Abstract
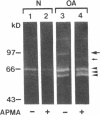
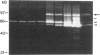
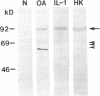
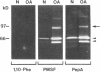
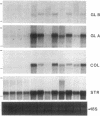
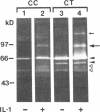
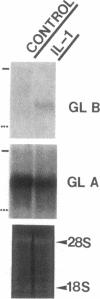
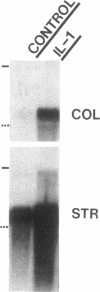
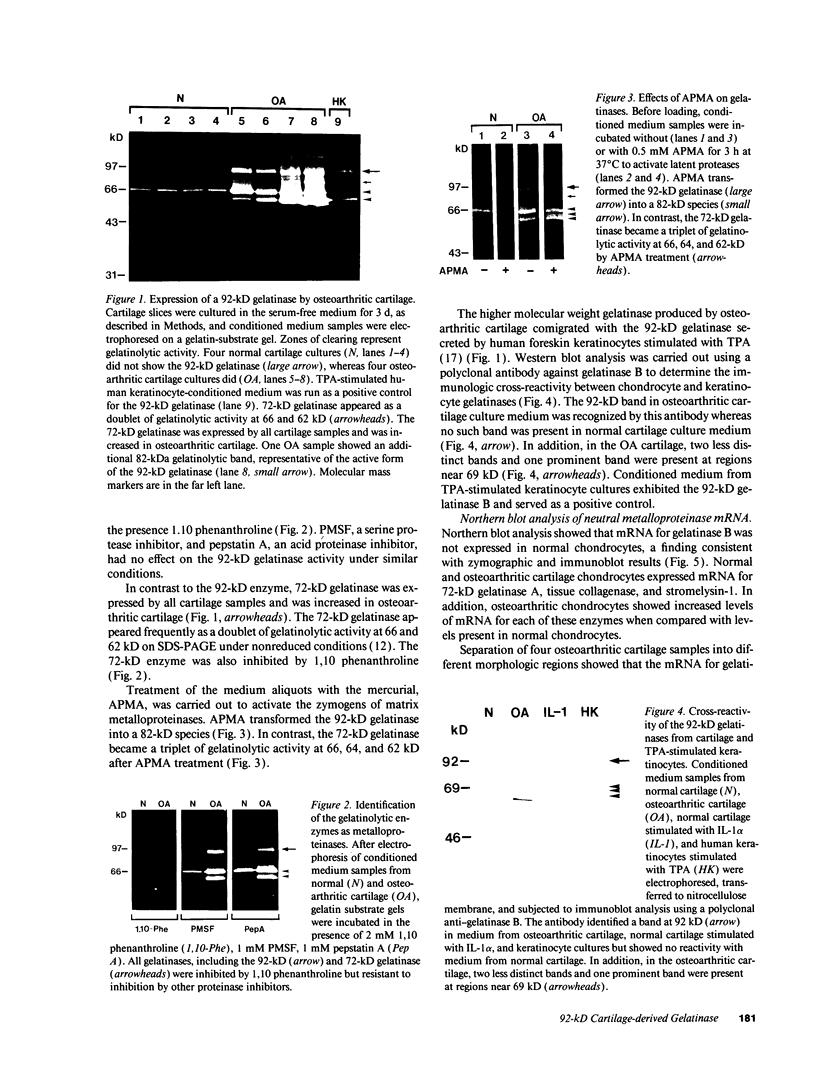
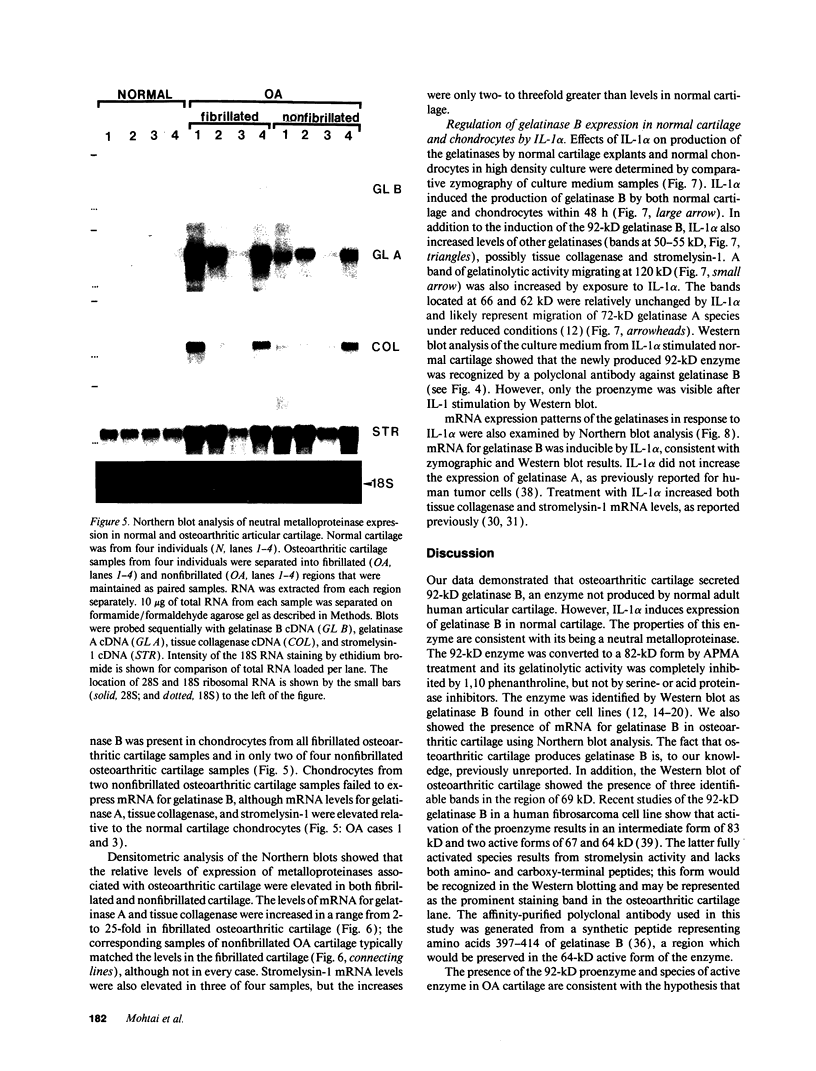
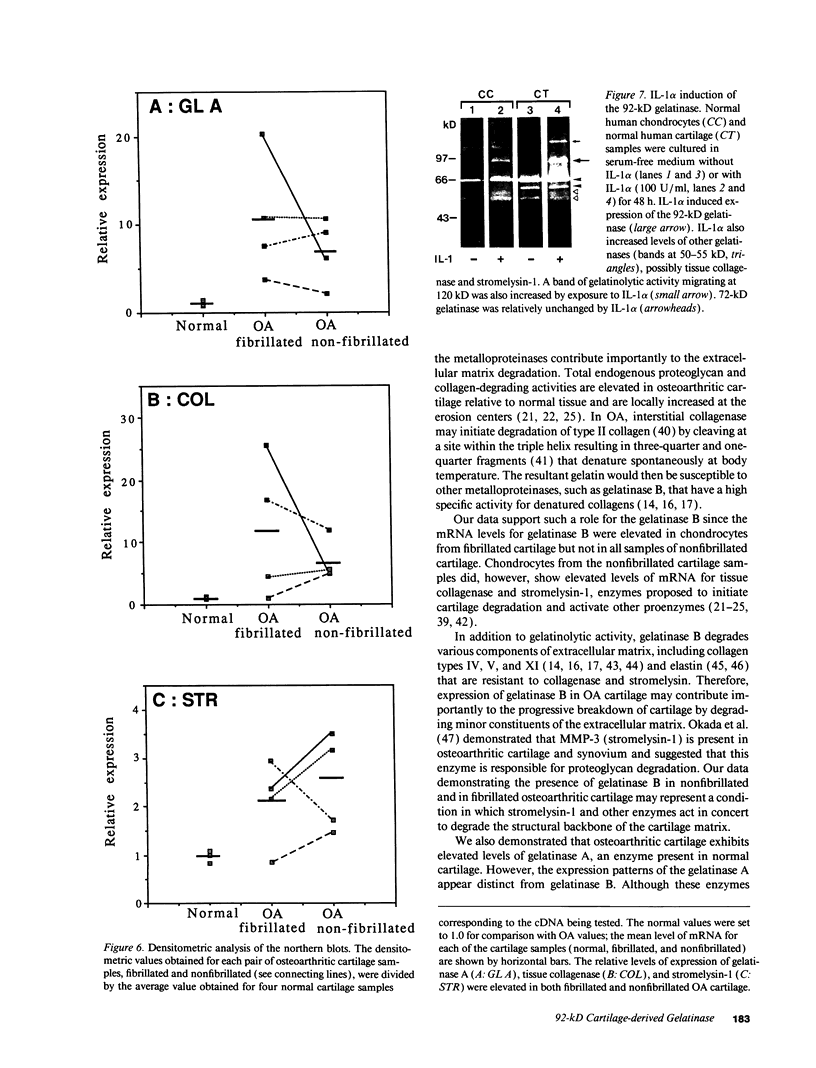
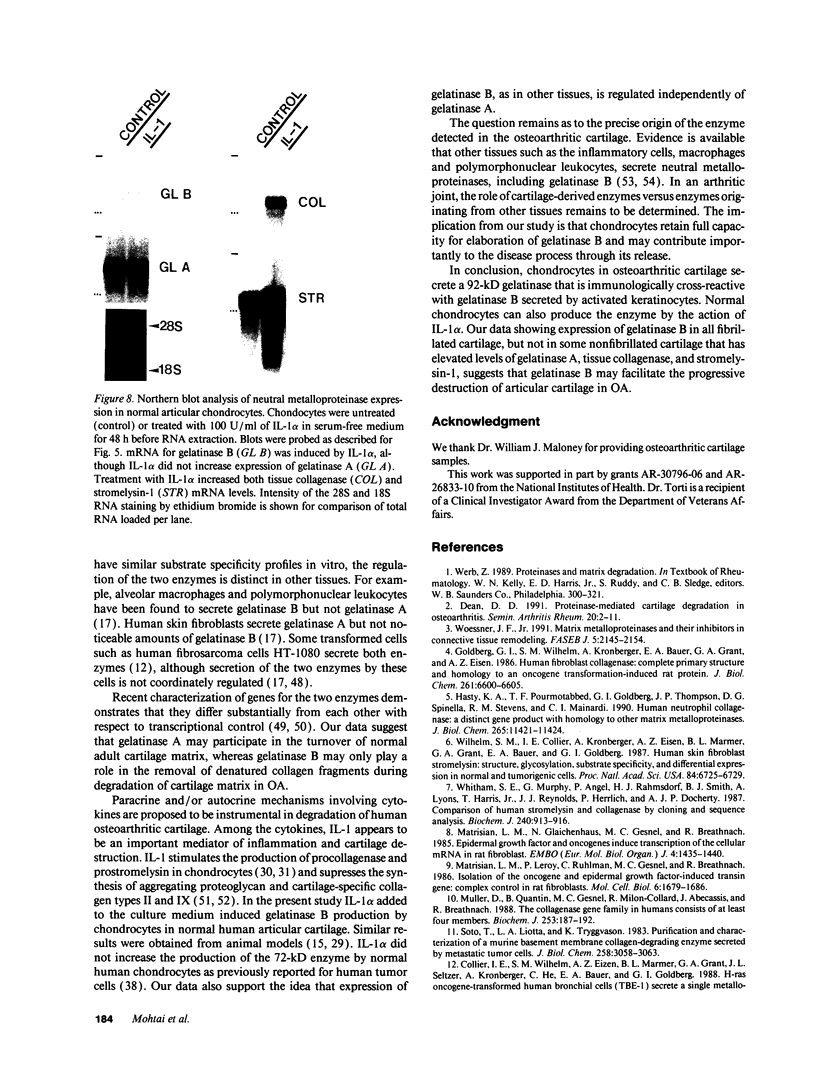
We report here that a 92-kD gelatinolytic metalloproteinase is expressed as protein and mRNA in human osteoarthritic cartilage, but not in normal adult articular cartilage. Western immunoblotting demonstrated that the 92-kD gelatinolytic activity corresponded to 92-kD type IV collagenase/gelatinase (gelatinase B); mRNA for gelatinase B was identified by Northern blotting. Chondrocytes from normal cartilage also exhibited mRNA for 72-kD type IV collagenase/gelatinase (gelatinase A), tissue collagenase, and stromelysin-1, and these mRNAs were increased in osteoarthritic cartilage. Regional analysis of osteoarthritic cartilage samples from four individuals revealed that gelatinase B mRNA was expressed in grossly fibrillated areas; two of four nonfibrillated cartilage samples failed to exhibit the mRNA, but did have increased levels of mRNA for other neutral metalloproteinases. IL-1 alpha treatment of normal human cartilage explants or isolated chondrocytes induced increased levels of gelatinase B and increased mRNA for tissue collagenase and stromelysin-1. Under identical conditions, mRNA