Abstract
Neuronostatin, a recently discovered peptide derived from the somatostatin preprohormone, significantly inhibited both food and water intake when administered centrally in adult male rats. Because neuronostatin is highly produced in the hypothalamus, an area of the brain through which important feeding circuits, including the central melanocortin system, communicate, we sought to determine if the anorexigenic and antidipsogenic effects of neuronostatin would be reversed by pretreatment with the melanocortin 3/4 receptor antagonist, SHU9119. SHU9119 pretreatment reversed the effect of neuronostatin on both food and water intake. We have shown recently that the central oxytocin system is a potential downstream mediator of the anorexignic action of alpha-MSH. We therefore tested whether the effects of neuronostatin also were dependent upon central oxytocin receptors. Neuronostatin-induced anorexia was not reversed by pretreatment with the oxytocin receptor antagonist, OVT, suggesting that neuronostatin acts through a unique subset of POMC neurons that do not signal via central oxytocin receptors.
Keywords: Neuronostatin, Melanocortins, Oxytocin, Appetite
1. Introduction
1.1 Neuronostatin
Neuronostatin is a recently discovered, 13 amino acid peptide that is derived from the somatostatin preprohormone. Unlike somatostatin, neuronostatin did not alter either basal or growth hormone releasing hormone- or ghrelin-stimulated growth hormone secretion from primary rat anterior pituitary cells (27). Additionally, neuronostatin failed to activate any of the five known somatostatin receptors in an in vitro system (27). Using a radioimmunoassay, neuronostatin immunoreactivity was detected in diverse metabolic, neuronal, and gastrointestinal tissues of rats, with highest levels in spleen, pancreas, and brain, particularly hypothalamus (27). In the hypothalamus, neuronostatin was detected in somatostatin-immunoreactive neurons, primarily in the periventricular nucleus (8). Because of the relatively high amount of neuronostatin present in the hypothalamus, an area of the brain known to be important in the control of food intake (2), we sought to determine if neuronostatin was involved in appetite regulation. When injected into the lateral cerebroventricle (i.c.v.) of adult male rats, neuronostatin led to an inhibition of both food and water intake that appeared to be dose-related (27).
1.2 The Central Melanocortin System
The central melanocortin system is an important neuronal circuit involved in the regulation of appetite and energy expenditure (6). This system is comprised of neurons, primarily in the arcuate nucleus of the hypothalamus, producing either alpha-melanocyte-stimulating hormone (alpha-MSH)/proopiomelanocortin (POMC) or neuropeptide Y (NPY)/agouti-related peptide (AgRP), as well as target neurons, located throughout the brain, expressing melanocortin 3/4 receptors (6). The central melanocortin system may interact with many other neuronal systems to control food intake, including serotonergic neurons (12), neurons producing brain-derived neurotrophic factor (BDNF) (1), and the central oxytocin system (24, 34).
Because the central melanocortin system mediates the anorexigenic activity of many peripherally- and hypothalamically-derived peptides [i.e. leptin (6); alpha-MSH (6); nesfatin-1 (21, 33)], we hypothesized that neuronostatin-induced anorexia and adipsia would be dependent upon the central melanocortin system. We found that the anorexigenic and antidipsogenic effects of neuronostatin were reversed by pretreatment with the melanocortin 3/4 receptor antagonist, SHU9119. We also found that these effects of neuronostatin were not dependent upon the central oxytocin system, as has been shown for other melanocortin-dependent peptides, including leptin (3) and nesfatin-1 (15, 34). These findings suggest that neuronostatin may act through a distinct subset of POMC neurons that, unlike nesfatin-1- and leptin-responsive POMC neurons, do not initiate central oxytocin signaling.
2. Materials and Methods
2.1 Animals
All procedures and protocols have been approved by the Saint Louis University Animal Use and Care Committee. Adult male rats (Harlan Sprague-Dawley, Indianapolis, IN) were housed under controlled conditions (23-25°C, lights on 0600-1800hr) with free access to food and water. Rats (225-300g) were anesthetized with a mixture of ketamine (60mg/ml; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (8mg/ml; TranquiVed, VedCo, Saint Joseph, MO) at a dose of 0.1 ml per 100 g body weight, as previously described (25). A stainless steel cannula (17 gauge, 23 mm) was implanted into the right lateral cerebroventricle (i.c.v.) using a stereotaxic device as previously described (34). Following surgery, animals were housed singly and body weights were monitored daily to ensure animals health and recovery of pre-surgery weight. Placement and patency of the i.c.v. cannula was verified by the dipsogenic effect of angiotensin II (26).
