Abstract
Alterations in serotonin (5-hydroxytriptamine, 5-HT), norepinephrine, and γ-aminobutyric acid have been linked to the pathophysiology of anxiety and depression, and medications that modulate these neurotransmitters are widely used to treat mood disorders. Recently, the neuropeptide substance P (SP) and its receptor, the neurokinin 1 receptor (NK1R), have been proposed as possible targets for new antidepressant and anxiolytic therapies. However, animal and human studies have so far failed to provide a clear consensus on the role of SP in the modulation of emotional states. Here we show that both genetic disruption and acute pharmacological blockade of the NK1R in mice result in a marked reduction of anxiety and stress-related responses. These behavioral changes are paralleled by an increase in the firing rate of 5-HT neurons in the dorsal raphe nucleus, a major source of serotonergic input to the forebrain. NK1R disruption also results in a selective desensitization of 5-HT1A inhibitory autoreceptors, which resembles the effect of sustained antidepressant treatment. Together these results indicate that the SP system powerfully modulates anxiety and suggest that this effect is at least in part mediated by changes in the 5-HT system.
Substance P (SP) and its receptor, the neurokinin 1 receptor (NK1R), participate in the neural processing of a range of noxious and stressful stimuli. In the spinal cord, SP contributes to nociception, and disruption of the NK1R decreases or ablates the late-phase response to peripheral injury (1–3). In the brainstem, SP modulates emesis, which can be decreased in animals and humans by NK1R antagonists (4). Peripheral inflammation in a variety of structures including gut, joints, and cutaneous tissue also partly depends on SP release and NK1R activation (5).
Recently, SP also has been implicated in the modulation of stress responses, mood, and anxiety, but its exact role remains unclear. Localized administration of SP in the central nervous system may produce anxiogenic or anxiolytic responses, depending on the animal species and location of injection (6–8). Conflicting results also have been obtained from the use of NK1R antagonists, and issues of drug access and species specificity have further clouded the roles of SP in stress-related behaviors (9). Interestingly, in humans, an NK1R antagonist has been reported to be an effective antidepressant and anxiolytic, although this result has not been replicated (10). It has been proposed that the antidepressant activity of NK1R antagonism may be independent of serotonergic and noradrenergic pathways and thus may represent an alternative therapeutic strategy for the treatment of mood disorders (10).
In the present study we used genetic disruption and pharmacological blockade of the NK1R to examine the roles of SP in the generation of anxiety-related behaviors in mice. Genetic disruption of NK1R function markedly reduced anxiety-related behaviors in the elevated plus maze (EPM), novelty suppressed feeding (NSF), and maternal separation paradigms. Pharmacological antagonism of the NK1R also decreased anxiety-related behaviors in wild-type mice, but has no effect on mice lacking the NK1R.
To determine whether the anxiolytic effect of NK1R disruption was independent of monoamine transmitter systems, we evaluated the function of serotonergic neurons in the dorsal raphe (DR) nucleus in wild-type and knockout animals. Mice lacking the NK1R displayed a 2-fold increase in the spontaneous firing rate of DR neurons, an effect that also was induced by systemic administration of an NK1 antagonist. This increase in firing rate was accompanied by desensitization of the autoregulatory presynaptic 5-hydroxytriptamine (5-HT) 1A receptor. Similar alterations in DR and 5-HT1A receptor functions also occur during chronic antidepressant treatment, and we hypothesize that these changes may contribute to the therapeutic activities of NK1R antagonists.
Materials and Methods
Gene Targeting and Verification of NK1R Deletion.
A cassette containing PGK-neo and the tetracycline trans-activator (11) was cloned into a 9-kb fragment derived from a 129/Sv genomic library. The PGK-neo cassette replaced the first exon from the ATG to a unique StuI site, resulting in a deletion of the first two transmembrane domains of the NK1R. Electroporated embryonic stem (ES) cells from 129/SvEv mice were selected with G418; ES cell DNA was digested with EcoRV and KpnI and screened for homologous recombination by using a 600-bp BstXI–NheI probe external to the 5′ homologous arm. Targeted ES cells were injected into blastocysts to produce chimeras, which were bred with 129/SvEv females to produce mutants on a pure 129/SvEv background. Deletion of the NK1R was confirmed by in situ hybridization, immunocytochemistry, and measurement of calcium transients in dorsal horn neurons exposed to SP (12).
Drugs.