levels for gelatinase A were not increased indicating that regulation of this enzyme in human articular chondrocytes is distinct from that of other metalloproteinases. Our data showing expression of gelatinase B in fibrillated cartilage suggest that it is a marker of progressive articular cartilage degradation in osteoarthritis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Corcoran M. L., Stetler-Stevenson W. G., Brown P. D., Wahl L. M. Interleukin 4 inhibition of prostaglandin E2 synthesis blocks interstitial collagenase and 92-kDa type IV collagenase/gelatinase production by human monocytes. J Biol Chem. 1992 Jan 5;267(1):515–519. [PubMed] [Google Scholar]
- Davis G. E., Martin B. M. A latent Mr 94,000 gelatin-degrading metalloprotease induced during differentiation of HL-60 promyelocytic leukemia cells: a member of the collagenase family of enzymes. Cancer Res. 1990 Feb 15;50(4):1113–1120. [PubMed] [Google Scholar]
- Dean D. D. Proteinase-mediated cartilage degradation in osteoarthritis. Semin Arthritis Rheum. 1991 Jun;20(6 Suppl 2):2–11. doi: 10.1016/0049-0172(91)90023-s. [DOI] [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. G., Mankin H. J., Jones H., Wright R., Crispen C., Vigliani G. Collagenase and collagenase inhibitors in osteoarthritic and normal cartilage. J Clin Invest. 1977 Feb;59(2):226–233. doi: 10.1172/JCI108632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K. A., Pourmotabbed T. F., Goldberg G. I., Thompson J. P., Spinella D. G., Stevens R. M., Mainardi C. L. