, Deeds LS, Haynesworth SE, et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34+/CD38− early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20(6):573–582. doi: 10.1634/stemcells.20-6-573. [DOI] [PubMed] [Google Scholar]
- 3.Schuller CE, Jankowski K, Mackenzie KL. Telomere length of cord blood-derived CD34+ progenitors predicts erythroid proliferative potential. Leukemia. 2007;21(5):983–991. doi: 10.1038/sj.leu.2404631. [DOI] [PubMed] [Google Scholar]
- 4.Gammaitoni L, Weisel KC, Gunetti M, et al. Elevated telomerase activity and minimal telomere loss in cord blood long-term cultures with extensive stem cell replication. Blood. 2004;103(12):4440–4448. doi: 10.1182/blood-2003-09-3079. [DOI] [PubMed] [Google Scholar]
- 5.Barker JN. Who should get cord blood transplants? Biol Blood Marrow Transplant. 2007;13(Suppl 1):78–82. [Google Scholar]
- 6.Brunstein CG, Wagner JE. Umbilical cord blood transplantation and banking. Annu Rev Med. 2006;57:403–417. doi: 10.1146/annurev.med.57.051804.123642. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman E. Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp Hematol. 2000;28(11):1197–1205. doi: 10.1016/s0301-472x(00)00540-3. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337(6):373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335(3):157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 10.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344(24):1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 11.Rocha V, Gluckman E. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1 Suppl 1):34–41. doi: 10.1016/j.bbmt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 13.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88(3):795–802. [PubMed] [Google Scholar]
- 14.Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR−/− hematopoietic stem progenitor cells shows a novel role of the C3a–C3aR axis in bone marrow homing. Leukemia. 2009;23(8):1455–1461. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reca R, Wysoczynski M, Yan J, Lambris JD, Ratajczak MZ. The role of third complement component (C3) in homing of hematopoietic stem/progenitor cells into bone marrow. Adv Exp Med Biol. 2006;586:35–51. doi: 10.1007/0-387-34134-X_3. [DOI] [PubMed] [Google Scholar]
- 16.Wysoczynski M, Reca R, Ratajczak J, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105(1):40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 17.Reca R, Mastellos D, Majka M, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101(10):3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 18.Wysoczynski M, Kucia M, Ratajczak J, Ratajczak MZ. Cleavage fragments of the third complement component (C3) enhance stromal derived factor-1 (SDF-1)-mediated platelet production during reactive postbleeding thrombocytosis. Leukemia. 2007;21(5):973–982. doi: 10.1038/sj.leu.2404629. [DOI] [PubMed] [Google Scholar]
- 19.Acharya M, Edkins AL, Ozanne BW, Cushley W. SDF-1 and PDGF enhance αvβ5-mediated ERK activation and adhesion-independent growth of human pre-B cell lines. Leukemia. 2009;23(10):1807–1817. doi: 10.1038/leu.2009.126. [DOI] [PubMed] [Google Scholar]
- 20.Schaumann DH, Tuischer J, Ebell W, Manz RA, Lauster R. VCAM-1-positive stromal cells from human bone marrow producing cytokines for B lineage progenitors and for plasma cells: SDF-1, flt3L, and BAFF. Mol Immunol. 2007;44(7):1606–1612. doi: 10.1016/j.molimm.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1–CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 22.Majka M, Ratajczak MZ. Biological role of the CXCR4-SDF-1 axis in normal human hematopoietic cells. Methods Mol Biol. 2006;332:103–114. doi: 10.1385/1-59745-048-0:101. [DOI] [PubMed] [Google Scholar]
- 23.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1–CXCR4 axis by the third complement component (C3) – implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34(8):986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34(8):967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23(9):1324–1332. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 26.Dar A, Goichberg P, Shinder V, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6(10):1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- 27.Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38(4):497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 28.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104(12):3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 29.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki O, Nishimura G, Okamoto R, et al. Induction of systemic bone changes by preconditioning total body irradiation for bone marrow transplantation. Pediatr Radiol. 2009;39(1):23–29. doi: 10.1007/s00247-008-1033-4. [DOI] [PubMed] [Google Scholar]
- 31.Kijowski J, Baj-Krzyworzeka M, Majka M, et al. The SDF-1–CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19(5):453–466. doi: 10.1634/stemcells.19-5-453. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T, Shen M, Fujikura D, et al. Functional role of death-associated protein 3 (DAP3) in anoikis. J Biol Chem. 2004;279(43):44667–44672. doi: 10.1074/jbc.M408101200. [DOI] [PubMed] [Google Scholar]