RP67580 and RP68651 (13) were dissolved in 0.1% HCl diluted in saline (0.9% NaCl); 8-hydroxy-2-(di-n-ptopylamino) tetralin (8-OH-DPAT) was dissolved in saline; and diazepam was dissolved in a 1:1 solution of saline and propylene glycol. Control animals were injected with the appropriate vehicle. All drugs were administered s.c. 30 min before placing the animals in the testing apparatus, except for the 8-OH-DPAT-induced hypothermia test, where 8-OH-DPAT was given 40 min after the first temperature measurement.
Animals.
Knockout, heterozygote, and wild-type 129/SvEv age-matched adult male mice (12–20 weeks) derived from heterozygote crossings (14) were used in all experiments, with the exception of the ultrasonic vocalizations (USV), which used 8-day-old pups of both sexes. Mice were housed 4–5 per cage in a 12-h (06:00–18:00) light/dark colony room at 22°C with freely available food and water.
Behavioral Studies.
Mice were genotyped after behavioral testing to remove the possibility of investigator bias. Mice were always tested during the light period. A total of 12–15 male animals per genotype were used in all tests. The EPM and the NSF were carried out during a 5-min period as described (15, 16) except for the testing apparatus of NSF that consisted of a plastic box, 50 × 50 × 20 cm. For the USV test 8-day-old litters were separated from the mother and maintained in an incubator at 34°C for a 30-min period. Two-minute behavioral sampling was carried out as described (17). The hypothermic effect of 8-OH DPAT was measured on animals singly housed for 24 h. The rectal temperature was recorded every 10 min with a Physitemp Bat 12 digital thermometer (Yellow Spring Instruments).
Immunohistochemistry and Imaging.
Animals were killed, and 20-μm brain sections were prepared and treated as described (18). Sections were incubated overnight at 4°C in 1:500 rabbit primary antibody raised against amino acids 393–407 of the rat NK1R (Chemicon) in combination with either 1:1,000 mouse monoclonal to tyrosine hydroxylase (Incstar, Stillwater, MN) or 1:6,000 mouse monoclonal to tryptophan hydroxylase [PH8, gift of M. D. Underwood, Columbia University (19)]. The primary antibody was labeled by using 1:1,000 CY3-conjugated donkey anti-rabbit or 1:1,000 FITC-conjugated donkey anti-mouse secondary antibodies (Jackson ImmunoResearch). All images were taken with a Bio-Rad MRC 1000 confocal laser scanning system coupled to a Zeiss Axiovert 100 inverted microscope. For each image acquisition, a Kalman average of four frames was used.
Corticosterone Test.
Animals were singly housed for 24 h and then a first blood sample was collected by making a small incision on the tip of the tail. Twenty four hours later the same animals were subjected to the EPM, and 30 min later tail blood was drawn. Serum corticosterone concentrations were measured with an 125I RIA kit (ICN).
Electrophysiological Experiments.
Experiments were carried out as described (20). Mice were anaesthetized with chloral hydrate (400 mg/kg, i.p., using a 4% solution) and placed in a stereotaxic frame (Kopf mouse adapter, Instruments, Tujunga, CA) with the skull positioned horizontally. Single- and double-barreled micropipettes were filled with, respectively, a 1 M and 1.5 M NaCl solution and used for recording DR 5-HT neurons. The impedance of the central barrel used for unitary or double-barreled recording typically ranged between 5 and 7 MΩ. The single- or double-barreled glass micropipettes were positioned 0.5–1 mm posterior to interaural line, on the midline, and lowered into the DR, usually attained a depth ranging between 2.5 and 3.5 mm from the brain surface (21), as initially confirmed on histological sections. The total number of spontaneously active 5-HT neurons and their average firing rate were assessed for a minimal period of 1 min. The stereotaxic coordinates for the CA3 pyramidal neuron layer initially were identified by using Fast Green deposits and visualized on histological sections. Subsequently these neurons were identified on-line by using these coordinates and by their large amplitude (0.5–1.2 mV), long duration (0.8–1.2 ms), single action potentials alternating with complex spike discharges (22). The multibarreled pipette, used for the microiontophoresis of hippocampus, were lowered at 2–3 mm lateral and 2.5–2.7 mm posterior to bregma into the CA3 region of dorsal hippocampus (21). Three of the side-barrels contained one of the following solutions: 5-HT (50 mM in NaCl, 200 mM, pH 4), 8-OH-DPAT (50 mM in NaCl, 200 mM, pH 4), quisqualate (1.5 mM in NaCl, 400 mM, pH 8). The fourth barrel used for automatic current balancing was filled with a 2 M NaCl solution. A variable ejection of quisqualate current (from −20 to −2 nA) was used to activate them within their physiological firing rate (8–15 Hz) (23). RP67580 (1.5 mg/kg) was administered s.c. after anesthesia. The recording was performed 30 min after the RP67580 injection.