2.2 Food and Water Intake Experiments
Food and water intake experiments were conducted using an ad libitum feeding model established by our laboratory (29). Briefly, rats were habituated to metabolic cages (Nalgene) for at least 3 days. During this time, food and water intakes and body weights were monitored daily to ensure animal health. On the day of experimentation, food and water were removed from the cages 10 minutes before i.c.v. injection with test substances at 1650 hours. Ten minutes following injection (at 1700), food and water were returned to the cages, and food and water intakes were recorded every 30 minutes until 2100, then again at 1200 and 1700 the following day. Experiments were conducted during this time frame (1700-2100, one hour during the light phase, 3 hours during the dark phase) to coincide with the light-entrained feeding cycle of our rat colony (33).
2.3 Neuronostatin and Antagonists
To test the hypothesis that the central melanocortin system mediates the effect of neuronostatin on food and water intake, rats were pretreated i.c.v. with either saline vehicle or the melanocortin 3/4 receptor antagonist, SHU9119 (300 pmole) (33), prior to i.c.v. administration of either saline vehicle or 300 pmole neuronostatin (27). To determine if the central oxytocin system is involved in neuronostatin-induced anorexia, animals were pretreated i.c.v. with either saline vehicle or the oxytocin receptor antagonist, [D-(CH2)5,Tyr(me)2,Orn8]-vasotocin (OVT, 8.7 nmole) (34), before treatment with either saline or neuronostatin i.c.v. We have demonstrated previously that this dose of OVT completely reverses the anorexigenic action of centrally administered oxytocin (34). All peptides were purchased from Phoenix Pharmaceuticals (Burlingame, CA).
2.4 Statistical Analysis
Doses were determined in previous experiments, as indicated. Data were analyzed using ANOVA with Scheffe’s multiple comparisons, and a p value of less than 0.05 was considered significant.
3. Results
3.1 Effect of SHU9119 Pretreatment on Neuronostatin-Induced Anorexia and Adipsia
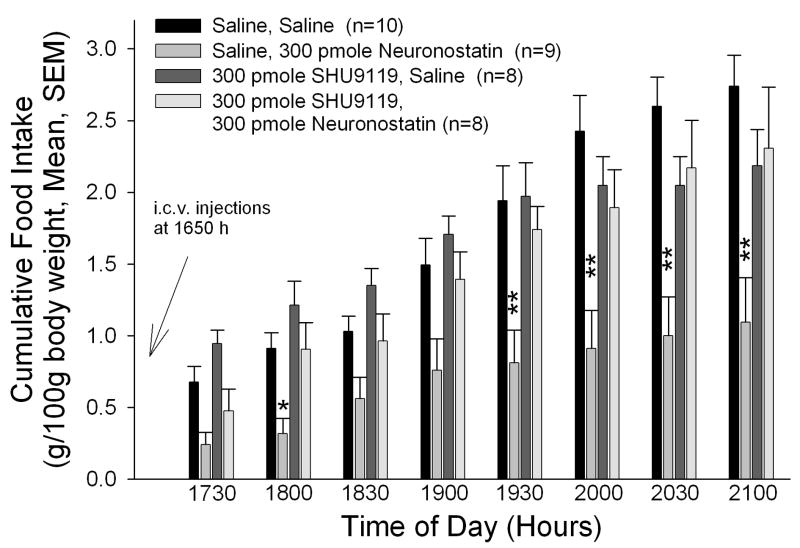
Central administration of neuronostatin led to significant decreases in food intake as previously described (27) (Figure 1A). While animals treated with saline prior to neuronostatin consumed less food than saline/saline-injected controls at all time intervals, this effect was most apparent during the later time points. Although 300 pmole SHU9119 did not significantly alter food intakes when injected prior to saline vehicle, pretreatment with SHU9119 prior to neuronostatin administration significantly inhibited the effect of neuronostatin on food intake.
