Abstract
Objective
To describe the prospective relationship of retinal vessel diameters with risk of hypertension in a multiethnic population-based cohort.
Methods
The Multi-Ethnic Study of Atherosclerosis is a population-based study of subclinical cardiovascular disease among white, African–American, Hispanic, and Chinese American adults aged 45–84 years. Retinal vessel diameters were measured using a standardized imaging software at the second examination (considered baseline in this analysis) and summarized as the central retinal artery/vein equivalent. Presence of retinopathy and retinal focal arteriolar narrowing and arteriovenous nicking was assessed by trained graders. Incidence of hypertension was defined among participants at risk as systolic blood pressure at least 140 mmHg, diastolic blood pressure at least 90 mmHg, or use of an antihypertensive medication.
Results
Of the initial 6237 participants at baseline, 2583 were at risk of hypertension. After 3.2±0.5 years of follow-up, 448 (17.3%) participants developed hypertension. After adjusting for age, sex, race/ethnicity, the average of mean arterial blood pressure in the first and second examination, and other vascular risk factors, persons with narrower retinal arteriolar diameter and wider venular diameter at baseline were more likely to develop hypertension [odds ratio per SD decrease in central retinal artery equivalent 1.20, 95% confidence intervals 1.02, 1.42; and odds ratio per SD increase in central retinal vein equivalent 1.18, 95% confidence interval 1.02, 1.37]. Persons with focal arteriolar narrowing were also more likely to develop hypertension (odds ratio 1.80, 95% confidence interval 1.09, 2.97).
Conclusion
Findings from this multiethnic population confirm that narrower retinal arteriolar diameter and wider venular diameter are associated with the development of hypertension independent of traditional risk factors.
Keywords: hypertension, microcirculation, retinal vessel diameter, retinopathy, the Multi-Ethnic Study of Atherosclerosis
Introduction
Microcirculatory dysfunction may play a major role in the pathogenesis of hypertension [1]. Narrowing of small arteries and arterioles [2–4] is a key pathological characteristic of essential hypertension and is known to increase peripheral vascular resistance [5]. It remains unclear, however, whether arteriolar narrowing precedes and contributes to the development of hypertension or is only a consequence of long-standing elevated blood pressure [5–7].
The retinal microvasculature allows direct visualization of small arteries and arterioles, thus offering a means to evaluate the relationship of structural microvascular changes with the development of hypertension [8–10]. Using computer-based techniques to measure retinal vessel diameters, four prospective studies [11–15] have shown that narrower retinal arterioles may precede clinical hypertension, even after adjusting for the effects of baseline blood pressure levels and other risk factors.
However, little is known about the potential ethnic variations in the association of retinal arteriolar narrowing and hypertension risk. Most of the previous studies were conducted on white populations [12–15], but it is well established that the prevalence and risk factors for hypertension differ substantially among different ethnic/racial populations [16]. Furthermore, there may be ethnic differences in the association between blood pressure and other markers of hypertensive end-organ damage, including hypertensive retinopathy [17] coronary artery disease [18], and left ventricular hypertrophy [19].
The purpose of this study is to determine the prospective association of retinal vessel diameter with the risk of hypertension among four major ethnic groups in the United States. We also examined relationships of retinal vessel diameters with progression, regression, and control of hypertension.
Methods
Study population
The Multi-Ethnic Study of Atherosclerosis is an ongoing prospective study of adults without a history of clinical cardiovascular disease selected from six US communities (Maryland, Illinois, North Carolina, California, New York, and Minnesota) [20]. Selection of the study population has been detailed elsewhere [20]. In brief, between July 2000 and August 2002, each site planned to examine approximately 1100 eligible participants, equally divided between men and women, according to site-specified racial and ethnic proportions. At the first examination (visit 1), there were 6814 participants (52.8% women), aged 45–84 years. The tenets of the Declaration of Helsinki were followed, and institutional review board approval was granted at each study site. Written informed consent was obtained from each participant.
The study population for this analysis was derived as follows. Fundus photography was performed at the second examination (visit 2; August 2002 to January 2004) [21–23], which we considered as the baseline for this report. At this second examination, 6237 (91.5%) participants returned, 6147 (90.2%) had retinal photography, and 5979 (87.7%) had photographs that were suitable for measurement of retinal vascular diameter. Of them, 2583 were free of hypertension at visit 2. We used hypertension status at the fourth examination (visit 4) as the study outcome after a follow-up period of mean (±SD) 3.2 (±0.5) years.