- 33.Broxmeyer HE, Kohli L, Kim CH, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and Gαi proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73(5):630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh MC, Baatar D, Collins G, et al. Dexamethasone augments CXCR4-mediated signaling in resting human T cells via the activation of the Src kinase Lck. Blood. 2009;113(3):575–584. doi: 10.1182/blood-2008-04-151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrussi L, Baldari CT. Intracellular mediators of CXCR4-dependent signaling in T cells. Immunol Lett. 2008;115(2):75–82. doi: 10.1016/j.imlet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Poggi A, Zancolli M, Catellani S, Borsellino G, Battistini L, Zocchi MR. Migratory pathways of γδ T cells and response to CXCR3 and CXCR4 ligands: adhesion molecules involved and implications for multiple sclerosis pathogenesis. Ann NY Acad Sci. 2007;1107:68–78. doi: 10.1196/annals.1381.008. [DOI] [PubMed] [Google Scholar]
- 37.Prasad A, Qamri Z, Wu J, Ganju RK. Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol. 2007;82(3):465–476. doi: 10.1189/jlb.1106678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janowska-Wieczorek A, Majka M, Kijowski J, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98(10):3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 39.Ratajczak MZ, Reca R, Wysoczynski M, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18(9):1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 40.Christopherson KW, II, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 41.Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 42.Kimura T, Boehmler AM, Seitz G, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103(12):4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 43.Nakahata T, Gross AJ, Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J Cell Physiol. 1982;113(3):455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- 44.Socolovsky M, Lodish HF, Daley GQ. Control of hematopoietic differentiation: lack of specificity in signaling by cytokine receptors. Proc Natl Acad Sci USA. 1998;95(12):6573–6575. doi: 10.1073/pnas.95.12.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116(5):1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 47.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7(4):333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 48.Antonchuk J, Sauvageau G, Humphries RK. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp Hematol. 2001;29(9):1125–1134. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- 49.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109(1):39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 50.Sauvageau G, Thorsteinsdottir U, Eaves CJ, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9(14):1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- 51.Thorsteinsdottir U, Sauvageau G, Humphries RK. Enhanced in vivo regenerative potential of HOXB4-transduced hematopoietic stem cells with regulation of their pool size. Blood. 1999;94(8):2605–2612. [PubMed] [Google Scholar]
- 52.Bhatia M, Bonnet D, Wu D, et al. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189(7):1139–1148. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zon LI. Self-renewal versus differentiation, a job for the mighty morphogens. Nat Immunol. 2001;2(2):142–143. doi: 10.1038/84239. [DOI] [PubMed] [Google Scholar]
- 54.Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2(2):172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 55.Yamane T, Kunisada T, Tsukamoto H, et al. Wnt signaling regulates hemopoiesis through stromal cells. J Immunol. 2001;167:765–772. doi: 10.4049/jimmunol.167.2.765. [DOI] [PubMed] [Google Scholar]
- 56.Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood. 1997;89(10):3624–3635. [PubMed] [Google Scholar]
- 57.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 58.Tanavde VM, Malehorn MT, Lumkul R, et al. Human stem-progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp Hematol. 2002;30(7):816–823. doi: 10.1016/s0301-472x(02)00818-4. [DOI] [PubMed] [Google Scholar]
- 59.Kojika S, Griffin JD. Notch receptors and hematopoiesis. Exp Hematol. 2001;29(9):1041–1052. doi: 10.1016/s0301-472x(01)00676-2. [DOI] [PubMed] [Google Scholar]
- 60.Milner LA, Kopan R, Martin DI, Bernstein ID. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood. 1994;83(8):2057–2062. [PubMed] [Google Scholar]
- 61.Ohishi K, Varnum-Finney B, Flowers D, Anasetti C, Myerson D, Bernstein ID. Monocytes express high amounts of Notch and undergo cytokine specific apoptosis following interaction with the Notch ligand, Delta-1. Blood. 2000;95(9):2847–2854. [PubMed] [Google Scholar]
- 62.Jones P, May G, Healy L, et al. Stromal expression of Jagged 1 promotes colony formation by fetal hematopoietic progenitor cells. Blood. 1998;92(5):1505–1511. [PubMed] [Google Scholar]