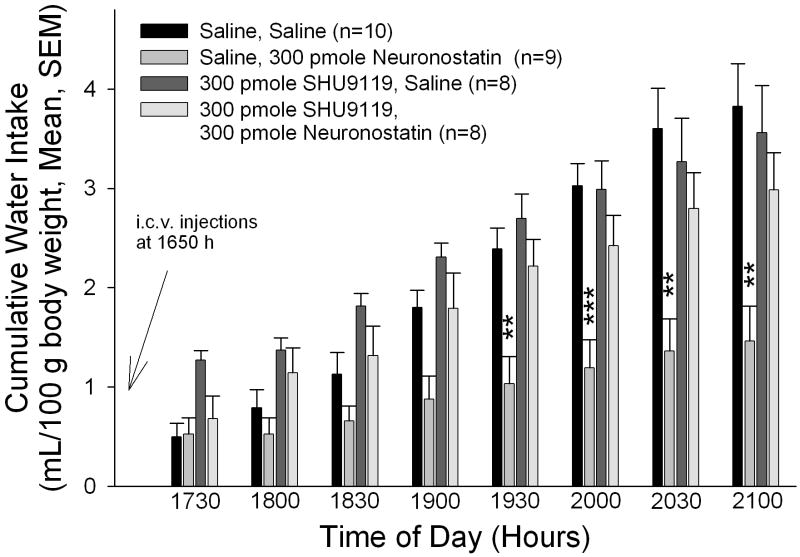
Figure 1. The anorexigenic and antidipsogenic effects of neuronostatin are reversed by pretreatment with SHU9119.
Rats were pretreated i.c.v. with either saline vehicle or vehicle containing 300 pmole SHU9119 prior to central administration of either saline or 300 pmole neuronostatin. SHU9119 pretreatment reversed the effect of neuronostatin on both food (A) and water (B) intakes. Data were analyzed by ANOVA with Scheffe’s multiple comparisons (*p<0.05, **p<0.01, ***p<0.001 vs. Saline controls).
Neuronostatin administration also led to an inhibition of water intake, although this effect did not attain significance until 1930 hours (Figure 1B). SHU9119 did not significantly alter water consumption when injected before saline vehicle. However, rats that received SHU9119 prior to neuronostatin drank significantly more water than animals pretreated with saline before neuronostatin administration.
3.2 Effect of OVT Pretreatment on Neuronostatin-Induced Anorexia and Adipsia
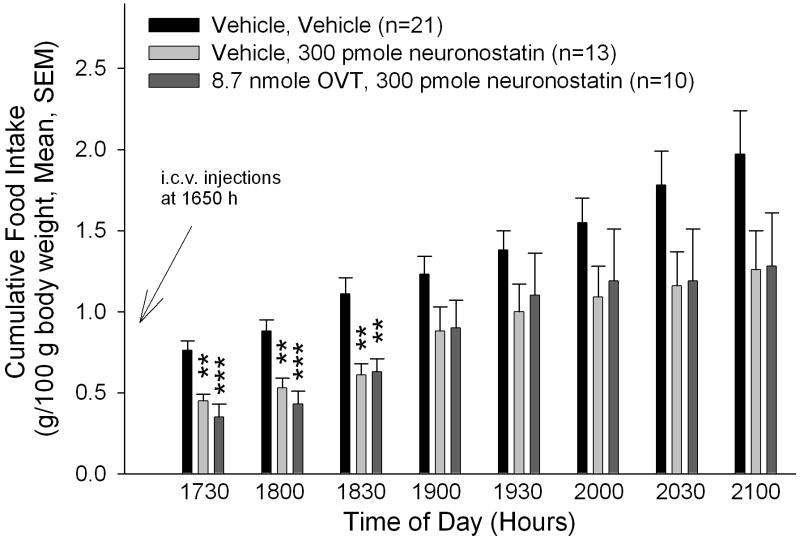
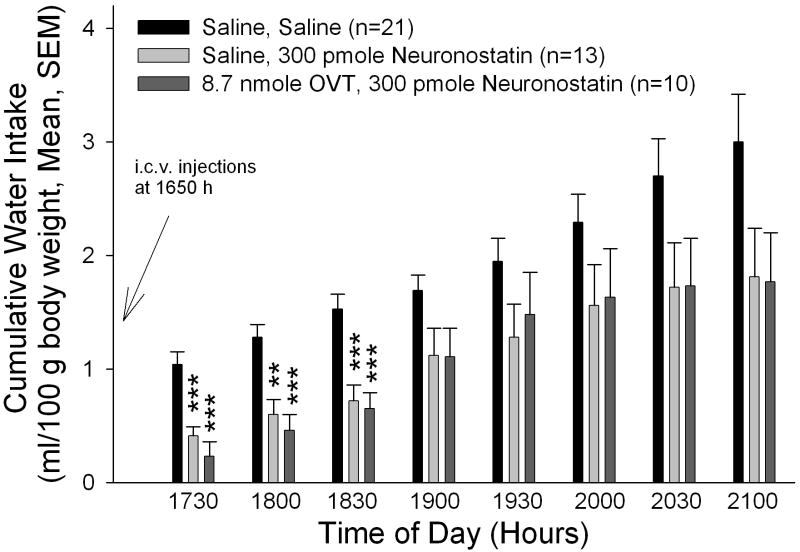
In our studies with another melanocortin-dependent anorexigen, nesfatin-1, we have shown that the central oxytocin system may act as a downstream mediator of the central melanocortin system (34). We therefore sought to determine if the anorexigenic effect of neuronostatin could be reversed by pretreatment with the oxytocin receptor antagonist, OVT. Animals that received saline before neuronostatin consumed less food and water than saline-injected controls (Figure 2A/B), as shown in the previous experiment. Importantly, OVT, at a dose that reversed an anorexigenic dose of oxytocin (34), did not significantly alter either food or water intake. Rats that were pretreated with OVT prior to neuronostatin administration consumed significantly less food and water than saline-injected controls, indicating that neuronostatin is not dependent upon central oxytocin receptors to exert its anorexigenic and antidipsogenic effects.