Measurement of retinal vascular diameter and retinal vascular signs
Fundus photography was performed according to a standardized protocol [21–23]. Participants were seated in a darkened room. Both eyes of each participant were photographed with a 45° 6.3 megapixel digital nonmydriatic camera. Two photographic fields were taken of each eye: the first centered on the optic disc [Early Treatment Diabetic Retinopathy Study (ETDRS) field 1] and the second centered on the fovea (ETDRS field 2) [24]. Images were sent from the centers of six study sites to the Ocular Epidemiology Reading Center at the University of Wisconsin, Madison, for measurement of retinal vascular diameter and assessment of other retinal disease. Retinal vascular diameter was measured with a computer-assisted program (IVAN, University of Wisconsin, Madison, USA), following a detailed protocol [8]. Trained graders were masked to participant clinical characteristics. Measurements from field 1 photograph of the right eye were used in this report, except that when retinal vascular diameter could not be measured in the right eye, the left eye photograph was used. For each photograph, the largest six arterioles and the largest six venules coursing through a zone between 0.5 and 1 disc diameter away from the optic disc margin were measured and summarized as the average central retinal artery and vein equivalents (CRAE and CRVE), using formulas developed by Hubbard et al. [8] and modified by Knudtson et al. [25]. In brief, assuming that a standard disc size is 1800 μm, we determined the diameter (μm) of individual retinal arterioles and venules. Calculation of CRAE and CRVE is based on branching coefficient; an empirical relationship between trunk and branches expressed as:
| (1) |
where w1 (μm) and w2 (μm) are the widths of daughter branches and W is the width of parent trunk. The branching coefficients for retinal arterioles and venules were determined from branching patterns in young normotensive adults as 1.28 and 1.11, respectively. Then, this formula was solved for arterioles and venules as below:
| (2) |
| (3) |
where w1 (μm) and w2 (μm) are the widths of daughter branches and is the estimate of parent trunk arteriole or venule. The biggest six measurements of retinal arterioles and venules were integrated using these two formulas. We repeated this calculation pairing up the largest vessels with the smallest until we reached a single number that represents the estimated calibre size of the central retinal artery and arterioles (or vein and venules) of the eye. Reproducibility of retinal vascular measurements has been reported previously, with intragrader and intergrader intraclass correlation coefficients ranging from 0.78 to 0.99 [8,22,26–28]. Presence of retinopathy signs was graded based on modification of the Airlie House Classification system [21]. For the current analysis, levels 10, 11, and 12 were defined as no retinopathy, and level 14 or above were defined as having retinopathy.
Retinal focal arteriolar narrowing and arteriovenous nicking were assessed in each of the four quadrants, excluding the area within 1/2 disc diameter surrounding the optic disc margin. For the assessment of focal arteriolar narrowing, the photograph graders examined all arterioles greater than or equal to 40 μ in diameter and evaluated the arteriole for constricted segments; only the constriction to 1/2 or less of the original diameter of proximal and distal vessel segments is considered definite. For the assessment of arteriovenous nicking, the grader examined all crossings of artery over vein and evaluated crossings where the venous blood column was narrowed on three or all four sides of the crossing.
Assessment of cardiovascular risk factors
Participants underwent interviews and assessments of cardiovascular risk factors during the course of the study [20]. Cardiovascular risk factors used for this analysis were collected at the second examination (baseline of this analysis) of the MESA. Resting blood pressure was measured three times with participants in the seated position (Dinamap model Pro 100 automated oscillometric sphygmomanometer; Critikon, GE Healthcare, Piscataway, New Jersey, USA). The MESA personnel assessed blood pressure and medication use during each MESA examination. They obtained three blood pressure measurements while seated, with each 5 min apart, using an automated sphygmomanometer. We calculated the mean of the last two measurements for analysis. The MESA personnel asked participants to bring all medications to each examination, and they assessed medication use by taking a medication inventory. As the device was programmed to output systolic and diastolic blood pressures, we calculated a mean arterial blood pressure (MABP) based on systolic and diastolic values. MABP was calculated not only at baseline visit (visit 2), but also at past visit (visit 1), as MABP=1/3×systolic BP+2/3×diastolic BP. Diabetes mellitus was defined as fasting glucose more than 6.99 mmol/l (126 mg/dl) or use of insulin or oral hypoglycemic medication. Height and weight were measured with participants wearing light clothing and no shoes, and the BMI was calculated as weight in kilograms divided by height in meter squared. The waist–hip ratio was defined as the ratio of the waist and hip circumferences.
A detailed questionnaire was used to obtain information about past medical history, cigarette smoking, alcohol consumption (defined as current and past/never), and medication use. For this current study, hypertension was defined following the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) as follows: [29] normal: SBP less than 120 mmHg, DBP less than 80; prehypertension: SBP 120–139 mmHg or DBP 80–89 mmHg; hypertension stage I: SBP 140–159 or DBP 90–99 mmHg; and hypertension stage II: SBP at least 160 mmHg or DBP at least 100 mmHg. People who were taking antihypertensive medication were categorized as having ‘hypertension stage II.’
Definition of incidence of hypertension
Incidence of hypertension was defined as being ‘normal or prehypertension at visit 1 (past) and visit 2 (baseline) examination without use of antihypertensive medication’ but being ‘hypertension stage 1 or stage 2 or use of antihypertensive medication at visit 4 (the follow-up visit of this report).’
Progression of hypertension was defined as an increase in the high blood pressure stages of two or more steps at the follow-up visit or the introduction of an antihypertensive medication at follow-up. Of all participants, 9.2% showed progression according to this definition.