- 63.Karanu FN, Murdoch B, Gallacher L, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192(9):1365–1372. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karanu FN, Murdoch B, Miyabayashi T, et al. Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood. 2001;97(7):1960–1967. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]
- 65.Li L, Milner LA, Deng Y, et al. The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity. 1998;8(1):43–55. doi: 10.1016/s1074-7613(00)80457-4. [DOI] [PubMed] [Google Scholar]
- 66.Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6(11):1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 67.Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99(7):2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 68.Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93(3):838–848. [PubMed] [Google Scholar]
- 69.Vas V, Szilagyi L, Paloczi K, Uher F. Soluble Jagged-1 is able to inhibit the function of its multivalent form to induce hematopoietic stem cell self-renewal in a surrogate in vitro assay. J Leukoc Biol. 2004;75(4):714–720. doi: 10.1189/jlb.1003462. [DOI] [PubMed] [Google Scholar]
- 70.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101(5):1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 71.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106(8):2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34+CD38− cord blood cells. J Clin Invest. 2002;110(8):1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med. 2005;201(9):1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kertesz Z, Vas V, Kiss J, et al. In vitro expansion of long-term repopulating hematopoietic stem cells in the presence of immobilized Jagged-1 and early acting cytokines. Cell Biol Int. 2006;30(5):401–405. doi: 10.1016/j.cellbi.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Holyoake TL, Nicolini FE, Eaves CJ. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp Hematol. 1999;27(9):1418–1427. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 76.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89(11):3919–3924. [PubMed] [Google Scholar]
- 77.Broxmeyer HE, Hangoc G, Cooper S, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci USA. 1992;89(9):4109–4113. doi: 10.1073/pnas.89.9.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu L, Xiao M, Shen RN, Grigsby S, Broxmeyer HE. Enrichment, characterization, and responsiveness of single primitive CD34 human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood. 1993;81(1):41–48. [PubMed] [Google Scholar]
- 79.Mayani H, Dragowska W, Lansdorp PM. Characterization of functionally distinct subpopulations of CD34+ cord blood cells in serum-free long-term cultures supplemented with hematopoietic cytokines. Blood. 1993;82(9):2664–2672. [PubMed] [Google Scholar]
- 80.Piacibello W, Sanavio F, Severino A, et al. Engraftment in nonobese diabetic severe combined immunodeficient mice of human CD34+ cord blood cells after ex vivo expansion: evidence for the amplification and self-renewal of repopulating stem cells. Blood. 1999;93(11):3736–3749. [PubMed] [Google Scholar]
- 81.Hao QL, Thiemann FT, Petersen D, Smogorzewska EM, Crooks GM. Extended long-term culture reveals a highly quiescent and primitive human hematopoietic progenitor population. Blood. 1996;88(9):3306–3313. [PubMed] [Google Scholar]
- 82.Hows JM, Bradley BA, Marsh JC, et al. Growth of human umbilical-cord blood in longterm haemopoietic cultures. Lancet. 1992;340(8811):73–76. doi: 10.1016/0140-6736(92)90396-k. [DOI] [PubMed] [Google Scholar]
- 83.Mayani H, Lansdorp PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood. 1994;83(9):2410–2417. [PubMed] [Google Scholar]
- 84.Steen R, Tjonnfjord GE, Egeland T. Comparison of the phenotype and clonogenicity of normal CD34+ cells from umbilical cord blood, granulocyte colony-stimulating factor-mobilized peripheral blood, and adult human bone marrow. J Hematother. 1994;3(4):253–262. doi: 10.1089/scd.1.1994.3.253. [DOI] [PubMed] [Google Scholar]
- 85.van de Ven C, Ishizawa L, Law P, Cairo MS. IL-11 in combination with SLF and G-CSF or GM-CSF significantly increases expansion of isolated CD34+ cell population from cord blood vs. adult bone marrow. Exp Hematol. 1995;23(12):1289–1295. [PubMed] [Google Scholar]
- 86.Piacibello W, Sanavio F, Garetto L, et al. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89(8):2644–2653. [PubMed] [Google Scholar]
- 87.Vormoor J, Lapidot T, Pflumio F, et al. Immature human cord blood progenitors engraft and proliferate to high levels in severe combined immunodeficient mice. Blood. 1994;83(9):2489–2497. [PubMed] [Google Scholar]
- 88.Rosler ES, Brandt JE, Chute J, Hoffman R. An in vivo competitive repopulation assay for various sources of human hematopoietic stem cells. Blood. 2000;96(10):3414–3421. [PubMed] [Google Scholar]