Autoradiography Studies.
The 8-OH-[3H]DPAT and 4-(2′-methoxyphenyl)-1-[2′-(n-2′-pyridinyl)-p-[125I]iodobenzamido]ethylpiperazine (p-[125I]MPPI) binding was performed as described (15) on brain sections from animals naïve to experimental manipulation. Six animals per genotype and six matched sections per brain were analyzed. Quantification was based on 3H and 125I internal standards (Arc, St. Louis) and carried out with the National Institutes of Health IMAGE 1.62 program.
Results and Discussion
A line of 129/SvEv mice lacking the NK1R (−/−) was generated by homologous recombination and found to develop and reproduce normally (see Materials and Methods). Lack of NK1R mRNA, protein, and function was demonstrated by in situ hybridization, immunocytochemistry, and calcium imaging of spinal cord neurons (data not shown; see Materials and Methods).
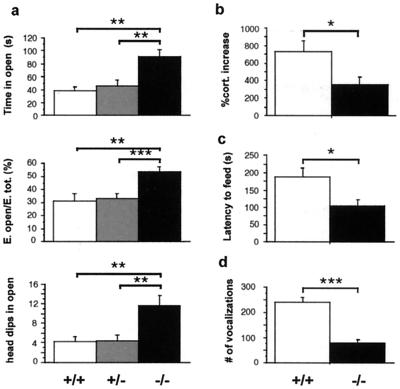
We subjected homozygous mutant (−/−), heterozygote (+/−), and wild-type (+/+) littermates to a series of behavioral tests designed to assess fear and anxiety-related behaviors. When placed in the EPM, mice face a conflict between exploration of a novel environment and fear of heights and open spaces. We have shown previously (17), using a factor analysis, that behavioral measures from the EPM load onto two independent categories: an anxiety-related factor including time in the open arms, entries in the open arms, and number of head dips in the open arms, and an activity-related factor including total number of entries and rearings. −/− mice displayed increased time, percentage of entries, and head dips in the open arms when compared with +/+ animals, indicating a decreased level of anxiety-related behaviors (24) (Fig. 1a). Overall activity levels were not affected by genotype, as indicated by total entries in the EPM and by total locomotion measured in an open field over a period of 1 h (data not shown). The EPM is a stressful task known to activate the hypothalamus-pituitary-adrenal axis (25). To assess whether the hormonal stress response also was altered in −/− mice, we measured serum corticosterone in mutant and +/+ mice in basal conditions and after a 10-min exposure to the EPM. Although basal levels were not significantly different between the two genotypes, the ability of EPM stress to increase corticosterone levels was significantly blunted in the −/− mice (Fig. 1b).
Figure 1.
Decreased anxiety-related behavior in NK1 −/− mice. (a) EPM: time, percentage of entries, and number of head dips in the open arms (mean ± SEM). −/− animals show a significant increase in all three measures compared with +/+ and +/− mice (n = 12–14 mice per genotype). (b) Serum corticosterone was measured 30 min after a 10-min trial in the EPM. Bar graph represents percentage of increase in corticosterone levels from baseline levels measured 1 day before the EPM trial (mean ± SEM, n = 7 mice per genotype). (c) NSF paradigm: latency to feed (mean ± SEM, n = 16 mice per genotype). (d) USV: ultrasonic calls emitted by 8-day-old pups during a 2-min separation from the litter (mean ± SEM, n = 13 mice per genotype). Significant differences between +/+ (white bars), +/− (gray bars), and −/− (black bars) are marked by *, P < 0.05; **, P < 0.01; or ***, P < 0.001 (unpaired t test).
To generalize our findings to other anxiety-related paradigms, we then subjected mice to another conflict test, the NSF test, in which food-deprived animals are presented with a food pellet placed in the center of a brightly lit open field. The mice must leave the corner of the box to approach the pellet and eat, and therefore face a conflict between hunger and fear of the center of the chamber. The latency to begin eating is shortened by anxiolytic and chronic antidepressant drug treatments and is thus an index of anxiety-related behaviors (ref. 16 and ‖ ). Mice lacking the NK1R displayed a significantly reduced latency to feed (Fig. 1c), whereas no difference was observed when animals were presented with food in the familiar environment of the home cage (data not shown). A third test of anxiety, the maternal separation-induced USV paradigm, measures the number of USV emitted by 8-day-old pups separated from their mother (26). −/− mice displayed a reduced number of vocalizations (Fig. 1d), indicating that pups also display a decrease in anxiety-related responses. Taken together, these results demonstrate that genetic disruption of NK1R function decreases anxiety-related responses. In another NK1R knockout line, decreased anxiety was found in the USV paradigm but not in the EPM (27, 28). This discrepancy may result from either experimental or genetic background differences (14).