In regard to regression of hypertension, as only 21 hypertensive persons (1%) showed a decrease of two or more stages of hypertension, we adopted a definition of regression based on drop in BP at visit 4 in persons defined as having hypertension at both visits 1 and 2. Thus, regression was defined as a decrease between visits 2 and 4 in systolic blood pressure of at least 10 mmHg or diastolic blood pressure at least 5 mmHg, without any use of antihypertensive medication at any visits. Of persons hypertensive, 23.1% showed regression according to this definition.
Controlled hypertension was defined as being ‘hypertension stage 1 or stage 2 at baseline’ and having BP in the ‘normal’ or ‘prehypertensive’ range at follow-up visit while using antihypertensive medications. Of those hypertensive participants, 6.4% were under control at follow-up according to this definition.
Statistical analysis
Cumulative incidence of hypertension was a primary dependent variable. Measurements of retinal vessel diameter were independent variables and were used as continuous (per SD change) or categorical (into quartiles) variables. Presence of focal arteriolar narrowing, arteriovenous nicking, and any signs of retinopathy were also used as dichotomous independent variables. Using Stata for Windows, version 10.1 (StataCorp, College Station, Texas, USA), we compared clinical characteristics by quartiles of CRAE using the analysis of variance. Covariates in multiple logistic regression models included age at baseline, sex, race (Model 1), and additional adjustment for glucose, BMI, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, presence of diabetes, duration of diabetes, current smoking at baseline, and CRAE (for models assessing CRVE) or CRVE (for models assessing CRAE; Model 2). We further adjusted for the average of past and baseline MABP in Model 3.
Similar analyses were repeated for regression of hypertension and progression of hypertension. Finally, change in area under the receiver operator characteristic (ROC) curve (AUC) was used to assess improvement in prediction using the models with or without retinal vessel measurements.
Results
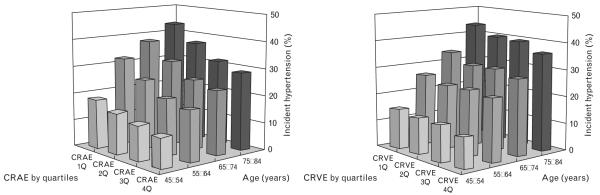
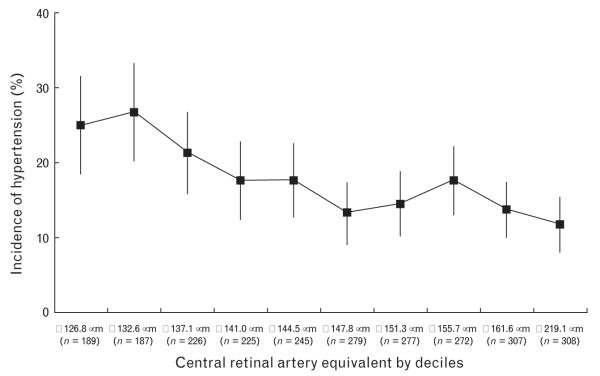
Table 1 shows baseline characteristics of study population. There were significant differences in age, systolic and diastolic blood pressure, sex, racial/ethnic group, and current smoker by quartiles of CRAE. Overall cumulative incidence of hypertension was 17.3% (448/2583). Figure 1 shows the frequency of incident hypertension by age groups and quartiles of CRAE or CRVE, adjusted for sex and race/ethnicities. There were overall trends of higher incidence of hypertension in older age groups and with smaller CRAE (P for trend <0.001 for both); although there were overall trends of higher incidence of hypertension in wider CRVE, this was not statistically significant (P for trend=0.3; Fig. 1). Figure 2 shows incidence of hypertension by deciles of CRAE, suggesting there is a near linear relationship between CRAE and incident hypertension.
Table 1.