- 89.Rebel VI, Miller CL, Eaves CJ, Lansdorp PM. The repopulation potential of fetal liver hematopoietic stem cells in mice exceeds that of their liver adult bone marrow counterparts. Blood. 1996;87(8):3500–3507. [PubMed] [Google Scholar]
- 90.Szilvassy SJ, Meyerrose TE, Ragland PL, Grimes B. Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood. 2001;98(7):2108–2115. doi: 10.1182/blood.v98.7.2108. [DOI] [PubMed] [Google Scholar]
- 91.Jaroscak J, Goltry K, Smith A, Waters-Pick B, Martin PL, Driscoll TA, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a Phase 1 trial using the AastromReplicell System. Blood. 2003;101(12):5061–5067. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 92.Kogler G, Nurnberger W, Fischer J, et al. Simultaneous cord blood transplantation of ex vivo expanded together with non-expanded cells for high risk leukemia. Bone Marrow Transplant. 1999;24(4):397–403. doi: 10.1038/sj.bmt.1701916. [DOI] [PubMed] [Google Scholar]
- 93.Pecora AL, Stiff P, Jennis A, et al. Prompt and durable engraftment in two older adult patients with high risk chronic myelogenous leukemia (CML) using ex vivo expanded and unmanipulated unrelated umbilical cord blood. Bone Marrow Transplant. 2000;25(7):797–799. doi: 10.1038/sj.bmt.1702222. [DOI] [PubMed] [Google Scholar]
- 94.Shpall E, Quinines R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Bone Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 95.Stiff PA, Parthasarathy M, Preti R, Chen B. Umbilical cord blood transplants in adults using a combination of unexpanded and ex vivo expanded cells: preliminary clinical observations. Blood. 1998;92(Suppl 1) (Abstract 646) [Google Scholar]
- 96.Peled T, Landau E, Mandel J, et al. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increases their engraftment potential in NOD/SCID mice. Exp Hematol. 2004;32(6):547–555. doi: 10.1016/j.exphem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 97.de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a Phase I/II clinical trial. Bone Marrow Transplant. 2008;41(9):771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McKenzie JL, Gan OI, Doedens M, Dick JE. Reversible cell surface expression of CD38 on CD34-positive human hematopoietic repopulating cells. Exp Hematol. 2007;35(9):1429–1436. doi: 10.1016/j.exphem.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 99.Haspel RL, Kao G, Yeap BY, et al. Preinfusion variables predict the predominant unit in the setting of reduced-intensity double cord blood transplantation. Bone Marrow Transplant. 2008;41(6):523–529. doi: 10.1038/sj.bmt.1705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haspel RL, Ballen KK. Double cord blood transplants: filling a niche? Stem Cell Rev. 2006;2(2):81–86. doi: 10.1007/s12015-006-0013-z. [DOI] [PubMed] [Google Scholar]
- 101.Hamza NS, Lisgaris M, Yadavalli G, et al. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br J Haematol. 2004;124(4):488–498. doi: 10.1046/j.1365-2141.2003.04792.x. [DOI] [PubMed] [Google Scholar]
- 102.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 103.van Heeckeren WJ, Fanning LR, Meyerson HJ, et al. Influence of human leucocyte antigen disparity and graft lymphocytes on allogeneic engraftment and survival after umbilical cord blood transplant in adults. Br J Haematol. 2007;139(3):464–474. doi: 10.1111/j.1365-2141.2007.06824.x. [DOI] [PubMed] [Google Scholar]
- 104.Hexner EO, Danet-Desnoyers GA, Zhang Y, et al. Umbilical cord blood xenografts in immunodeficient mice reveal that T cells enhance hematopoietic engraftment beyond overcoming immune barriers by stimulating stem cell differentiation. Biol Blood Marrow Transplant. 2007;13(10):1135–1144. doi: 10.1016/j.bbmt.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 105.Christopherson KW, II, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage-human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/β2null immunodeficient mice. Stem Cells Dev. 2007;16(3):355–360. doi: 10.1089/scd.2007.9996. [DOI] [PubMed] [Google Scholar]
- 106.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16(3):347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 107.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 109.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Graves SS, Hogan W, Kuhr CS, et al. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110(1):418–423. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.George TJ, Sugrue MW, George SN, Wingard JR. Factors associated with parameters of engraftment potential of umbilical cord blood. Transfusion. 2006;46(10):1803–1812. doi: 10.1111/j.1537-2995.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- 112.Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Enhanced engraftment of umbilical cord blood-derived stem cells in NOD/SCID mice by cotransplantation of a second unrelated cord blood unit. Exp Hematol. 2005;33(10):1249–1256. doi: 10.1016/j.exphem.2005.06.019. [DOI] [PubMed] [Google Scholar]
Website
- 201.National Marrow Donor Program. www.nmdpresearch.org.