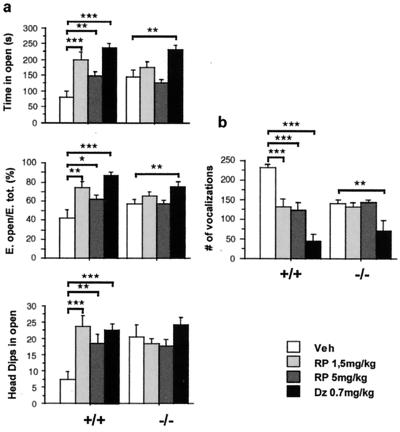
To determine whether a similar behavioral effect could be obtained by acute pharmacological blockade of NK1Rs, the rat and mouse-selective NK1R antagonist RP67580 (29) was used in the EPM paradigm. In this test, RP67580 acted as a potent anxiolytic in +/+ mice, but was ineffective in −/− animals (Fig. 2a). RP68651, an inactive enantiomer of RP67580, had no effect on anxiety-related behavior in wild-type mice (not shown), indicating that the anxiolytic activity of RP67580 reflects NK1R antagonism rather than nonspecific pharmacological effects. Interestingly, RP67580 was as effective as the classic anxiolytic agent diazepam, which was used to validate the EPM as an anxiety-related test (Fig. 2a). Diazepam also was effective in −/− mice, suggesting that the SP system is not required for γ-aminobutyric-mediated anxiolysis. The dose of diazepam was arrived at by determining the minimum dose required to exert an anxiolytic effect in EPM. A complete dose–response study to establish the maximal effect from both compounds would be necessary to better compare the relative efficacy of RP67580 with diazepam.
Figure 2.
Effect of the NK1R antagonist RP67580 on anxiety-related behaviors. (a) EPM: diazepam (DZ) and both doses of RP67580 (RP) significantly decreased anxiety-related measures in +/+ mice, whereas only diazepam was effective in −/− (n = 9–10 mice group). (b) USV: the two doses of RP67580 produced a significant decrease in the number of vocalizations in +/+ mice whereas they had no effect in the −/− mice. Diazepam caused a significant decrease in vocalization in both genotypes. ANOVA revealed a main effect of treatment and an interaction of genotype and treatment in all measures presented. Significant differences between groups were calculated by Fisher post hoc analysis (*, P < 0.05; **, P < 0.01; or ***, P < 0.001).
The anxiolytic activity of RP67580 also was assessed in the USV paradigm; whereas vocalizations were decreased in +/+ mice by RP67580, this drug had no effect in −/− mice (Fig. 2b). In contrast, diazepam had an anxiolytic effect in both genotypes. As in the case of the EPM, the effect of RP67580 on anxiety-related responses was similar to the effect of the genetic disruption of the NK1R. In contrast to what observed in the EPM, the effect of diazepam on +/+ mice was more pronounced than that of RP67580, suggesting a partial dissociation between these two tests. These experiments indicate that NK1Rs modulate anxiety-related behaviors and suggest that the phenotype of the −/− mice is attributable to the absence of the receptor rather than to developmental compensations.
We next assessed whether NK1R antagonism was able to modify the function of the serotonergic system, a neurotransmitter known to modulate mood and anxiety. Thus, we examined the in vivo function of 5-HT neurons in the DR, the major source of 5-HT projections to forebrain structures. We recorded the firing rate of 5-HT neurons identified by their activity pattern in +/+, −/−, and RP67580-pretreated +/+ mice. Their firing rates were significantly increased both in −/− animals and +/+ mice acutely pretreated with RP67580, indicating that 5-HT transmission may be enhanced by both a genetic disruption and an acute blockade of NK1Rs (Fig. 3a). An increase of 5-HT transmission is a common consequence to most antidepressant agents but requires prolonged treatment. In the case of RP67580, an enhancement of the firing rate of 5-HT neurons was observed within 30 min of administering the antagonist. Consistent with our data, Lanfumey and colleagues** recently showed in a different strain of NK1R knockouts that the 5-HT outflow produced in the frontal cortex by paroxetine administration was 4- to 6-fold higher in the mutants than in control wild types. This finding suggests that in NK1 −/− mice the increased basal firing activity of 5-HT neurons in the DR is likely to be accompanied by an enhanced release in the terminal fields of serotonergic projections. Other studies have shown that local administration of SP in the DR leads to an increase of 5-HT neuron activity and that this effect is mediated by glutamatergic inputs (‡‡ and ref. 30). The apparent discrepancy between these results and our present data may be explained by the fact that we are looking at a global effect of NK1 antagonism on the central nervous system rather than local effects on 5-HT neurons in the DR when other inputs have been bypassed.