Baseline demographic and clinical characteristics of study population by quartiles of the central retinal artery equivalent
| Variable | CRAE 1Q: ≤135.1μm (n=488) |
CRAE 2Q: 135.1–144.5μm (n=584) |
CRAE 3Q: 144.5–153.4μm (n=695) |
CRAE 4Q: ≥153.4μm (n=748) |
P |
|---|---|---|---|---|---|
| Mean (SD) | |||||
| Age (years) | 62.2 (10.0) | 60.0 (9.4) | 58.4 (8.4) | 57.8 (8.59) | <0.001 |
| SBP, Visit 1 (mmHg) | 117.5 (11.6) | 114.5 (12.1) | 112.0 (11.8) | 110.0 (13.0) | <0.001 |
| DBP Visit 1 (mmHg) | 70.8 (8.5) | 69.6 (8.6) | 68.5 (8.3) | 66.5 (8.6) | <0.001 |
| SBP, Visit 2 (mmHg) | 116.9 (11.8) | 114.4 (12.3) | 111.9 (12.3) | 108.6 (13.2) | <0.001 |
| DBP Visit 2 (mmHg) | 70.2 (8.4) | 69.4 (8.6) | 68.0 (8.5) | 65.4 (8.6) | <0.001 |
| Total cholesterol (mg/dl) | 195.5 (35.8) | 194.5 (34.4) | 196.7 (33.6) | 194.6 (36.2) | 0.626 |
| HDL cholesterol (mg/dl) | 53.0 (15.7) | 52.8 (15.2) | 51.8 (15.2) | 52.5 (15.7) | 0.566 |
| Triglycerides (mg/dl) | 122.7 (73.3) | 124.9 (67.6) | 133.2 (83.6) | 124.7 (86.6) | 0.087 |
| Glucose (mg/dl) | 99.7 (23.9) | 98.1 (16.6) | 100.0 (27.1) | 101.2 (31.6) | 0.183 |
| BMI (kg/m2) | 27.1 (4.8) | 27.2 (4.8) | 27.3 (4.9) | 27.2 (5.2) | 0.878 |
| n (%) | |||||
| Female | 221 (45.3) | 277 (47.4) | 368 (53.0) | 445 (59.5) | <0.001 |
| Race/ethnicities | |||||
| White | 263 (53.9) | 269 (46.1) | 309 (44.5) | 287 (38.4) | <0.001 |
| African–American | 66 (13.5) | 115 (19.7) | 131 (18.9) | 168 (22.5) | |
| Hispanic | 88 (18.0) | 112 (19.2) | 164 (23.6) | 195 (26.1) | |
| Chinese American | 71 (14.6) | 88 (15.1) | 91 (13.1) | 98 (13.1) | |
| Prevalence of diabetes | 28 (5.8) | 30 (5.1) | 43 (6.2) | 61 (8.2) | 0.123 |
| Current smoker | 46 (9.4) | 69 (11.8) | 99 (14.2) | 149 (19.9) | <0.001 |
1Q–4Q, lowest to highest quartiles; CRAE, the central retinal artery equivalent; HDL, high-density lipoprotein cholesterol.
Fig. 1.
Incidence of hypertension (%) by age groups and vessel diameters (adjusted for sex and race). CRAE, central retinal artery equivalent; CRVE, central retinal vein equivalent; 1Q–4Q, lowest to highest quartiles.
Fig. 2.
Incidence of hypertension (%) by deciles of central retinal artery equivalent.
Central retinal artery equivalent and incidence of hypertension
In the whole study sample, smaller CRAE was associated with 34% higher odds of incident hypertension per 1 SD decrease in CRAE [odds ratio (OR) 1.34, 95% confidence interval (CI) 1.18, 1.52] and two-fold higher odds of hypertension when the narrowest is compared with the widest quartile (OR 1.92, 95% CI 1.41, 2.62), after adjusting for age, sex, and race/ethnicities (Table 2, Model 1). After adjusting for CRVE and the average of MABP at the first and second examinations as well as glucose, BMI, total cholesterol, HDL cholesterol, triglycerides, presence of diabetes, duration of diabetes, and current smoking (Model 3), this association remained significant (per SD decrease in CRAE, OR 1.20, 95% CI 1.02, 1.42; the smallest quartile vs. the widest quartile, OR 1.47, 95% CI 1.01, 2.14). In the ethnic subgroups, this association was significant in white, but not in African–American, Hispanic, or Chinese groups (Table 2).
Table 2.
Baseline retinal arteriolar and venular diameter and incident hypertension in the Multi-Ethnic Study of Atherosclerosis with 3-year follow-upa
| Central retinal artery equivalent | |||||
|---|---|---|---|---|---|
| No. of participants | Incident hypertension (%) | Model 1: OR (95% CI)b | Model 2: OR (95% CI)b | Model 3: OR (95% CI)b | |
| By quartiles (ranges in μm) | |||||
| Total | 2515 | ||||
| 1Q: ≤135.1 | 488 | 118 (24.2) | 1.92 (1.41, 2.62) | 2.54 (1.78, 3.63) | 1.47 (1.01, 2.14) |
| 2Q: 135.1 – 144.5 | 584 | 110 (18.8) | 1.45 (1.07, 1.97) | 1.74 (1.26, 2.42) | 1.17 (0.83, 1.66) |
| 3Q: 144.5 – 153.4 | 695 | 103 (14.8) | 1.14 (0.85, 1.54) | 1.28 (0.94, 1.76) | 1.03 (0.74, 1.43) |
| 4Q: ≥153.4 | 748 | 100 (13.4) | 1.0 | 1.0 | 1.0 |
| Per SD decrease | |||||
| Total | 2515 | – | 1.34 (1.18, 1.52) | 1.57 (1.35, 1.83) | 1.20 (1.02, 1.42) |
| White | 1128 | – | 1.33 (1.11, 1.61) | 1.58 (1.24, 2.01) | 1.29 (1.01, 1.66) |
| African–American | 480 | – | 1.28 (0.98, 1.67) | 1.53 (1.11, 2.11) | 1.04 (0.72, 1.49) |
| Hispanic | 559 | – | 1.41 (1.08, 1.85) | 1.74 (1.27, 2.40) | 1.32 (0.94, 1.85) |
| Chinese American | 348 | – | 1.29 (0.87, 1.91) | 1.38 (0.84, 2.25) | 0.98 (0.56, 1.69) |
|
| |||||
| Central retinal vein equivalent | |||||
|
| |||||
| By quartiles (ranges in μm) | |||||
| Total | 2521 | ||||
| 1Q: ≤199.4 | 592 | 94 (15.9) | 1.0 | 1.0 | 1.0 |
| 2Q: 199.4 – 213.9 | 677 | 115 (17.0) | 1.12 (0.83, 1.53) | 1.53 (1.10, 2.14) | 1.38 (0.98, 1.96) |