Figure 2. Neuronostatin-induced anorexia and adipsia are not reversed by pretreatment with OVT.
Rats were pretreated i.c.v. with either saline vehicle or vehicle containing 8.7 nmole OVT prior to central administration of either saline or 300 pmole neuronostatin. OVT pretreatment did not reverse the effect of neuronostatin on either food (A) and water (B) intakes. Data were analyzed by ANOVA with Scheffe’s multiple comparisons (*p<0.05, **p<0.01, ***p<0.001 vs. Saline controls).
4. Discussion
4.1 Oxytocin as a Downstream Mediator of Melanocortin Signaling
Several lines of evidence suggest that the central oxytocin system is a downstream mediator of the central melanocortin system. Like the central melanocortin system, central oxytocin is important in both appetite (22) and cardiovascular function (19, 32). Additionally, melanocortin agonists led to an increase in the central release of oxytocin (24), and also to an increase in c-fos expression in oxytocin-immunoreactive neurons (5). Furthermore, we have shown recently that the anorexigenic effect of alpha-MSH was reversed by pretreatment with OVT (34).
In the present studies, we found that neuronostatin recruits the central melanocortin system, but not the central oxytocin system, to exert is anorexigenic and antidipsogenic actions, since neuronostaitn-induced anorexia and adipsia were reversed by pretreatment with SHU9119, but not OVT. In contrast, the anorexigenic effect of other melanocortin-dependent feeding peptides, including leptin (3) and nesfatin-1 (15, 34), are dependent upon the central oxytocin system.