Figure 3.
Effects of NK1R antagonism on 5-HT function. (a) The spontaneous firing rate (mean ± SEM, Hz) of 5-HT neurons in the DR was increased in the −/− mice and +/+ mice pretreated with RP67580 at a dose of 1.5 mg/kg. The numbers inside the bars indicate the number of cells tested. **, P < 0.01; ***, P < 0.001 (t test). (b) Percentage of inhibition of spontaneous firing of 5-HT neurons in the DR (Left) or quisqualate-induced firing of CA3 neurons in the hippocampus (Right) by microiontophoretic application of 8-OH-DPAT (mean ± SEM). The x axis indicates the intensity (in nAmp) of microiontophoretic currents used to deliver 8-OH-DPAT. 8-OH-DPAT has little effect on the firing of 5-HT neurons in −/− mice but produces a dose-dependent inhibition of firing in +/+ mice (Left). The ANOVA indicates a significant main effect of genotype (P < 0.01). The firing of CA3 neurons is equally inhibited by 8-OH-DPAT in the two genotypes (Right). (c) 8-OH-[3H]DPAT binding (mean ± SEM, pmol/mg of tissue) in DR and hippocampus. *, P < 0.05 (unpaired t test, n = 4–5). (d) Body temperature (mean ± SEM, n = 7). Arrow indicates the time of administration of either vehicle (Veh) or 8-OH-DPAT (DPAT) at the doses indicated. The repeated-measure ANOVA revealed a significant interaction of genotype and treatment (P < 0.01).
The firing rate of DR neurons is modulated by the local release of 5-HT, which activates the inhibitory 5-HT1A autoreceptor. 5-HT1A autoreceptors subsequently can be desensitized after prolonged agonist binding (31). To determine whether alterations in 5-HT1A receptor function could be associated with the observed enhancement of firing rate, we examined the effect of the selective 5-HT1A receptor agonist 8-OH-DPAT on the firing of 5-HT neurons in the DR. In +/+ animals, direct microiontophoretic application of 8-OH-DPAT onto 5-HT neurons produced a dose-dependent inhibition of their firing. This effect was markedly reduced in the −/− animals (Fig. 3b). In addition to its role as a presynaptic autoreceptor, the 5-HT1A receptor is located at postsynaptic sites, particularly in the hippocampus. 8-OH-DPAT applied in the hippocampus exerted an inhibitory effect on the quisqualate-evoked firing activity of CA3 neurons in both +/+ and −/− mice (Fig. 3b). These observations indicate that NK1R disruption results in a marked decrease in the function of presynaptic 5-HT1A autoreceptors whereas the function of postsynaptic hippocampal 5-HT1A receptors is unchanged. In preliminary studies on rats, we have seen that short- and long-term treatment with another NK1R antagonist, CP-96,345, significantly increased the spontaneous firing activity of DR neurons, and that this increase was associated with an attenuation of somatodendritic 5HT1A autoreceptor responsiveness.††
Consistent with these data, radioligand binding studies using the 5-HT1A receptor agonist [3H]-8-OH-DPAT showed a significant decrease of the specific binding sites in the DR, but no difference in hippocampus (Fig. 3c) and hypothalamus (not shown). Interestingly, the binding of 4-(2′-methoxyphenyl)-1-[2′-(n-2′-pyridinyl)-p-[125I]iodobenzamido]ethylpiperazine (p-[125I]MPPI), a 5-HT1A receptor antagonist, was identical in +/+ and −/− mice, suggesting that the decrease in [3H]-8-OH-DPAT binding observed in the DR of −/− mice reflects a decrease in G protein-coupled 5-HT1A receptors rather than a reduction in total receptor numbers.