| 3Q: 213.9 – 228.2 | 643 | 109 (17.0) | 1.10 (0.80, 1.51) | 1.63 (1.14, 2.32) | 1.36 (0.94, 1.96) |
| 4Q: ≥228.2 | 609 | 114 (18.7) | 1.23 (0.90, 1.71) | 1.99 (1.35, 2.93) | 1.68 (1.13, 2.52) |
| Per SD increase | |||||
| Total | 2521 | – | 1.05 (0.94, 1.18) | 1.27 (1.10, 1.46) | 1.18 (1.02, 1.37) |
| White | 1131 | – | 1.07 (0.90, 1.28) | 1.31 (1.05, 1.65) | 1.22 (0.97, 1.54) |
| African–American | 480 | – | 1.05 (0.83, 1.33) | 1.27 (0.95, 1.70) | 1.25 (0.91, 1.72) |
| Hispanic | 563 | – | 1.05 (0.82, 1.34) | 1.28 (0.95, 1.71) | 1.15 (0.84, 1.57) |
| Chinese American | 347 | – | 1.04 (0.73, 1.48) | 1.14 (0.74, 1.78) | 1.12 (0.69, 1.81) |
CI, confidence interval; CRAE, central retinal artery equivalent; CRVE, central retinal vein equivalent; OR, odds ratio. Model 1: Adjusting for age, sex, and race/ethnicity. Model 2: Model 1 and glucose, BMI, total cholesterol, high-density lipoprotein cholesterol, triglyceride, presence of diabetes, current smoking, and CRAE for CRVE or CRVE for CRAE at baseline. Model 3: Model 2 and average of past and baseline mean arterial blood pressure.
Defined as being normal or prehypertension at visit 1 and visit 2 without use of antihypertensive medication and being hypertension stage 1 or stage 2 or use of antihypertensive medication at visit 4.
OR: odds ratio; 95% CI: 95% confidence interval. 1Q–4Q: lowest to highest quartiles.
Central retinal vein equivalent and incidence of hypertension
Wider CRVE was also significantly associated with higher incidence of hypertension after adjusting for MABP and other covariables in Model 3 in the entire study sample (per 1 SD increase in CRVE, OR 1.18, 95% CI 1.02, 1.37; the widest quartile vs. the narrowest quartile, OR 1.68, 95% CI 1.13, 2.52; Table 2). Although the direction of the association was positive in all of the four racial/ethnic groups, it did not reach statistical significance in any of them (Table 2).
As C-reactive protein, interleukin-6, and plasma fibrinogen level were found to be associated with CRVE in our previous report [22], we further adjusted for C-reactive protein, interleukin-6, and plasma fibrinogen level at the first examination and this did not alter the significant associations of CRAE or CRVE with incidence of hypertension in the whole study sample (data not shown). There were no significant interactions between vessel measurements (CRAE or CRVE in quartiles) and race/ethnicities on the incidence of hypertension (data not shown).
Retinopathy, focal arteriolar narrowing, or arteriovenous nicking and incidence of hypertension
Participants with retinopathy signs at baseline had 50% higher odds of incident hypertension compared with those without retinopathy signs, after adjusting for age, sex, and race/ethnicity (OR 1.51, 95% CI 1.13, 2.03; Table 3, Model 1). However, this association diminished after adjusting for the average of MABP in the past and other cardiovascular risk factors (Model 3). Associations were not significant in diabetic and nondiabetic subgroups analyzed separately (adjusted OR for diabetic group 1.55, 95% CI 0.67, 3.59 and for nondiabetic group 1.31, 95% CI 0.92, 1.86). Focal arteriolar narrowing of the retinal arterioles was associated with an increased incidence of hypertension after adjusting for the average of MABP and other cardiovascular risk factors (OR 1.80, 95% CI 1.09, 2.97; Table 3), whereas arteriovenous nicking was not significantly associated with the incidence of hypertension. There were no significant racial/ethnic differences in associations of retinopathy, focal arteriolar narrowing, or arteriovenous nicking with incident hypertension.
Table 3.
Presence of focal retinal signs at baseline and incident hypertension
| No. of participants | Incident hypertension (%) | Model 1: OR (95% CI)a | Model 2: OR (95% CI)a | Model 3: OR (95% CI)a | |
|---|---|---|---|---|---|
| Retinopathy | |||||
| Present | 307 | 72 (23.5) | 1.51 (1.13, 2.03) | 1.39 (1.02, 1.89) | 1.37 (0.99, 1.89) |
| Absent | 2239 | 367 (16.4) | 1.00 | 1.00 | 1.00 |
| Focal arteriolar narrowing | |||||
| Present | 87 | 33 (37.9) | 2.66 (1.68, 4.22) | 2.58 (1.61, 4.11) | 1.80 (1.09, 2.97) |
| Absent | 2455 | 407 (16.6) | 1.00 | 1.00 | 1.00 |
| Arteriovenous nicking | |||||
| Present | 62 | 18 (29.0) | 1.56 (0.88, 2.77) | 1.51 (0.85, 2.71) | 1.20 (0.65, 2.22) |
| Absent | 2503 | 427 (17.1) | 1.00 | 1.00 | 1.00 |
The Multi-Ethnic Study of Atherosclerosis, 3-year follow-up. Model 1: Adjusting for age, sex, and race/ethnicity. Model 2: Model 1 and glucose, BMI, total cholesterol, high-density lipoprotein cholesterol, triglyceride, presence of diabetes, duration of diabetes, and current smoking. Model 3: Model 2 and average of past and baseline mean arterial blood pressure.