4.2 POMC Subpopulations
One explanation for these observations is that these peptides interact with different subpopulations of POMC neurons, which project to different downstream neuronal circuits. Indeed, there is evidence that the cluster of POMC neurons originating in the arcuate nucleus of the hypothalamus is divided into neuronal subpopulations that express different sets of receptors and release different neurotransmitters. For example, only 67% of POMC neurons express the leptin receptor (11). Leptin stimulates transcription of POMC through activation of signal transducer and activator of transcription 3 (STAT3) proteins, but the activated form of STAT3, phospho-STAT3 is detected only in 37% of POMC neurons treated with leptin (20). A subpopulation of POMC neurons coexpressing pituitary adenylate cyclase-activating polypeptide (PACAP) is located in the ventrolateral arcuate nucleus, and these account for about 20% of POMC neurons in the hypothalamus (9). An additional subpopulation of POMC neurons, comprising about 37% of all POMC neurons, coexpress choline acetyltransferase, indicating that these neurons are cholinergic (17). These cholinergic POMC neurons are specifically located in the ventral aspect of the arcuate nucleus (17). Furthermore, a recent report (31) has shown that there are distinct subtypes of POMC neurons that respond to either insulin or leptin. These data suggest that not only do distinct phenotypic groups of POMC neurons exist, but they are organized regionally within the arcuate nucleus, and therefore may project to differing sites of melanocortin receptor expression.
4.3 Potential Involvement of CRF Neurons
There is also evidence that the central melanocortin system interacts with CRF (corticotropin releasing factor) neurons. For example, the anorexigenic effect of NDP-MSH, a melanocortin agonist, was reversed by pretreatment with the non-selective CRF antagonist, astressin, and NDP-MSH did not alter early phase food intake in mice lacking CRF (13). Additionally, melanocortin agonists led to increases in CRH mRNA levels in the PVN (14) and in plasma ACTH (7). However, pretreatment with a melanocortin 4 receptor antagonist failed to affect CRF-induced anorexia (30) and centrally-injected CRF reduced food intake in melanocortin 4 receptor deficient mice (16), suggesting that the interaction between POMC and CRF neurons is a one-way circuit.
Nesfatin-1, a melanocortin-dependent anorexigen, was shown to colocalize with CRF in parvocellular elements of the PVN where it has been hypothesized to act as a paracrine or autocrine factor (10). A role for nesfatin-1 in stress-related behavior has been hypothesized (18, 33). Indeed, nesfatin-1-induced anorexia can be blocked by pretreatment with a CRF2 receptor antagonist (28), adding to the evidence that this endogenous neuropeptide signals through multiple neuronal circuitries. CRF neurons may lie upstream of the central oxytocin system, as pretreatment with the oxytocin receptor antagonist, OVT, abrogated CRF-induced anorexia (23). Likewise, central administration of CRF led to an increase in oxytocin release, but did not affect the release of vasopressin (4). Together these data suggest that nesfatin-1 may signal sequentially through POMC, CRF, and oxytocin neurons. It is possible that neuronostatin activates a POMC-CRF circuit that does not require the involvement of oxytocin neurons. On the other hand, no significant effects of neuronostatin were observed on spontaneous locomotor activity (27), suggesting that the CNS actions of neuronostatin diverge from those of nesfatin-1 not only in being oxytocin-independent, but perhaps also independent of central CRF pathways as well.
4.4 Summary
In summary, we have identified a mechanism of neuronostatin-induced anorexia and adipsia. Although the actions of neuronostatin are dependent upon the central melanocortin system, they are not dependent upon the central oxytocin system. These data suggest that multiple subpopulations of POMC neurons exist which respond to anorexigenic peptides, such as neuronostatin and nesfatin-1, utilizing in response separate downstream signaling circuits.