5-HT1A receptor function was further evaluated in awake animals by assessing the effect of 8-OH-DPAT on body temperature. Systemic administration of 8-OH-DPAT is known to elicit a sudden and profound hypothermia, an effect that in mice is thought to be mainly mediated by presynaptic 5-HT1A receptor activation (32, 33). Although the hypothermic effect of a 0.5 mg/kg dose of 8-OH-DPAT was comparable in both genotypes, the administration of a lower dose (0.16 mg/kg) resulted in a less pronounced hypothermic effect in −/− than in +/+ mice. This finding is consistent with a decrease in presynaptic 5-HT1A receptor function (Fig. 3d).
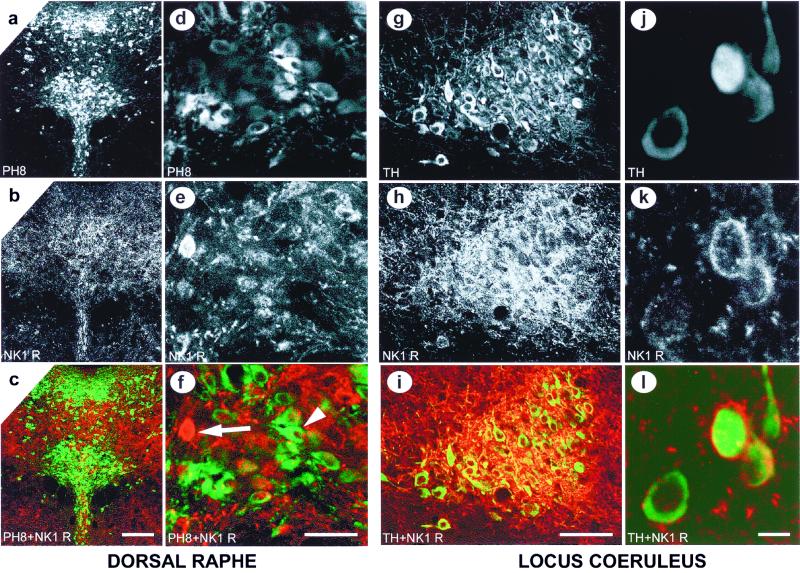
To investigate the possibility of a direct effect of SP transmission on 5-HT neurons, immunocytochemical identification of serotonergic neurons using the PH8 antitryptophan hydroxylase antibody (19) was compared with the location of neurons expressing the NK1R in the DR. We detected an extensive network of NK1R-positive fibers in the raphe area, but we found little overlap between serotonergic neuron and NK1Rs (Fig. 4 a–f). This finding suggests that NK1Rs are probably not located on serotonergic neurons. However, NK1Rs may still affect serotonergic function via non-5-HT neurons in the DR.
Figure 4.
Localization of NK1Rs in the DR and LC. (a–f) Immunofluorescent double labeling of tryptophan hydroxylase (PH8, in green) and NK1R (in red) in the DR. The arrowhead indicates a neuron stained with the PH8 antibody (f), while the arrow shows a NK1R-positive neuron in the same area (f). [Scale bar: 200 μm (a–c) and 50 μm (d–f).] (g–l) Immunofluorescent double labeling of tyrosine hydroxylase (TH, in green) and NK1R (in red) in the LC. [Scale bar: 100 μm (g–i) and 10 μm (j–l).]
Another structure known to modulate the activity of DR neurons is the locus coeruleus (LC), which exerts a stimulatory effect on serotonergic neurons (34). In addition, we recently demonstrated that the function of noradrenergic neurons in the LC can be modulated by NK1R antagonists (35). We therefore performed an immunocytochemical double labeling of tyrosine hydroxylase and NK1Rs in the LC, which revealed high levels of NK1Rs on noradrenergic neurons (Fig. 4 g–l). Thus, NK1R antagonists may exert their anxiolytic effect by modulating the activity of noradrenergic neurons, which in turn modulate serotonergic function. In this regard, we have shown that NK1R antagonism attenuates α-2 adrenoreceptor responsiveness in the LC (39). However, it remains to be investigated whether α-2 desensitization results in an increased LC firing rate in our mice.