OR: odds ratio; 95% CI: 95% confidence interval.
Progression and regression of hypertension
After adjusting for past and baseline MABP and other cardiovascular risk factors, narrower CRAE and wider CRVE were associated with 49 and 46% increased odds of progression of hypertension stage, respectively (Table 4). Wider CRAE was associated with 49% increased odds of regression of hypertension after adjusting for the same covariates, including MABP (OR for widest vs. narrowest quartile 1.49, 95% CI 1.06, 2.09; Table 4). In the same model, wider CRAE was not significantly associated with controlled hypertension.
Table 4.
Baseline vessel measurements and secondary outcomes (progression of hypertension, regression of hypertension, and controlled hypertension)
| Cumulative incidence (%) | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Progression of hypertension (n=5373)a | ||||
| Narrower CRAE (1Q vs. 4Q) | 152 (11.2)/112 (8.5) | 1.37 (1.05, 1.78) | 1.52 (1.13, 2.05) | 1.49 (1.09, 2.02) |
| Wider CRVE (4Q vs. 1Q) | 125 (9.3)/116 (8.5) | 1.15 (0.88, 1.52) | 1.47 (1.07, 2.03) | 1.46 (1.06, 2.02) |
| Regression of hypertension (n=2369)b | ||||
| Wider CRAE (4Q vs. 1Q) | 157 (22.9)/120 (25.8) | 0.90 (0.68, 1.19) | 1.06 (0.77, 1.47) | 1.49 (1.06, 2.09) |
| Narrower CRVE (1Q vs. 4Q) | 136 (23.7)/112 (20.1) | 1.22 (0.90, 1.64) | 1.22 (0.86, 1.73) | 1.37 (0.96, 1.95) |
| Controlled hypertension (n=2376)c | ||||
| Wider CRAE (4Q vs. 1Q) | 39 (8.2)/37 (5.2) | 1.64 (1.02, 2.63) | 1.92 (1.12, 3.29) | 1.45 (0.83, 2.53) |
| Narrower CRVE (1Q vs. 4Q) | 38 (6.0)/36 (5.9) | 1.02 (0.62, 1.67) | 1.40 (0.80, 2.47) | 1.34 (0.75, 2.36) |
The Multi-Ethnic Study of Atherosclerosis, 3-year follow-up. Model 1: Adjusting for age, sex, and race/ethnicity. Model 2: Model 1 and glucose, BMI, total cholesterol, high-density lipoprotein cholesterol, triglyceride, presence of diabetes, current smoking, and CRAE for CRVE or CRVE for CRAE. Model 3: Model 2 and average of past and baseline mean arterial blood pressure CRAE, central retinal artery equivalent; CRVE, central retinal vein equivalent; 1Q–4Q, lowest to highest quartiles.
Defined as having two-step or more increase in the stage at follow-up compared with baseline in hypertension stage of normal, prehypertension, stage I hypertension, and stage II hypertension or introduction of an antihypertensive medication.
Defined as having hypertension stage I or II at baseline and having systolic blood pressure decreased at least 10 mmHg, diastolic blood pressure decreased at least 5mmHg between visit 2 and visit 4, without any use of antihypertensive medication at any visits.
Defined as having hypertension stage I or II at baseline and having normal or prehypertension at follow-up visit with antihypertensive treatment.
Change in area under receiver operator characteristic curve by models
Adding CRAE and CRVE to the model predicting hypertension from age, sex, race/ethnicities, BMI, medical history, and baseline MABP did not improve the AUC significantly (AUC: 0.775 vs. 0.778; P=0.08).
Discussion
In this prospective multiethnic population-based study, we found that narrower retinal arteriolar diameter was associated with increased risk of hypertension development and progression. We also found that participants with wider retinal venular diameter and focal arteriolar narrowing were more likely to develop hypertension.