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990 Jul 15;265(20):11421–11424. [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Huhtala P., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Tryggvason K. Completion of the primary structure of the human type IV collagenase preproenzyme and assignment of the gene (CLG4) to the q21 region of chromosome 16. Genomics. 1990 Mar;6(3):554–559. doi: 10.1016/0888-7543(90)90486-e. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Tuuttila A., Chow L. T., Lohi J., Keski-Oja J., Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991 Sep 5;266(25):16485–16490. [PubMed] [Google Scholar]
- Ishibashi M., Ito A., Sakyo K., Mori Y. Procollagenase activator produced by rabbit uterine cervical fibroblasts. Biochem J. 1987 Jan 15;241(2):527–534. doi: 10.1042/bj2410527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. G., Smith R. L. Stimulation of adult chondrocyte metabolism by a thyroid-derived factor. J Orthop Res. 1990 Mar;8(2):227–233. doi: 10.1002/jor.1100080211. [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Peeters-Joris C., Vaes G. Production of gelatin-degrading matrix metalloproteinases ('type IV collagenases') and inhibitors by articular chondrocytes during their dedifferentiation by serial subcultures and under stimulation by interleukin-1 and tumor necrosis factor alpha. Biochim Biophys Acta. 1991 Aug 13;1094(1):8–18. doi: 10.1016/0167-4889(91)90020-x. [DOI] [PubMed] [Google Scholar]