The SP and 5-HT systems have long been associated with the modulation of numerous behavioral responses. In the clinical setting, enhancement of 5-HT transmission and more recently antagonism of SP both have been reported to have anxiolytic and antidepressant effects (10). Although it has been proposed that the anxiolytic effects of NK1R antagonism may be independent of the serotonergic system (10), the present results suggest a powerful and unsuspected interaction between these neurotransmitter systems. Although previous studies have provided conflicting effects on anxiety of SP injection in specific brain areas, the present study suggests that a systemwide antagonism of NK1Rs is associated with anxiolysis. We show here that NK1R antagonism results in decreased anxiety, enhanced firing of 5-HT neurons, and attenuation of presynaptic 5-HT1A receptor function. These effects mimic some of the key behavioral and cellular consequences of the long-term administration of antidepressant drugs. Specifically, most long-term antidepressant treatments produce an increase in serotonergic activity. In addition, the administration of selective serotonin reuptake inhibitors (SSRI) has been shown to result in desensitization of 5-HT1A autoreceptors (36, 37). This modification occurs progressively and has been suggested to underlie the slow onset of the therapeutic action of these drugs. Therefore, the inactivation of presynaptic 5-HT1A receptors has been the rationale for the use of 5-HT1A receptor antagonists like pindolol to accelerate the onset of action of SSRIs (38), even though not all studies have replicated this result (39). In our study, however, all of the behavioral, physiological, and biochemical changes produced by the NK1R antagonist RP67580 take place acutely. We hypothesize therefore that SP antagonism may provide an avenue for the development of faster acting antidepressant and anxiolytic therapies.
Acknowledgments
We thank B. Dauer, J. Gingrich, C. Gross, S. Kasir, and S. Schacher for comments and suggestions, M. D. Underwood for the gift of the PH8 antibody, L. Zablow for his help with the confocal microscope, and L. Ravizza and F. Bogetto for their support. This work was funded by grants from the National Institute of Mental Health, National Alliance for Research on Schizophrenia and Depression, and Bristol-Myers Squibb (to R.H.) and the National Institute of Mental Health (to M.J.S.H.).
Abbreviations
- 5-HT
5-hydroxytriptamine
- NK1
neurokinin 1
- NK1R
NK1 receptor
- SP
substance P
- DR
dorsal raphe
- EPM
elevated plus maze
- USV
ultrasonic vocalizations
- NSF
novelty suppressed feeding
- 8-OH-DPAT
8-hydroxy-2-(di-n-ptopylamino) tetralin
- LC
locus coeruleus
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041596398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041596398
Santarelli, L., Gross, C., Schwartz, J. H. & Hen, R. (1999) Soc. Neurosci. Abstr. 25, 32.13.
Lanfumey, L., Froger, N., Lena, I., Andre, J., Hunt S., De Felipe C., Gardier, A. M. & Hamon, M. (2000) Soc. Neurosci. Abstr. 26, 47.7.
Liu, R.-J. & Aghajanian, G. (2000) Soc. Neurosci. Abstr. 26, 720.9.
Haddjeri, N. & Blier, P. (2000) Soc. Neurosci. Abstr. 26, 690.4.
References
- 1.De Felipe C, Herrero J F, O'Brien J A, Palmer J A, Doyle C A, Smith A J, Laird J M, Belmonte C, Cervero F, Hunt S P. Nature (London) 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 2.King T E, Heath M J, Debs P, Davis M B, Hen R, Barr G A. NeuroReport. 2000;11:587–591. doi: 10.1097/00001756-200002280-00031. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y Q, Mantyh P W, Carlson E J, Gillespie A M, Epstein C J, Basbaum A I. Nature (London) 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 4.Saria A. Eur J Pharmacol. 1999;375:51–60. doi: 10.1016/s0014-2999(99)00259-9. [DOI] [PubMed] [Google Scholar]
- 5.Holzer P. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 6.File S E. Pharmacol Biochem Behav. 1997;58:747–752. doi: 10.1016/s0091-3057(97)90002-2. [DOI] [PubMed] [Google Scholar]
- 7.de Araujo J E, Huston J P, Brandao M L. Exp Brain Res. 1998;123:84–89. doi: 10.1007/s002210050547. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira R M, Santos A R, Ribeiro S J, Calixto J B, Rae G A, De Lima T C. Eur J Pharmacol. 1996;311:7–14. doi: 10.1016/0014-2999(96)00390-1. [DOI] [PubMed] [Google Scholar]