In previous studies, narrower retinal arteriolar diameter and smaller arteriovenous ratio were consistently associated with the incidence of hypertension [11–15]. Smaller arteriovenous ratio reflects either narrower retinal arteriolar diameter or wider venular diameter. Unlike the association of retinal arteriolar narrowing and hypertension, the association of wider retinal venular diameter and hypertension has not been consistently found. A significant association between narrower venular diameter and incident hypertension was initially reported in the Rotterdam Eye Study [15], but this was diminished after additional adjustment with retinal arteriolar diameter [30]. Also, retinal venular diameter was not significantly associated with incidence of severe hypertension in the Blue Mountains Eye Study [14]. We found that adding the diameter of the fellow retinal vessel (CRAE for CRVE and vice-versa) enhanced the association between narrower retinal arteriolar diameter or wider venular diameter and incidence of hypertension consistently (model 1 vs. model 2, Tables 2 and 4). Our findings support the view that narrower retinal arteriolar diameter and wider retinal venular diameter are both associated with the incidence of hypertension in the multiethnic population. As wider retinal venular diameter is associated with inflammation markers [22,31] and the metabolic syndrome [32,33], these findings could explain at least partly the association of wider venules with the development of hypertension [34]. However, in our current study, further adjustment of C-reactive protein, interleukin-6, and plasma fibrinogen level did not alter this association. We cannot exclude the possibility that this association is the result of residual confounding by unknown factors that we did not collect information on and have not adjusted for.
We found that wider retinal arteriolar diameter was associated with regression of hypertension. Similarly, the Blue Mountains Eye Study showed that wider retinal arteriolar diameter predicted better response to hypertension treatment [14]. A recent study reported that antihypertensive treatment could reverse retinal arteriolar narrowing, as indicated by length-to-diameter ratio, in hypertensive patients [35]. The clinical implications of these findings include the possibilities that retinal vessel diameter changes are indicators for susceptibility to the development of hypertension, and its regression or better treatment response.
Although the Beaver Dam Eye Study found association between retinopathy and incident hypertension [36], we could not confirm this association in the MESA population. Our results were consistent with those reported by the Atherosclerosis Risk in Communities Study [11]. Though differences in these associations across the four ethnic groups were not statistically significant, the association between retinal vascular diameter and incidence of hypertension was significant only in whites but not in African–Americans, Hispanics, or Chinese Americans (Table 2). It is likely this reflects smaller numbers in these subgroups.
The pathophysiological mechanisms underlying these associations are not entirely clear. Retinal arteriolar narrowing has been associated with reduced large artery compliance [26,27], which contributes to systolic hypertension. In the MESA, narrower retinal arteriolar diameter was associated with increased aortic stiffness [26], as determined from magnetic resonance imaging, and reduced large artery elasticity, as determined from pulse contour analysis. These associations remained significant even after adjusting for age, blood pressure (past and current), and other vascular risk factors [26]. However, the nature of these associations (causal or not) cannot be determined by these previous studies, as they were based on cross-sectional data [26]. It has also been hypothesized that the association of arteriolar narrowing and reduced large artery compliance could be due to arterial and arteriolar wall thickening, which may be accompanied by similar remodeling in the larger arteries [27].
Strength of our study is the use of a community-based, multiethnic cohort. Also, we used a standardized imaging technique to measure retinal vascular diameters, which enables us to compare the results with other studies that used the same methodology. However, we cannot differentiate whether the vessel diameter change was temporary or structural because our measurement was based on a photograph taken at just one point at time. Other limitations include a relatively short follow-up period and the fact that the outcomes were only assessed at a single follow-up time point. It is also possible that our observed associations were confounded by unmeasured characteristics or resulted from misclassification of hypertension [37]. In addition, ethnic-related variations in the measurements of vessel diameters [38] may affect our results in this multiethnic population. Although we had hoped to be able to determine whether differences exist among the four racial/ethnic groups, our study lacked statistical power to detect any statistically meaningful differences. We had estimated the association of retinal vessels and incidence of hypertension would be OR of 1.8 or above, with statistical power of 0.80 or above. However, the associations we found were less than OR of 1.7. However, our findings suggest that further study to examine racial/ethnic differences in a larger group of study participants is warranted.
In summary, we report an association of baseline retinal vascular diameter and retinopathy signs with the incidence of hypertension over a 3-year period in a multiethnic community-based cohort free of cardiovascular disease at baseline. These findings support a microvascular involvement to the etiology of hypertension.
Acknowledgement
The present study is supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 and Grant R01HL69979 (RK TYW) from the National Heart, Lung and Blood Institute and a NIH Intramural Research Award Z01EY00403 (MFC) from the National Eye Institute, USA.
Abbreviations
- 1Q–4Q
lowest to highest quartiles
- AUC
the area under the receiver operator characteristics curve
- BMES
the Blue Mountains Eye Study
- CI
confidence interval
- CRAE
the Central Retinal Artery Equivalent
- CRVE
the Central Retinal Vein Equivalent
- ETDRS
the Early Treatment Diabetic Retinopathy Study
- HDL
high-density lipoprotein
- JNC-7
the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure
- MABP
mean arterial blood pressure
- MESA
the Multi-Ethnic Study of Atherosclerosis
- OR
odds ratio
- ROC
the receiver operator characteristics
Footnotes
There are no conflicts of interest.