Acknowledgments
This work was supported by NIH Grant HL66023 (WKS) and a predoctoral fellowship [NIH Grant 5T32GM008306] to GLCY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system food intake control. Endocrinology. 2009;150:2646–53. doi: 10.1210/en.2008-1184. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neuroscience and Biobehavioral Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 3.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol. 2004;287:R87–96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bruhn TO, Sutton SW, Plotsky PM, Vale WW. Central administration of corticotropin-releasing factor modulates oxytocin secretion in the rat. Endocrinology. 1986;119:1558–63. doi: 10.1210/endo-119-4-1558. [DOI] [PubMed] [Google Scholar]
- 5.Caquineau C, Leng G, Guan XMM, Jian M, Van Der Ploeg L, Douglas AJ. Effects of alpha-melanocyte-stimulating hormone on magnocellular oxytocin neurons and their activation at intromission in male rats. J Neuroendocrinol. 2006;18:685–91. doi: 10.1111/j.1365-2826.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 6.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 7.Dhillo WS, Small CJ, Seal LJ, Kim MS, Stanley SA, Murphy KG, Ghatei MA, Bloom SR. The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology. 2002;75:209–16. doi: 10.1159/000054712. [DOI] [PubMed] [Google Scholar]
- 8.Dun SL, Brailoiu GC, Tica AA, Yang J, Chang JK, Brailoiu E, Dun NJ. Neuronostatin is co-expressed with somatostatin and mobilizes calcium in cultured rat hypothalamic neurons. Neuroscience. 2010;166:455–63. doi: 10.1016/j.neuroscience.2009.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durr K, Norsted E, Gomuc B, Suarez E, Hannibal J, Meister B. Presence of pituitary adenylate cyclase-activating polypeptide (PACAP) defines a subpopulation of hypothalamic POMC neurons. Brain Res. 2007;1186:203–11. doi: 10.1016/j.brainres.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreacivity in the rat CNS. Neuroscience. 2008;156:563–79. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 11.Funahashi H, Yamada S, Kageyama H, Takenoya F, Guan JL, Shioda S. Coexistence of leptin- and orexin-receptors in feeding-regulating neurons in the hypothalamic arcuate nucleus—a triple labeling study. Peptides. 2003;24:687–94. doi: 10.1016/s0196-9781(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 12.Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JM, Cone RD, Elmquist JK. Central serotonin and melanocortin pathways regulating energy homeostasis. Annals NY Acad Sci. 2003;994:169–74. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawashima S, Sakihara S, Kageyama K, Nigawara T, Suda T. Corticotropin-releasing factor (CRF) is involved in the acute anorexic effect of alpha-melanocyte-stimulating hormone: a study using CRF-deficient mice. Peptides. 2008;29:2169–74. doi: 10.1016/j.peptides.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci. 2003;23:7863–72. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh-I S, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metabolism. 2009;10:355–65. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 17.Meister B, Gomuc B, Suarez E, Ishii Y, Durr K, Gillberg L. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. Eur J Neurosci. 2006;24:2731–40. doi: 10.1111/j.1460-9568.2006.05157.x. [DOI] [PubMed] [Google Scholar]
- 18.Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rats. Psychopharmacology. 2008;201:115–23. doi: 10.1007/s00213-008-1252-2. [DOI] [PubMed] [Google Scholar]
- 19.Michelini LC, Marcelo MC, Amico J, Morris M. Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. Am J Physiol. 2003;284:H2269–76. doi: 10.1152/ajpheart.00774.2002. [DOI] [PubMed] [Google Scholar]
- 20.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjobaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–31. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 21.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 22.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbails JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–8. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- 23.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am J Physiol. 1991;260:R448–52. doi: 10.1152/ajpregu.1991.260.2.R448. [DOI] [PubMed] [Google Scholar]
- 24.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan X, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–8. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samson WK, Murphy TC, Resch ZT. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am J Physiol. 1998;274:R1505–9. doi: 10.1152/ajpregu.1998.274.5.R1505. [DOI] [PubMed] [Google Scholar]
- 26.Samson WK, White MM, Price CP, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol. 2007;292:R637–43. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- 27.Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GLC, Klein C, Lyn R, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan X, Chang JK, Hsueh AJW. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem. 2008;283:31949–59. doi: 10.1074/jbc.M804784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Lambrecht NWG, Tache Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–9. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor MM, Bagley SL, Samson WK. Intermedin/adrenomedullin-2 acts within central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol. 2005;288:R919–27. doi: 10.1152/ajpregu.00744.2004. [DOI] [PubMed] [Google Scholar]
- 30.Vergoni AV, Bertolini A, Wikberg JE, Schioth HB. Corticotropin-releasing factor (CRF) induced anorexia is not influenced by a melanocortin 4 receptor blockage. Peptides. 1999;20:509–13. doi: 10.1016/s0196-9781(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 31.Williams KW, Margatho LO, Lee CE Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–9. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wsol A, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Kowalewski S, Puchalska L. Oxytocin in the cardiovascular responses to stress. J Physiol Pharmacol. 2008;59:123–7. [PubMed] [Google Scholar]
- 33.Yosten GLC, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol. 2009;297:R330–6. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yosten GLC, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol. 2010;298:R1642–7. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]