- Lyons J. G., Birkedal-Hansen B., Moore W. G., O'Grady R. L., Birkedal-Hansen H. Characteristics of a 95-kDa matrix metalloproteinase produced by mammary carcinoma cells. Biochemistry. 1991 Feb 12;30(6):1449–1456. doi: 10.1021/bi00220a001. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P., Cloutier J. M., Howell D. S., Ghandur-Mnaymneh L., Woessner J. F., Jr Neutral proteases capable of proteoglycan digesting activity in osteoarthritic and normal human articular cartilage. Arthritis Rheum. 1984 Mar;27(3):305–312. doi: 10.1002/art.1780270310. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll U. M., Youngleib G. L., Rosinski K. B., Quigley J. P. Tumor promoter-stimulated Mr 92,000 gelatinase secreted by normal and malignant human cells: isolation and characterization of the enzyme from HT1080 tumor cells. Cancer Res. 1990 Oct 1;50(19):6162–6170. [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., McGarrity A. M., Reynolds J. J., Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 1989 Mar;92(Pt 3):487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Partial purification of collagenase and gelatinase from human polymorphonuclear leucocytes. Analysis of their actions on soluble and insoluble collagens. Biochem J. 1982 Apr 1;203(1):209–221. doi: 10.1042/bj2030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata Y., Enghild J. J., Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992 Feb 25;267(6):3581–3584. [PubMed] [Google Scholar]
- Okada Y., Gonoji Y., Naka K., Tomita K., Nakanishi I., Iwata K., Yamashita K., Hayakawa T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992 Oct 25;267(30):21712–21719. [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Okada Y., Shinmei M., Tanaka O., Naka K., Kimura A., Nakanishi I., Bayliss M. T., Iwata K., Nagase H. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992 Jun;66(6):680–690. [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Howell D. S., Ghandur-Mnaymneh L., Enis J. E., Woessner J. F., Jr Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983 Jan;26(1):63–68. doi: 10.1002/art.1780260110. [DOI] [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Tryggvason K. Purification and characterization of a murine basement membrane collagen-degrading enzyme secreted by metastatic tumor cells. J Biol Chem. 1983 Mar 10;258(5):3058–3063. [PubMed] [Google Scholar]
- Sapolsky A. I., Keiser H., Howell D. S., Woessner J. F., Jr Metalloproteases of human articular cartilage that digest cartilage proteoglycan at neutral and acid pH. J Clin Invest. 1976 Oct;58(4):1030–1041. doi: 10.1172/JCI108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky A. I., Sheff M. F., Matsuta K., Howell D. S., Moskowitz R. W., Goldberg V. M., Norby D. P., Malemud C. J. 'Gelatinase-like' activity from articular chondrocytes in monolayer culture. Biochim Biophys Acta. 1983 Apr 5;762(2):227–231. doi: 10.1016/0167-4889(83)90075-7. [DOI] [PubMed] [Google Scholar]
- Schnyder J., Payne T., Dinarello C. A. Human monocyte or recombinant interleukin 1's are specific for the secretion of a metalloproteinase from chondrocytes. J Immunol. 1987 Jan 15;138(2):496–503. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Yamagata S., Tanaka R., Ito Y., Shimizu S. Gelatinases of murine metastatic tumor cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):228–234. doi: 10.1016/s0006-291x(89)80202-5. [DOI] [PubMed] [Google Scholar]