References
- 1.Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006;48:1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- 2.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 3.Rizzoni D, Castellano M, Porteri E, Bettoni G, Muiesan ML, Agabiti-Rosei E. Vascular structural and functional alterations before and after the development of hypertension in SHR. Am J Hypertens. 1994;7:193–200. doi: 10.1093/ajh/7.2.193. [DOI] [PubMed] [Google Scholar]
- 4.Norrelund H, Christensen KL, Samani NJ, Kimber P, Mulvany MJ, Korsgaard N. Early narrowed afferent arteriole is a contributor to the development of hypertension. Hypertension. 1994;24:301–308. doi: 10.1161/01.hyp.24.3.301. [DOI] [PubMed] [Google Scholar]
- 5.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 6.Mulvany MJ. Are vascular abnormalities a primary cause or secondary consequence of hypertension? Hypertension. 1991;18:I52–I57. doi: 10.1161/01.hyp.18.3_suppl.i52. [DOI] [PubMed] [Google Scholar]
- 7.Vicaut E. Hypertension and the microcirculation: a brief overview of experimental studies. J Hypertens Suppl. 1992;10:S59–S68. doi: 10.1097/00004872-199207005-00009. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the atherosclerosis risk in communities study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–435. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–2317. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 11.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Klein BE, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith W, Wang JJ, Wong TY, Rochtchina E, Klein R, Leeder SR, Mitchell P. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension. 2004;44:442–447. doi: 10.1161/01.HYP.0000140772.40322.ec. [DOI] [PubMed] [Google Scholar]
- 14.Wang JJ, Rochtchina E, Liew G, Tan AG, Wong TY, Leeder SR, et al. The long-term relation among retinal arteriolar narrowing, blood pressure, and incident severe hypertension. Am J Epidemiol. 2008;168:80–88. doi: 10.1093/aje/kwn100. [DOI] [PubMed] [Google Scholar]
- 15.Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A, de Jong PT. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension. 2006;47:189–194. doi: 10.1161/01.HYP.0000199104.61945.33. [DOI] [PubMed] [Google Scholar]
- 16.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the Multiethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Duncan BB, Nieto FJ, Klein BE, Couper DJ, et al. Racial differences in the prevalence of hypertensive retinopathy. Hypertension. 2003;41:1086–1091. doi: 10.1161/01.HYP.0000064181.63546.53. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multiethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 19.Thomas KL, East MA, Velazquez EJ, Tuttle RH, Shaw LK, O’Connor CM, Peterson ED. Outcomes by race and etiology of patients with left ventricular systolic dysfunction. Am J Cardiol. 2005;96:956–963. doi: 10.1016/j.amjcard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AV Diez, Folsom AR, et al. Multiethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, et al. Diabetic retinopathy in a multiethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multiethnic Study of Atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multiethnic Study of Atherosclerosis. Ophthalmology. 2006;113:373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Grading diabetic retinopathy from stereoscopic color fundus photographs – an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 25.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 26.Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, et al. Aortic distensibility and retinal arteriolar narrowing: the Multiethnic Study of Atherosclerosis. Hypertension. 2007;50:617–622. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 27.Cheung N, Islam FM, Jacobs DR, Jr, Sharrett AR, Klein R, Polak JF, et al. Arterial compliance and retinal vascular caliber in cerebrovascular disease. Ann Neurol. 2007;62:618–624. doi: 10.1002/ana.21236. [DOI] [PubMed] [Google Scholar]
- 28.Cheung N, Bluemke DA, Klein R, Sharrett AR, Islam FM, Cotch MF, et al. Retinal arteriolar narrowing and left ventricular remodeling: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2007;50:48–55. doi: 10.1016/j.jacc.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 30.Liew G, Wong TY, Mitchell P, Wang JJ. Are narrower or wider retinal venules associated with incident hypertension? Hypertension. 2006;48:e10. doi: 10.1161/01.HYP.0000231652.97173.4c. author reply e11. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 32.Wong TY, Duncan BB, Golden SH, Klein R, Couper DJ, Klein BE, et al. Associations between the metabolic syndrome and retinal microvascular signs: the atherosclerosis risk in communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki R, Tielsch JM, Wang JJ, Wong TY, Mitchell P, Tano Y, et al. The metabolic syndrome and retinal microvascular signs in a Japanese population: the Funagata Study. Br J Ophthalmol. 2008;92:161–166. doi: 10.1136/bjo.2007.127449. [DOI] [PubMed] [Google Scholar]
- 34.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 35.Hughes AD, Stanton AV, Jabbar AS, Chapman N, Martinez-Perez ME, McG Thom SA. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. J Hypertens. 2008;26:1703–1707. doi: 10.1097/HJH.0b013e328304b072. [DOI] [PubMed] [Google Scholar]
- 36.Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98–107. [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Dov IZ. Retinal vessel narrowing: a prehypertensive or masked hypertensive state? Hypertension. 2006;47:e19. doi: 10.1161/01.HYP.0000218339.34795.18. author reply e19. [DOI] [PubMed] [Google Scholar]
- 38.Rochtchina E, Wang JJ, Taylor B, Wong TY, Mitchell P. Ethnic variability in retinal vessel caliber: a potential source of measurement error from ocular pigmentation? – the Sydney Childhood Eye Study. Invest Ophthalmol Vis Sci. 2008;49:1362–1366. doi: 10.1167/iovs.07-0150. [DOI] [PubMed] [Google Scholar]