- 9.Rupniak N M, Kramer M S. Trends Pharmacol Sci. 1999;20:485–490. doi: 10.1016/s0165-6147(99)01396-6. [DOI] [PubMed] [Google Scholar]
- 10.Kramer M S, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek J J, Reines S A, Liu G, Snavely D, Wyatt-Knowles E, et al. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 11.Stark K L, Oosting R S, Hen R. Biol Psychiatry. 1998;44:163–168. doi: 10.1016/s0006-3223(98)00040-7. [DOI] [PubMed] [Google Scholar]
- 12.Heath M J, Lints T J, Lee C J, Dodd J. J Physiol (London) 1995;486:139–148. doi: 10.1113/jphysiol.1995.sp020798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garret C, Carruette A, Fardin V, Moussaoui S, Peyronel J F, Blanchard J C, Laduron P M. Proc Natl Acad Sci USA. 1991;88:10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips T J, Hen R, Crabbe J C. Psychopharmacology (Berlin) 1999;147:5–7. doi: 10.1007/s002130051128. [DOI] [PubMed] [Google Scholar]
- 15.Ramboz S, Oosting R, Amara D A, Kung H F, Blier P, Mendelsohn M, Mann J J, Brunner D, Hen R. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodnoff S R, Suranyi-Cadotte B, Quirion R, Meaney M J. Psychopharmacology. 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- 17.Brunner D, Buhot M C, Hen R, Hofer M. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- 18.Lucas J J, Segu L, Hen R. Mol Pharmacol. 1997;51:755–763. doi: 10.1124/mol.51.5.755. [DOI] [PubMed] [Google Scholar]
- 19.Underwood M D, Khaibulina A A, Ellis S P, Moran A, Rice P M, Mann J J, Arango V. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 20.Rueter L E, Tecott L H, Blier P. Naunyn-Schmiedeberg's Arch Pharmacol. 2000;35:484–491. doi: 10.1007/s002109900181. [DOI] [PubMed] [Google Scholar]
- 21.Franklin K B, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 22.Kandel E R, Spencer W A. J Neurophysiol. 1961;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- 23.Ranck J B. Behavioral Correlates and Firing Repertoires of Neurons in the Dorsal Hippocampal Formation and Septum of Unrestrained Rats. New York: Plenum; 1975. [DOI] [PubMed] [Google Scholar]
- 24.Lister R G. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers R J, Haller J, Holmes A, Halasz J, Walton T J, Brain P F. Physiol Behav. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 26.Hofer M A. Psychoneuroendocrinology. 1996;21:203–217. doi: 10.1016/0306-4530(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 27.Rupniak N M, Carlson E C, Harrison T, Oates B, Seward E, Owen S, de Felipe C, Hunt S, Wheeldon A. Neuropharmacology. 2000;39:1413–1421. doi: 10.1016/s0028-3908(00)00052-6. [DOI] [PubMed] [Google Scholar]
- 28.Murtra P, Sheasby A M, Hunt S P, De Felipe C. Nature (London) 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- 29.Fong T M, Yu H, Strader C D. J Biol Chem. 1992;267:25668–25671. [PubMed] [Google Scholar]
- 30.Gradin K, Qadri F, Nomikos G G, Hillegaart V, Svensson T H. Eur J Pharmacol. 1992;218:363–367. doi: 10.1016/0014-2999(92)90194-9. [DOI] [PubMed] [Google Scholar]
- 31.Blier P, de Montigny C. Neuroscience. 1985;16:949–955. doi: 10.1016/0306-4522(85)90107-1. [DOI] [PubMed] [Google Scholar]
- 32.Bill D J, Knight M, Forster E A, Fletcher A. Br J Pharmacol. 1991;103:1857–1864. doi: 10.1111/j.1476-5381.1991.tb12342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin G M, De Souza R J, Green A R. Neuropharmacology. 1985;24:1187–1194. doi: 10.1016/0028-3908(85)90153-4. [DOI] [PubMed] [Google Scholar]
- 34.Peyron C, Luppi P H, Fort P, Rampon C, Jouvet M. J Comp Neurol. 1996;364:402–413. doi: 10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Haddjeri N, Blier P. NeuroReport. 2000;11:1323–1327. doi: 10.1097/00001756-200004270-00035. [DOI] [PubMed] [Google Scholar]
- 36.Blier P, de Montigny C, Tardif D. Psychopharmacology. 1984;84:242–249. doi: 10.1007/BF00427453. [DOI] [PubMed] [Google Scholar]
- 37.Blier P, de Montigny C. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 38.Perez V, Gilaberte I, Faries D, Alvarez E, Artigas F. Lancet. 1997;349:1594–1597. doi: 10.1016/S0140-6736(96)08007-5. [DOI] [PubMed] [Google Scholar]
- 39.Berman R M, Anand A, Cappiello A, Miller H L, Hu X S, Oren D A, Charney D S. Biol Psychiatry. 1999;45:1170–1177. doi: 10.1016/s0006-3223(98)00383-7. [DOI] [PubMed] [Google Scholar]